The Reality Monitoring Deficit as a Common Neuropsychological Correlate of Schizophrenic and Affective Psychosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Psychopatological Assessment
2.3. Source Monitoring Task
2.4. Data Analysis
3. Results
| Mood disorder | Schizophrenia | Controls | |||||
|---|---|---|---|---|---|---|---|
| Delusional (n = 21) | Non delusional (n = 23) | Delusional (n = 35) | Non delusional (n = 19) | (n = 50) | F | p | |
| Sex (M/F) | 7/14 | 5/18 | 18/17 | 10/9 | 23/27 | ||
| Age | 39.33 | 39.43 | 34.11 | 34.95 | 32.28 | 2.39 | 0.053 |
| Age at onset | 29.20 | 28.84 | 22.21 | 24.95 | 5.13 | 0.003 | |
| FVPS score | 42.33 | 40.71 | 52.17 | 40.90 | 33.95 | 6.47 | 0.000 |
| IPSAQ Externalizing Bias | 3.00 | 2.57 | 3.08 | 4.36 | 5.30 | 1.94 | 0.111 |
| IPSAQ Personalizing Bias | 0.58 | 0.51 | 0.56 | 0.48 | 0.39 | 1.86 | 0.124 |
| Mood disorder | Schizophrenia | Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| Delusional (n = 21) | Non delusional (n = 23) | Delusional (n = 35) | Non delusional (n = 19) | (n = 50) | F | p | ||
| Stem | ||||||||
| d' | 2.68(0.83) | 1.16(2.11) | 2.55(0.66) | 2.13(1.27) | 3.00(0.69) | 9.99 | < 0.001 | |
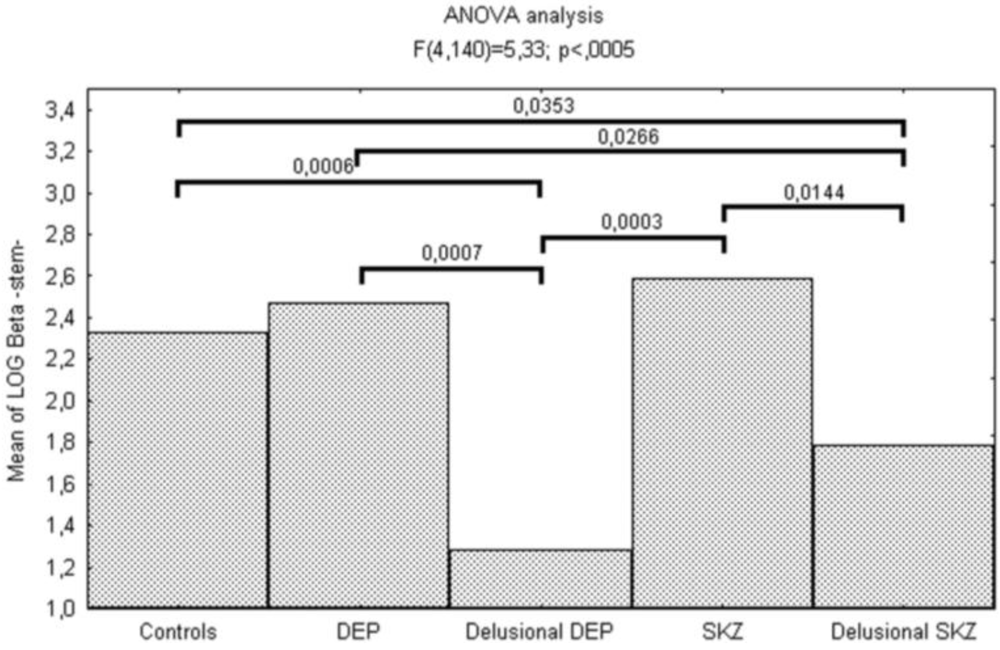
| log β | 1.28(1.99) | 2.47(0.72) | 1.78(1.24) | 2.59(0.68) | 2.32(0.80) | 5.33 | < 0.001 | |
| Picture | ||||||||
| d' | 3.87(0.97) | 4.03(0.79) | 3.06(1.03) | 3.20(1.57) | 4.24(0.72) | 9.11 | < 0.001 | |
| log β | −0.04(1.32) | 0.42(1.19) | 0.62(1.30) | 1.19(1.33) | 0.99(1.26) | 3.36 | 0.03 | |
| Heard | ||||||||
| d' | 1.41(0.59) | 1.78(0.71) | 1.22(0.88) | 1.34(0.54) | 2.13(0.62) | 10.79 | < 0.001 | |
| log β | 1.51(0.79) | 1.31(0.58) | 1.32(1.01) | 1.65(0.79) | 1.76(0.86) | 1.83 | 0.12 | |
4. Discussion
5. Conclusions
Conflict of Interest
References
- Waters, F.; Allen, P.; Aleman, A.; Fernyhough, C.; Woodward, T.S.; Badcock, J.C.; Barkus, E.; Johns, L.; Varese, F.; Menon, M.; et al. Auditory hallucinations in schizophrenia and nonschizophrenia populations: A review and integrated model of cognitive mechanisms. Schizophr. Bull. 2012, 38, 683–693. [Google Scholar]
- Johnson, M.K.; Hashtroudi, S.; Lindsay, D.S. Source monitoring. Psychol. Bull. 1993, 114, 3–28. [Google Scholar]
- Johnson, M.K.; Foley, M.A.; Leach, K. The consequences for memory of imagining in another person’s voice. Mem. Cognit. 1988, 16, 337–342. [Google Scholar]
- Johnson, M.K. Discriminating the origins of information. In Delusional Beliefs; Oltmanns, T.F., Maher, B.A., Eds.; Wiley: New York, NY, USA, 1988; pp. 34–65. [Google Scholar]
- Johnson, M.K.; Foley, M.A.; Suengas, A.G.; Raye, C.L. Phenomenal characteristics of memories for perceived and imagined autobiographical events. J. Exp. Psychol. Gen. 1988, 117, 371–376. [Google Scholar]
- Hashtroudi, S.; Johnson, M.K.; Chrosniak, L.D. Aging and source monitoring. Psychol. Aging 1989, 4, 106–112. [Google Scholar]
- Lindsay, D.S.; Johnson, M.K.; Kwon, P. Developmental changes in memory source monitoring. J. Exp. Child Psychol. 1991, 52, 297–318. [Google Scholar]
- Bentall, R.P. The illusion of reality: A review and integration of psychological research on hallucinations. Psychol. Bull. 1990, 107, 82–95. [Google Scholar]
- Baker, C.A.; Morrison, A.P. Cognitive processes in auditory hallucinations: Attributional biases and metacognition. Psychol. Med. 1998, 28, 1199–1208. [Google Scholar]
