Reasoning about “Capability”: Wild Robins Respond to Limb Visibility in Humans
Abstract
:1. Introduction
2. Methods
2.1. Study Area and Subjects
2.2. Procedure
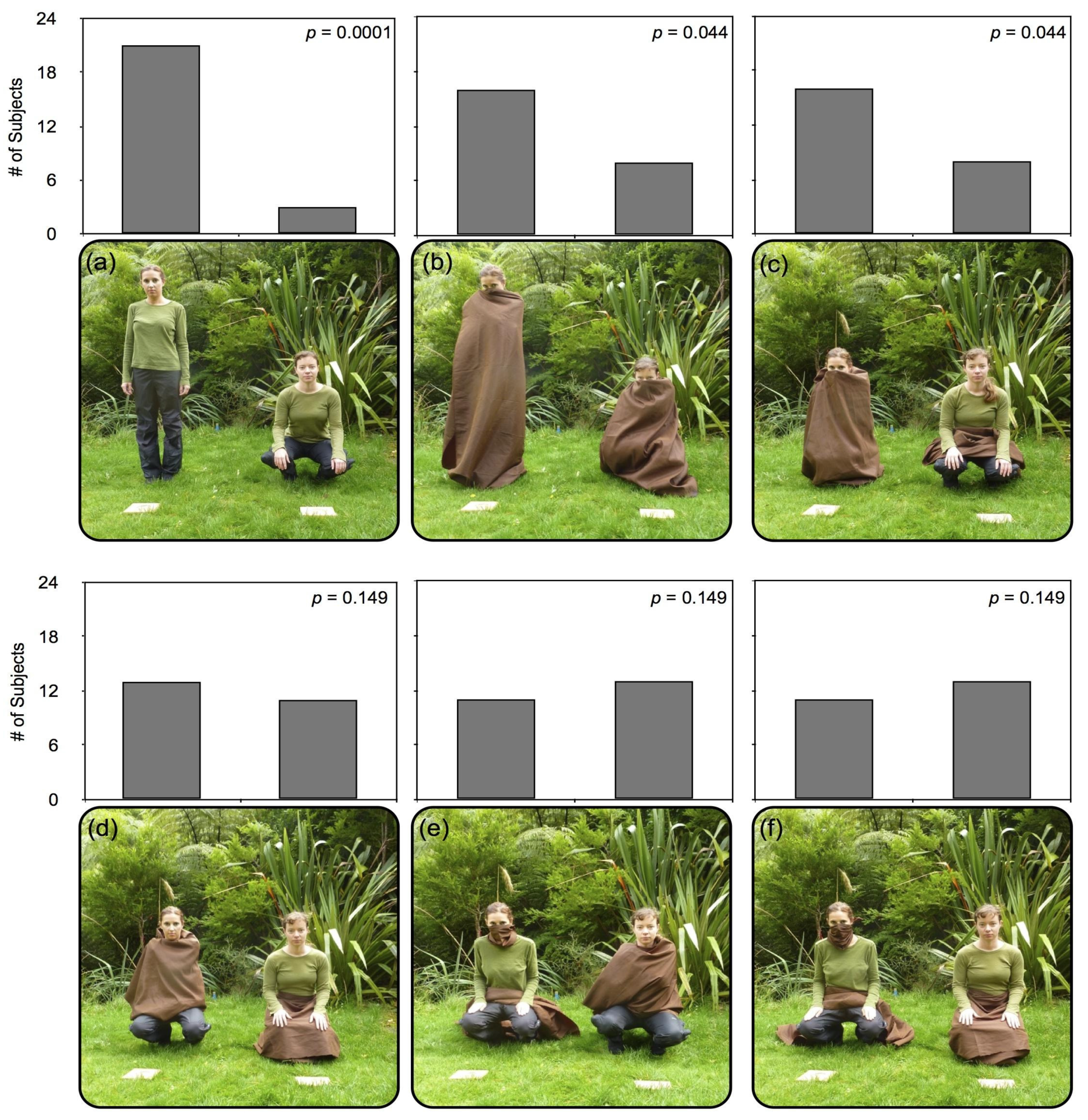
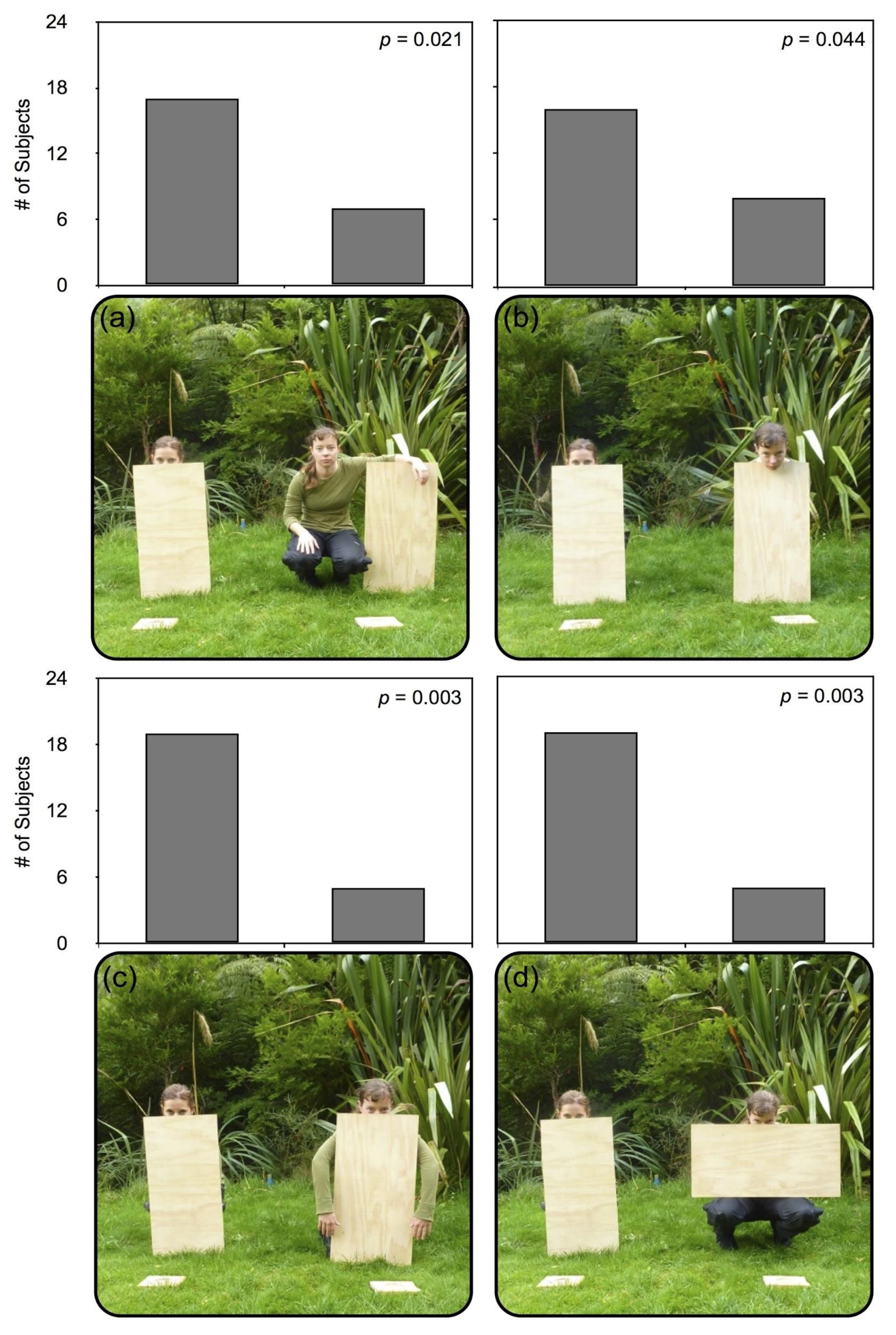
3. Experiment 1
3.1. Procedure
3.2. Results
4. Experiment 2
4.1. Procedure
4.2. Results
5. Discussion
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Spelke, E.S.; Kinzler, K.D. Core knowledge. Dev. Sci. 2007, 10, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Agrillo, C.; Regolin, L.; Vallortigara, G. Can young chicks take into account the observer’s perspective? Perception 2004, 33, 110–111. [Google Scholar]
- Flombaum, J.I.; Santos, L.R. Rhesus monkeys attribute perceptions to others. Curr. Biol. 2005, 15, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Hare, B.; Call, J.; Tomasello, M. Do chimpanzees know what conspecifics know? Anim. Behav. 2001, 61, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Behne, T.; Carpenter, M.; Call, J.; Tomasello, M. Unwilling versus unable: Infants’ understanding of intentional action. Dev. Psychol. 2005, 41, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Call, J.; Hare, B.; Carpenter, M.; Tomasello, M. “Unwilling” versus “unable”: Chimpanzees’ understanding of human intentional action. Dev. Sci. 2004, 7, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Péron, F.; Rat-Fischer, L.; Nagle, L.; Bovet, D. “Unwilling” versus “unable”: Do grey parrots understand human intentional actions? Interact. Stud. 2010, 11, 428–441. [Google Scholar]
- Phillips, W.; Barnes, J.L.; Mahajan, N.; Yamaguchi, M.; Santos, L.R. “Unwilling” versus “unable”: Capuchin monkeys’ (Cebus apella) understanding of human intentional action. Dev. Sci. 2009, 12, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Bateman, P.; Fleming, P. Who are you looking at? Hadeda ibises use direction of gaze, head orientation and approach speed in their risk assessment of a potential predator. J. Zool. 2011, 285, 316–323. [Google Scholar] [CrossRef]
