Role of Polyamines in Asthma Pathophysiology
Abstract
:1. Introduction
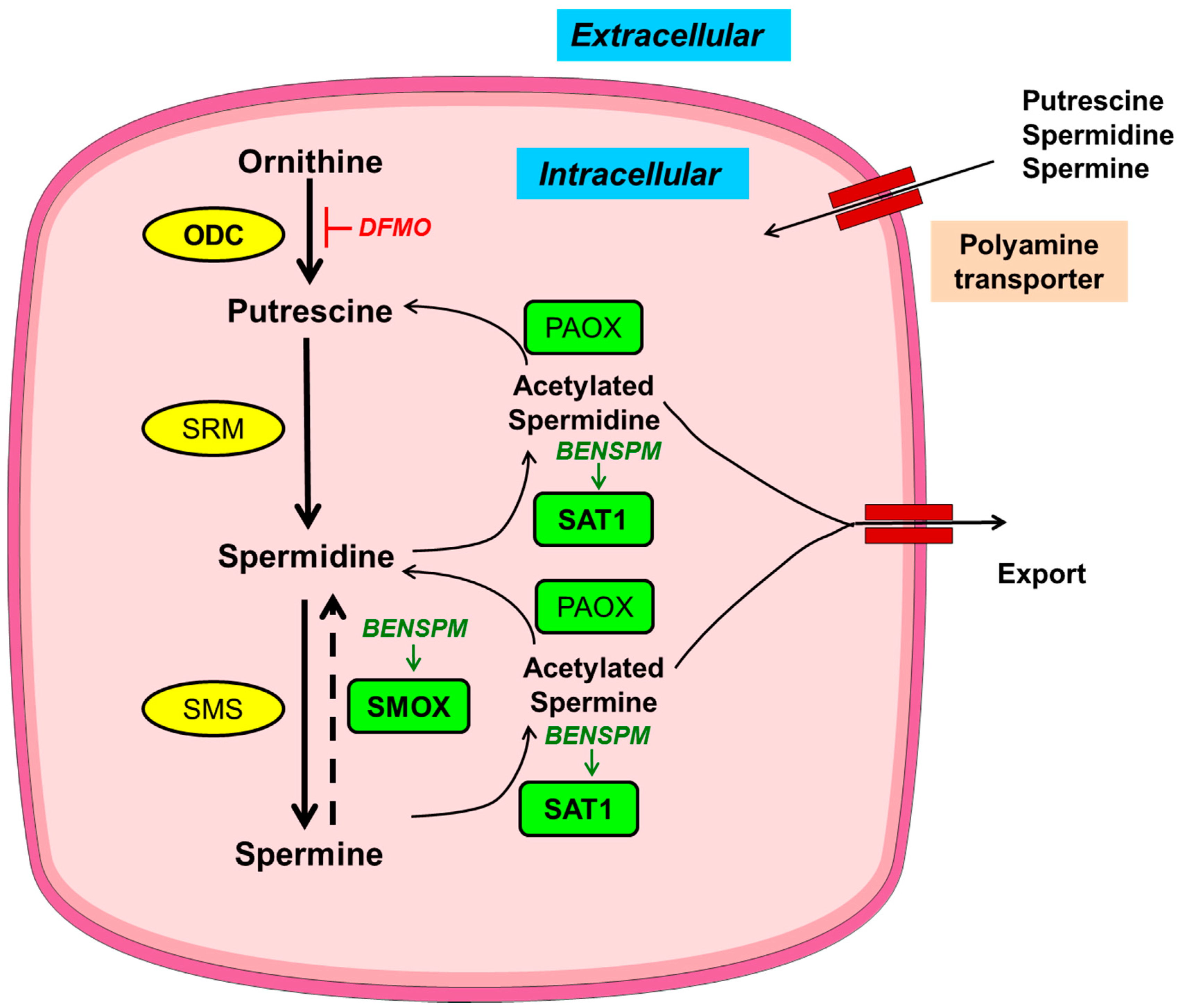
2. Homeostasis of Polyamines
3. Asthma Pathophysiology
4. Alteration of Polyamines in Asthma
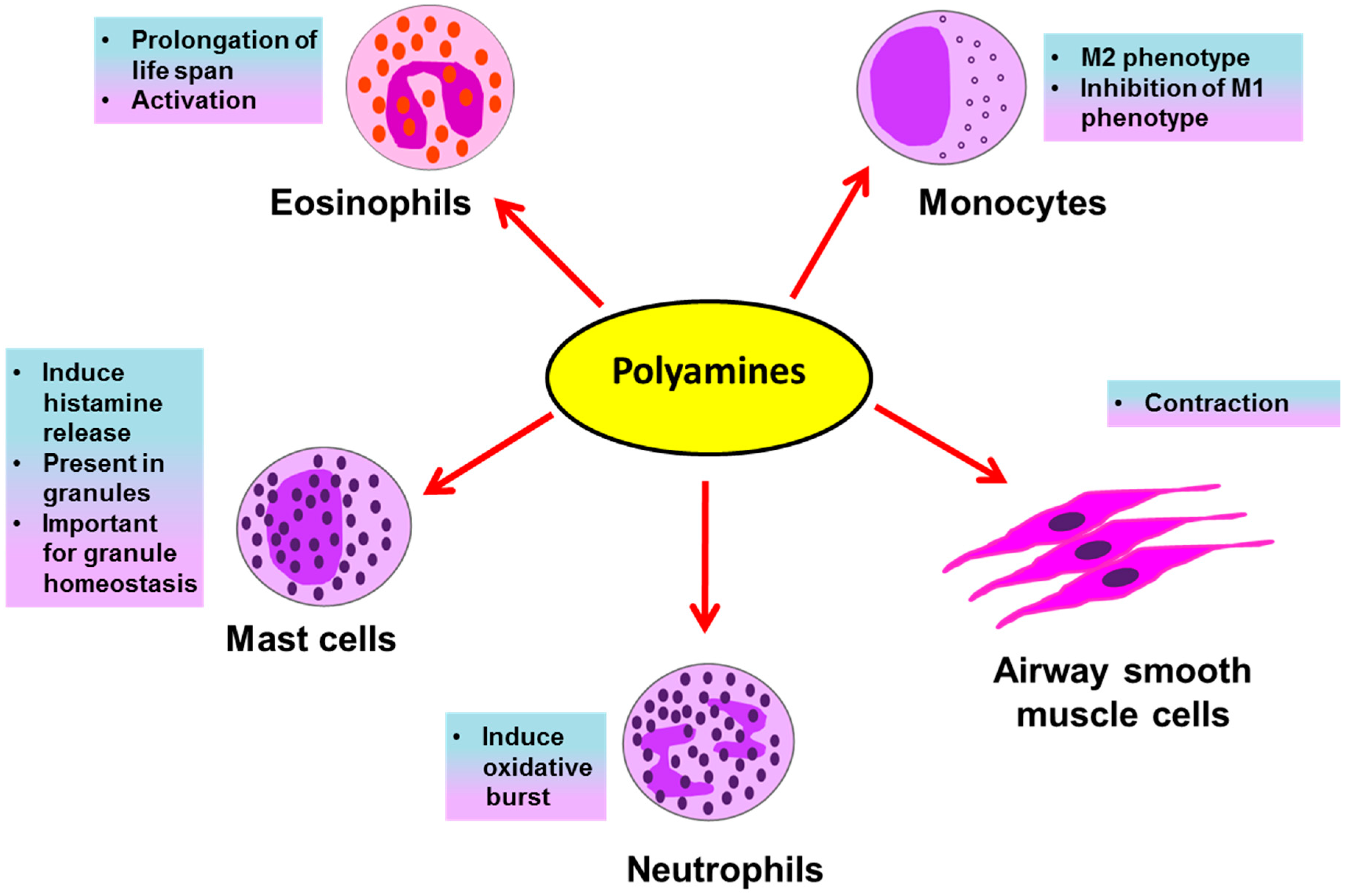
5. Polyamines Affect Immune Cells in Asthma
5.1. Polyamines and Mast Cells
5.2. Polyamines and Eosinophils
5.3. Polyamines and Neutrophils
5.4. Polyamines and Monocytes/Macrophages
6. Polyamines and Structural Airway Smooth Muscle Cells
7. Discussion
8. Conclusions and Future Perspective
Acknowledgments
Conflicts of Interest
References
- Dobell, C. Antony van Leeuwenhoek and His “Little Animals”; Being Some Account of the Father of Protozoology and Bateriology and His Multifarious Discoveries in These Disciplines; John Bale, Sons & Danielson Ltd.: London, UK, 1932. [Google Scholar]
- Woster, P.M. Polyamine Cell Signaling; Humana Press: New York, NY, USA, 2006; pp. 3–24. [Google Scholar]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef] [PubMed]
- Tabor, C.W.; Tabor, H. Polyamines. Annu. Rev. Biochem. 1984, 53, 749–790. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in aging and disease. Aging 2011, 3, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Functions of Polyamines in Mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [PubMed]
- Seiler, N.; Raul, F. Polyamines and apoptosis. J. Cell. Mol. Med. 2005, 9, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Nowotarski, S.L.; Woster, P.M.; Casero, R.A., Jr. Polyamines and cancer: Implications for chemotherapy and chemoprevention. Expert Rev. Mol. Med. 2013, 15, e3. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Replicative senescence: An old lives’ tale? Cell 1996, 84, 497–500. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Baines, K.J.; Fu, J.; Scott, H.A.; Gibson, P.G. The Neutrophilic Inflammatory Phenotype Is Associated With Systemic Inflammation in Asthma. Chest 2012, 142, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, M.; Shimizu, Y.; Tsukagoshi, H.; Ueki, M. Elevated levels of peripheral-blood, naturally occurring aliphatic polyamines in bronchial asthmatic patients with active symptoms. Allergy 1992, 47, 638–643. [Google Scholar] [CrossRef] [PubMed]
- North, M.L.; Grasemann, H.; Khanna, N.; Inman, M.D.; Gauvreau, G.M.; Scott, J.A. Increased ornithine-derived polyamines cause airway hyperresponsiveness in a mouse model of asthma. Am. J. Respir. Cell Mol. Biol. 2013, 48, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.M.; Fraser, A.V.; Hughes, A. A perspective of polyamine metabolism. Biochem. J. 2003, 376, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Seiler, N.; Delcros, J.G.; Moulinoux, J.P. Polyamine transport in mammalian cells. Int. J. Biochem. Cell Biol. 1996, 28, 843–861. [Google Scholar] [CrossRef]