- Keefe, R.S.; Arnold, M.C.; Bayen, U.J.; McEvoy, J.P.; Wilson, W.H. Source-monitoring deficits for self-generated stimuli in schizophrenia: Multinomial modeling of data from three sources. Schizophr. Res. 2002, 57, 51–67. [Google Scholar]
- Anselmetti, S.; Cavallaro, R.; Bechi, M.; Angelone, S.M.; Ermoli, E.; Cocchi, F.; Smeraldi, E. Psychopathological and neuropsychological correlates of source monitoring impairment in schizophrenia. Psychiatr. Res 2007, 150, 51–59. [Google Scholar]
- Harvey, P.D. Reality monitoring in mania and schizophrenia. The association of thought disorder and performance. J. Nerv. Ment. Dis. 1985, 173, 67–73. [Google Scholar]
- Nienow, T.M.; Docherty, N.M. Internal source monitoring and thought disorder in schizophrenia. J. Nerv. Ment. Dis. 2004, 192, 696–700. [Google Scholar]
- Nathaniel-James, D.A.; Frith, C.D. Confabulation in schizophrenia: Evidence of a new form? Psychol. Med. 1996, 26, 391–399. [Google Scholar]
- Brebion, G.; Smith, M.J.; Amador, X.; Malaspina, D.; Gorman, J.M. Word recognition, discrimination accuracy, and decision bias in schizophrenia: Association with positive symptomatology and depressive symptomatology. J. Nerv. Ment. Dis. 1998, 186, 604–609. [Google Scholar]
- Keefe, R.S.; Arnold, M.C.; Bayen, U.J.; Harvey, P.D. Source monitoring deficits in patients with schizophrenia; a multinomial modelling analysis. Psychol. Med. 1999, 29, 903–914. [Google Scholar]
- Martinez-Aran, A.; Vieta, E.; Colom, F.; Reinares, M.; Benabarre, A.; Gasto, C.; Salamero, M. Cognitive dysfunctions in bipolar disorder: Evidence of neuropsychological disturbances. Psychother. Psychosom. 2000, 69, 2–18. [Google Scholar]
- Bearden, C.E.; Hoffman, K.M.; Cannon, T.D. The neuropsychology and neuroanatomy of bipolar affective disorder: A critical review. Bipolar. Disord. 2001, 3, 106–150. [Google Scholar]
- Murphy, F.C.; Sahakian, B.J. Neuropsychology of bipolar disorder. Br. J. Psychiatr. Suppl. 2001, 41, s120–s127. [Google Scholar]
- Quraishi, S.; Frangou, S. Neuropsychology of bipolar disorder: A review. J. Affect. Disord. 2002, 72, 209–226. [Google Scholar]
- Basso, M.R.; Bornstein, R.A. Neuropsychological deficits in psychotic versus nonpsychotic unipolar depression. Neuropsychology 1999, 13, 69–75. [Google Scholar]
- Ilsley, J.E.; Moffoot, A.P.; O'Carroll, R.E. An analysis of memory dysfunction in major depression. J. Affect. Disord. 1995, 35, 1–9. [Google Scholar]
- Benedetti, F.; Anselmetti, S.; Marcello, F.; Daniele, R.; Roberto, C.; Cristina, C.; Enrico, S. Reality monitoring and paranoia in remitted delusional depression. Clin. Psychiatr. 2005, 23, 199–205. [Google Scholar]
- Waters, F.; Woodward, T.; Allen, P.; Aleman, A.; Sommer, I. Self-recognition deficits in schizophrenia patients with auditory hallucinations: A meta-analysis of the literature. Schizophr. Bull. 2012, 38, 741–750. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994; pp. 339–345. [Google Scholar]
- Fenigstein, A.; Vanable, P.A. Paranoia and self-consciousness. J. Pers. Soc. Psychol. 1992, 62, 129–138. [Google Scholar]