- Bräuer, J.; Call, J.; Tomasello, M. All great ape species follow gaze to distant locations and around barriers. J. Comp. Psychol. 2005, 119, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Bugnyar, T.; Stöwe, M.; Heinrich, B. Ravens, Corvus corax, follow gaze direction of humans around obstacles. Proc. R. Soc. London B: Biol. Sci. 2004, 271, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Lyons, N.J.; Cole, H.L.; Goldsmith, A.R. Subtle cues of predation risk: Starlings respond to a predator’s direction of eye-gaze. Proc. R. Soc. London B: Biol. Sci. 2008, 275, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Jaime, M.; Lopez, J.P.; Lickliter, R. Bobwhite quail (Colinus virginianus) hatchlings track the direction of human gaze. Anim. Cogn. 2009, 12, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Horner, V.; Whiten, A. Causal knowledge and imitation/emulation switching in chimpanzees (Pan troglodytes) and children (Homo sapiens). Anim. Cogn. 2005, 8, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.; Subiaul, F. Do chimpanzees know what others can and cannot do? Reasoning about “capability”. Anim. Cogn. 2009, 12, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Povinelli, D.J.; Perilloux, H.K.; Reaux, J.E.; Bierschwale, D.T. Young and juvenile chimpanzees’ (Pan troglodytes) reactions to intentional versus accidental and inadvertent actions. Behav. Processes 1998, 42, 205–218. [Google Scholar] [CrossRef]
- Myowa-Yamakoshi, M.; Scola, C.; Hirata, S. Humans and chimpanzees attend differently to goal-directed actions. Nat. Commun. 2012, 3, 693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Bayern, A.M.; Emery, N.J. Jackdaws respond to human attentional states and communicative cues in different contexts. Curr. Biol. 2009, 19, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Hare, B.; Tomasello, M. Domestic dogs (Canis familiaris) use human and conspecific social cues to locate hidden food. J. Comp. Psychol. 1999, 113, 173–177. [Google Scholar] [CrossRef]
- Miklösi, A.; Polgárdi, R.; Topál, J.; Csányi, V. Use of experimenter-given cues in dogs. Anim. Cogn. 1998, 1, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.P.; Litchfield, C.A. Dingoes (Canis dingo) can use human social cues to locate hidden food. Anim. Cogn. 2010, 13, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Pack, A.A.; Herman, L.M. Bottlenosed dolphins (Tursiops truncatus) comprehend the referent of both static and dynamic human gazing and pointing in an object-choice task. J. Comp. Psychol. 2004, 118, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, J.; Riedel, J.; Call, J.; Tomasello, M. Domestic goats, Capra hircus, follow gaze direction and use social cues in an object choice task. Anim. Behav. 2005, 69, 11–18. [Google Scholar] [CrossRef]