- Babbar, N.; Gerner, E.W. Targeting polyamines and inflammation for cancer prevention. Clin. Cancer Prev. 2011, 188, 49–64. [Google Scholar] [CrossRef]
- Muth, A.; Madan, M.; Archer, J.J.; Ocampo, N.; Rodriguez, L.; Phanstiel, O. Polyamine transport inhibitors: Design, synthesis, and combination therapies with difluoromethylornithine. J. Med. Chem. 2014, 57, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; McCann, P.P. Polyamine metabolism and function. Am. J. Physiol. 1982, 243, C212–C221. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Spermidine/spermine-N1 -acetyltransferase: A key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E995–E1010. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.W.; Bernacki, R.J.; Miller, J.; Bergeron, R.J. Antitumor activity of N1,N11-bis(ethyl)norspermine against human melanoma xenografts and possible biochemical correlates of drug action. Cancer Res. 1993, 53, 581–586. [Google Scholar] [PubMed]
- Ramakrishna, L.; de Vries, V.C.; Curotto de Lafaille, M.A. Cross-roads in the lung: Immune cells and tissue interactions as determinants of allergic asthma. Immunol. Res. 2012, 53, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Free, M.E.; Whitehead, G.S.; Maruoka, S.; Wilson, R.H.; Nakano, K.; Cook, D.N. Pulmonary CD103+ dendritic cells prime Th2 responses to inhaled allergens. Mucosal Immunol. 2012, 5, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. Innate and adaptive immune responses in asthma. Nat. Med. 2012, 18, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Lebman, D.A.; Coffman, R.L. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J. Exp. Med. 1988, 168, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R.; Okuda, M.; Yssel, H.; Okumura, K.; Ra, C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc epsilonRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J. Clin. Investig. 1997, 99, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Wills-Karp, M. Interleukin-13 in asthma pathogenesis. Curr. Allergy Asthma Rep. 2004, 4, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Homer, R.J.; Wang, Z.; Chen, Q.; Geba, G.P.; Wang, J.; Zhang, Y.; Elias, J.A. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Investig. 1999, 103, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Perkins, C.; Wills-Karp, M.; Finkelman, F.D. IL-4 induces IL-13-independent allergic airway inflammation. J. Allergy Clin. Immunol. 2006, 118, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Canonica, G.W. The role of interleukin 5 in asthma. Expert Rev. Clin. Immunol. 2016, 12, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Girodet, P.