- Kinderman, P.; Bentall, R.P. Causal attributions in paranoia and depression: Internal, Personal, and situational attributions for negative events. J. Abnorm. Psychol. 1997, 106, 341–345. [Google Scholar]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatr. 1960, 23, 56–62. [Google Scholar]
- Battig, W.; Montague, W. Category norms for verbal items in 56 categories: A replication and extension of the Connecticut category norms. J. Exp. Psychol. 1969, 89, 1–46. [Google Scholar]
- Dell'Acqua, R.; Lotto, L.; Job, R. Naming times and standardized norms for the Italian PD/DPSS set of 266 pictures: Direct comparisons with American, English, French, and Spanish published databases. Behav. Res. Meth. Instrum. Comput. 2000, 32, 588–615. [Google Scholar]
- Harvey, L. RScorePlus, v.5.3.4. Parameter estimation of signal detection models. Available online: http://psych.colorado.edu/~lharvey/ (accessed on 4 October 2006).
- Dunbar, G.C.; Lishman, W.A. Depression, Recognition-memory and hedonic tone a signal detection analysis. Br. J. Psychiatr. 1984, 144, 376–382. [Google Scholar]
- Mogg, K.; Mathews, A.; Weinman, J. Memory bias in clinical anxiety. J. Abnorm. Psychol. 1987, 96, 94–98. [Google Scholar]
- Brown, H.D.; Kosslyn, S.M.; Breiter, H.C.; Baer, L.; Jenike, M.A. Can patients with obsessive-compulsive disorder discriminate between percepts and mental images? A signal detection analysis. J. Abnorm. Psychol. 1994, 103, 445–454. [Google Scholar]
- Kadlec, H. Statistical properties of d' and beta estimates of signal detection theory. Psychol. Meth. 1999, 4, 22–43. [Google Scholar]
- Achim, A.M.; Weiss, A.P. No evidence for a differential deficit of reality monitoring in schizophrenia: A meta-analysis of the associative memory literature. Cogn. Neuropsychiatr. 2008, 13, 369–384. [Google Scholar]
- Schatzberg, A.F. Non-schizophrenic psychoses: Common and distinguishing features. J. Psychiatr. Res. 2004, 38, 1–2. [Google Scholar]
- Ketter, T.A.; Wang, P.W.; Becker, O.V.; Nowakowska, C.; Yang, Y. Psychotic bipolar disorders: Dimensionally similar to or categorically different from schizophrenia? J. Psychiatr. Res. 2004, 38, 47–61. [Google Scholar]
- Kendler, K.S.; McGuire, M.; Gruenberg, A.M.; O'Hare, A.; Spellman, M.; Walsh, D. The Roscommon Family Study. IV. Affective illness, Anxiety disorders, and alcoholism in relatives. Arch. Gen. Psychiatr. 1993, 50, 952–960. [Google Scholar]
- Wang, P.W.; Ketter, T.A. Biology and recent brain imaging studies in affective psychoses. Curr. Psychiatr. Rep. 2000, 2, 298–304. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Radaelli, D.; Benedetti, F.; Cavallaro, R.; Colombo, C.; Smeraldi, E. The Reality Monitoring Deficit as a Common Neuropsychological Correlate of Schizophrenic and Affective Psychosis. Behav. Sci. 2013, 3, 244-252. https://doi.org/10.3390/bs3020244
Radaelli D, Benedetti F, Cavallaro R, Colombo C, Smeraldi E. The Reality Monitoring Deficit as a Common Neuropsychological Correlate of Schizophrenic and Affective Psychosis. Behavioral Sciences. 2013; 3(2):244-252. https://doi.org/10.3390/bs3020244
Chicago/Turabian StyleRadaelli, Daniele, Francesco Benedetti, Roberto Cavallaro, Cristina Colombo, and Enrico Smeraldi. 2013. "The Reality Monitoring Deficit as a Common Neuropsychological Correlate of Schizophrenic and Affective Psychosis" Behavioral Sciences 3, no. 2: 244-252. https://doi.org/10.3390/bs3020244