- Maros, K.; Gácsi, M.; Miklósi, Á. Comprehension of human pointing gestures in horses (Equus caballus). Anim. Cogn. 2008, 11, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Péron, F.; Chardard, C.; Nagle, L.; Bovet, D. Do African grey parrots (Psittacus erithacus) know what a human experimenter does and does not see? Behav. Processes 2011, 87, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Giret, N.; Miklósi, Á.; Kreutzer, M.; Bovet, D. Use of experimenter-given cues by African gray parrots (Psittacus erithacus). Anim. Cogn. 2009, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Powlesland, R. The Foraging Behaviour of the South Island Robin; Te Rau Press: Gisborne, New Zealand, 1981. [Google Scholar]
- Menzies, I.J.; Burns, K. Temporal shifts in the pair-bond dynamics of New Zealand robins (Petroica australis). N. Z. J. Ecol. 2010, 34, 265–268. [Google Scholar]
- Van Horik, J.; Burns, K. Cache spacing patterns and reciprocal cache theft in New Zealand robins. Anim. Behav. 2007, 73, 1043–1049. [Google Scholar] [CrossRef]
- Flack, J.D. The use of frontal spot and crown feathers in inter-and intraspecific display by the South Island robin, Petroica australis australis. Notornis 1976, 23, 90–105. [Google Scholar]
- Hare, B.; Tomasello, M. Chimpanzees are more skilful in competitive than in cooperative cognitive tasks. Anim. Behav. 2004, 68, 571–581. [Google Scholar] [CrossRef]
- Garland, A.; Low, J.; Armstrong, N.; Burns, K.C. Wild robins (Petroica longipes) respond to human gaze. Anim. Cogn. 2014, 17, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, S.M.; Amiot, C.; Mclean, I.G.; Lovegrove, T.G.; Armstrong, D.P.; Brunton, D.H.; Ji, W. Losing anti-predatory behaviour: A cost of translocation. Austral Ecol. 2012, 37, 413–418. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; Ripmeester, E.A. Birdsong and anthropogenic noise: Implications and applications for conservation. Mol. Ecol. 2008, 17, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Sol, D.; Lefebvre, L. Behavioural flexibility predicts invasion success in birds introduced to New Zealand. Oikos 2000, 90, 599–605. [Google Scholar] [CrossRef]