-O.; Nguyen, D.; Mancini, J.D.; Hundal, M.; Zhou, X.; Israel, E.; Cernadas, M. Alternative Macrophage Activation Is Increased in Asthma. Am. J. Respir. Cell Mol. Biol. 2016, 55, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Martin, J.G. Structural aspects of airway remodeling in asthma. Curr. Allergy Asthma Rep. 2008, 8, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. Epithelium dysfunction in asthma. J. Allergy Clin. Immunol. 2007, 120, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yin, G.; Liu, J.; Liu, X.; Zhao, S. Epithelial apoptosis and loss in airways of children with asthma. J. Asthma Off. J. Assoc. Care Asthma 2011, 48, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Bartemes, K.R.; Kita, H. Dynamic role of epithelium-derived cytokines in asthma. Clin. Immunol. 2012, 143, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Avila, P.C. Plasticity of airway epithelial cells. J. Allergy Clin. Immunol. 2011, 128, 1225–1226. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, N.; King, N.E.; Laporte, J.; Yang, M.; Mishra, A.; Pope, S.M.; Muntel, E.E.; Witte, D.P.; Pegg, A.A.; Foster, P.S.; et al. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J. Clin. Investig. 2003, 111, 1863–1874. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V. Eosinophilic and Neutrophilic Inflammation in Asthma: Insights from Clinical Studies. Proc. Am. Thorac. Soc. 2009, 6, 256–259. [Google Scholar] [CrossRef] [PubMed]
- White, M.V. The role of histamine in allergic diseases. J. Allergy Clin. Immunol. 1990, 86, 599–605. [Google Scholar] [CrossRef]
- Vargas, L.; Patiño, P.J.; Montoya, F.; Vanegas, A.C.; Echavarría, A.; García de Olarte, D. A study of granulocyte respiratory burst in patients with allergic bronchial asthma. Inflammation 1998, 22, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Sampson, A.P. The role of eosinophils and neutrophils in inflammation. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2000, 30 (Suppl. 1), 22–27. [Google Scholar] [CrossRef]
- Reuter, S.; Stassen, M.; Taube, C. Mast cells in allergic asthma and beyond. Yonsei Med. J. 2010, 51, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, M.; Uno, D.; Kobayashi, S. Naturally occurring aliphatic polyamines-induced histamine release from rat peritoneal mast cells. Allergy 1991, 46, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, M.; Uno, D.; Hanawa, K.; Kobayashi, S. Polyamines stimulate the phosphorylation of phosphatidylinositol in rat mast cell granules. Allergy 1990, 45, 262–267. [Google Scholar] [CrossRef] [PubMed]
- García-Faroldi, G.; Rodríguez, C.E.; Urdiales, J.L.; Pérez-Pomares, J.M.; Dávila, J.C.; Pejler, G.; Sánchez-Jiménez, F.; Fajardo, I. Polyamines Are Present in Mast Cell Secretory Granules and Are Important for Granule Homeostasis. PLoS ONE 2010, 5, e15071. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Harada, Y.; Moriyama, S.; Furuta, K.; Tanaka, S.; Miyaji, T.; Omote, H.; Moriyama, Y.; Hiasa, M. Vesicular Polyamine Transporter Mediates Vesicular Storage and Release of Polyamine from Mast Cells. J. Biol. Chem. 2017, 292, 3909–3918. [Google Scholar] [CrossRef] [PubMed]