- Call, J. Apes know that hidden objects can affect the orientation of other objects. Cognition 2007, 105, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Kundey, S.M.; De Los Reyes, A.; Taglang, C.; Baruch, A.; German, R. Domesticated dogs’ (Canis familiaris) use of the solidity principle. Anim. Cogn. 2010, 13, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Pattison, K.F.; Miller, H.C.; Rayburn-Reeves, R.; Zentall, T. The case of the disappearing bone: Dogs’ understanding of the physical properties of objects. Behav. Processes 2010, 85, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.R.; Sulkowski, G.M.; Spaepen, G.M.; Hauser, M.D. Object individuation using property/kind information in rhesus macaques (Macaca mulatta). Cognition 2002, 83, 241–264. [Google Scholar] [CrossRef]
- Bird, C.D.; Emery, N.J. Rooks perceive support relations similar to six-month-old babies. Proc. R. Soc. London B: Biol. Sci. 2010, 277, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Pollok, B.; Prior, H.; Güntürkün, O. Development of object permanence in food-storing magpies (Pica pica). J. Comp. Psychol. 2000, 114, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Milos, N.; Vallortigara, G. Piagetian object permanence and its development in Eurasian jays (Garrulus glandarius). Anim. Cogn. 2007, 10, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Povinelli, D.J. Folk Physics for Apes: The Chimpanzee’s Theory of How the World Works; Oxford University Press: Oxford, UK, 2000; pp. 206–270. [Google Scholar]
- Helme, A.E.; Call, J.; Clayton, N.S.; Emery, N.J. What do bonobos (Pan paniscus) understand about physical contact? J. Comp. Psychol. 2006, 120, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Chiandetti, C.; Vallortigara, G. Intuitive physical reasoning about occluded objects by inexperienced chicks. Proc. R. Soc. London B: Biol. Sci. 2011, 278, 2621–2627. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.R.; Rutledge, R.B.; Gray, R.D. The right tool for the job: what strategies do wild New Caledonian crows use? Anim. Cogn. 2006, 9, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.H.; Elliffe, D.; Hunt, G.R.; Gray, R.D. Complex cognition and behavioural innovation in New Caledonian crows. Proc. R. Soc. London B: Biol. Sci. 2010, 277, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.H.; Elliffe, D.M.; Hunt, G.R.; Emery, N.J.; Clayton, N.S.; Gray, R.D. New Caledonian crows learn the functional properties of novel tool types. PLoS ONE 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.H.; Miller, R.; Gray, R.D. New Caledonian crows reason about hidden causal agents. Proc. Natl. Acad. Sci. USA 2012, 109, 16389–16391. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.H.; Hunt, G.R.; Medina, F.S.; Gray, R.D. Do New Caledonian crows solve physical problems through causal reasoning? Proc. R. Soc. London B: Biol. Sci. 2009, 276, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Udell, M.A.; Dorey, N.R.; Wynne, C.D. Wolves outperform dogs in following human social cues. Anim. Behav. 2008, 76, 1767–1773. [Google Scholar] [CrossRef]
- Hare, B.; Brown, M.; Williamson, C.; Tomasello, M. The domestication of social cognition in dogs. Science 2002, 298, 1634–1636. [Google Scholar] [CrossRef] [PubMed]
- Miklósi, Á.; Kubinyi, E.; Topál, J.; Gácsi, M.; Virányi, Z.; Csányi, V. A simple reason for a big difference: Wolves do not look back at humans, but dogs do. Curr. Biol. 2003, 13, 763–766. [Google Scholar] [CrossRef]
- Pika, S.; Bugnyar, T. The use of referential gestures in ravens (Corvus corax) in the wild. Nat. Commun. 2011, 2, 560. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Tomonaga, M. The perception of self-agency in chimpanzees (Pan troglodytes). Proc. R. Soc. London B: Biol. Sci. 2011. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garland, A.; Low, J. Reasoning about “Capability”: Wild Robins Respond to Limb Visibility in Humans. Behav. Sci. 2016, 6, 15. https://doi.org/10.3390/bs6030015
Garland A, Low J. Reasoning about “Capability”: Wild Robins Respond to Limb Visibility in Humans. Behavioral Sciences. 2016; 6(3):15. https://doi.org/10.3390/bs6030015
Chicago/Turabian StyleGarland, Alexis, and Jason Low. 2016. "Reasoning about “Capability”: Wild Robins Respond to Limb Visibility in Humans" Behavioral Sciences 6, no. 3: 15. https://doi.org/10.3390/bs6030015