- Ilmarinen, P.; Moilanen, E.; Erjefält, J.S.; Kankaanranta, H. The polyamine spermine promotes survival and activation of human eosinophils. J. Allergy Clin. Immunol. 2015, 136, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Rihs, S.; Braun, R.K.; Betz, S.; Bruijnzeel, P.L. Increased expression of CD11b and functional changes in eosinophils after migration across endothelial cell monolayers. J. Immunol. 1993, 150, 4061–4071. [Google Scholar] [PubMed]
- Combadière, C.; el Benna, J.; Pedruzzi, E.; Hakim, J.; Périanin, A. Stimulation of the human neutrophil respiratory burst by formyl peptides is primed by a protein kinase inhibitor, staurosporine. Blood 1993, 82, 2890–2898. [Google Scholar] [PubMed]
- Mann, B.S.; Chung, K.F. Blood neutrophil activation markers in severe asthma: Lack of inhibition by prednisolone therapy. Respir. Res. 2006, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, C.; Georgountzos, A.; Caldarera, I.; Flamigni, F.; Ligabue, A. Polyamines stimulate superoxide production in human neutrophils activated by N-fMet-Leu-Phe but not by phorbol myristate acetate. Biochim. Biophys. Acta 1987, 930, 135–139. [Google Scholar] [CrossRef]
- Melgert, B.N.; ten Hacken, N.H.; Rutgers, B.; Timens, W.; Postma, D.S.; Hylkema, M.N. More alternative activation of macrophages in lungs of asthmatic patients. J. Allergy Clin. Immunol. 2011, 127, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Jeong, J.J.; Nyenhuis, S.; Berdyshev, E.; Chung, S.; Ranjan, R.; Karpurapu, M.; Deng, J.; Qian, F.; Kelly, E.A.B.; et al. Recruited Alveolar Macrophages, in Response to Airway Epithelial–Derived Monocyte Chemoattractant Protein 1/CCL2, Regulate Airway Inflammation and Remodeling in Allergic Asthma. Am. J. Respir. Cell Mol. Biol. 2015, 52, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, J.; Lamers, W.H.; Koehler, E.S.; Geuns, J.M.C.; Alhonen, L.; Uimari, A.; Pirnes-Karhu, S.; Van Overmeire, E.; Morias, Y.; Brys, L.; et al. Pivotal Advance: Arginase-1-independent polyamine production stimulates the expression of IL-4-induced alternatively activated macrophage markers while inhibiting LPS-induced expression of inflammatory genes. J. Leukoc. Biol. 2012, 91, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Noble, P.B.; Pascoe, C.D.; Lan, B.; Ito, S.; Kistemaker, L.E.M.; Tatler, A.L.; Pera, T.; Brook, B.S.; Gosens, R.; West, A.R. Airway smooth muscle in asthma: Linking contraction and mechanotransduction to disease pathogenesis and remodelling. Pulm. Pharmacol. Ther. 2014, 29, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.R.A.; Roth, M.; Tamm, M.; Hughes, M.; Ge, Q.; King, G.; Burgess, J.K.; Black, J.L. Airway Smooth Muscle Cell Proliferation Is Increased in Asthma. Am. J. Respir. Crit. Care Med. 2001, 164, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Halayko, A.J.; Salari, H.; MA, X.; Stephens, N.L. Markers of airway smooth muscle cell phenotype. Am. J. Physiol. 1996, 270, L1040–L1051. [Google Scholar] [CrossRef] [PubMed]
- Gunst, S.J.; Tang, D.D. The contractile apparatus and mechanical properties of airway smooth muscle. Eur. Respir. J. 2000, 15, 600–616. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Grasemann, H.; Shehnaz, D.; Enomoto, M.; Leadley, M.; Belik, J.; Ratjen, F. L-ornithine derived polyamines in cystic fibrosis airways. PLoS ONE 2012, 7, e46618. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Baron, C.B.; Griffiths, T.; Greeley, P.; Coburn, R.F. Effects of polyamines and calcium and sodium ions on smooth muscle cytoskeleton-associated phosphatidylinositol (4)-phosphate 5-kinase. J. Cell. Physiol. 1998, 177, 161–173. [Google Scholar] [CrossRef]
- Chen, C.; Kudo, M.; Rutaganira, F.; Takano, H.; Lee, C.; Atakilit, A.; Robinett, K.S.; Uede, T.; Wolters, P.J.; Shokat, K.M.; et al. Integrin α9β1 in airway smooth muscle suppresses exaggerated airway narrowing. J. Clin. Investig. 2012, 122, 2916–2927. [Google Scholar] [CrossRef] [PubMed]
- Childs, A.C.; Mehta, D.J.; Gerner, E.W. Polyamine-dependent gene expression. Cell. Mol. Life Sci. 2003, 60, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.A.; Gilmour, S.K. High levels of intracellular polyamines promote histone acetyltransferase activity resulting in chromatin hyperacetylation. J. Cell. Biochem. 2000, 77, 345–360. [Google Scholar] [CrossRef]
- Hardbower, D.M.; Asim, M.; Luis, P.B.; Singh, K.; Barry, D.P.; Yang, C.; Steeves, M.A.; Cleveland, J.L.; Schneider, C.; Piazuelo, M.B.; et al. Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications. Proc. Natl. Acad. Sci. USA 2017, 114, E751–E760. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Viar, M.J.; Li, J.; Shi, H.J.; McCormack, S.A.; Johnson, L.R. Polyamines are necessary for normal expression of the transforming growth factor-beta gene during cell migration. Am. J. Physiol. 1997, 272, G713–G720. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kumar, M.; Negi, V.; Pattnaik, B.; Prakash, Y.S.; Agrawal, A.; Ghosh, B. MicroRNA-326 regulates profibrotic functions of transforming growth factor-β in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2014, 50, 882–892. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, V. Role of Polyamines in Asthma Pathophysiology. Med. Sci. 2018, 6, 4. https://doi.org/10.3390/medsci6010004
Jain V. Role of Polyamines in Asthma Pathophysiology. Medical Sciences. 2018; 6(1):4. https://doi.org/10.3390/medsci6010004
Chicago/Turabian StyleJain, Vaibhav. 2018. "Role of Polyamines in Asthma Pathophysiology" Medical Sciences 6, no. 1: 4. https://doi.org/10.3390/medsci6010004




