Testing the Glucose Hypothesis among Capuchin Monkeys: Does Glucose Boost Self-Control?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Apparatus
2.3. General Procedure
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baumeister, R.F.; Bratslavsky, E.; Muraven, M.; Tice, D.M. Ego depletion: Is the active self a limited resource? J. Personal. Soc. Psychol. 1998, 74, 1252–1265. [Google Scholar] [CrossRef]
- Baumeister, R.F. Yielding to temptation: Self-control failure, impulsive purchasing, and consumer behavior. J. Consum. Res. 2002, 28, 670–676. [Google Scholar] [CrossRef]
- Baumeister, R.F.; Heatherton, T.F.; Tice, D.M. Losing Control: How and Why People Fail at Self-Regulation; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Muraven, M.; Tice, D.M.; Baumeister, R.F. Self-control as a limited resource: Regulatory depletion patterns. J. Personal. Soc. Psychol. 1998, 74, 774–789. [Google Scholar] [CrossRef]
- Hagger, M.S.; Chantelle, W.; Stiff, C.; Chatzisarantis, N.L. Ego depletion and the strength model of self-control: A meta-analysis. Psychol. Bull. 2010, 136, 495–525. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, R.F.; Vohs, K.D.; Tice, D.M. The strength model of self-control. Curr. Dir. Psychol. Sci. 2007, 16, 351–355. [Google Scholar] [CrossRef]
- Gailliot, M.T.; Baumeister, R.F. The physiology of willpower: Linking blood glucose to self-control. Personal. Soc. Psychol. Rev. 2007, 11, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Parker, P.Y. Breakfast, blood glucose, and cognition. Am. J. Clin. Nutr. 1998, 67, 772S–778S. [Google Scholar] [PubMed]
- Benton, D.; Owens, D.S.; Parker, P.Y. Blood glucose influences memory and attention in young adults. Neuropsychologia 1994, 32, 595–607. [Google Scholar] [CrossRef]
- Benton, D.; Parker, P.Y.; Donohoe, R.T. The supply of glucose to the brain and cognitive functioning. J. Biol. Sci. 1996, 28, 463–479. [Google Scholar] [CrossRef]
- Donohoe, R.T.; Benton, D. Blood glucose control and aggressiveness in females. Personal. Indiv. Differ. 1999, 26, 905–911. [Google Scholar] [CrossRef]
- Donohoe, R.T.; Benton, D. Glucose tolerance predicts performance on tests of memory and cognition. Physiol. Behav. 2000, 71, 395–401. [Google Scholar] [CrossRef]
- Fairclough, S.H.; Houston, K. A metabolic measure of mental effort. Biol. Psychol. 2004, 66, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.Y.; Benton, D. The influence of a glucose drink on a demanding working memory task. Physiol. Behav. 1999, 67, 69–74. [Google Scholar] [CrossRef]
- Owens, D.S.; Benton, D. The impact of raising blood glucose on reaction times. Neuropsychobiology 1994, 30, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.B.; Harper, S.; Kennedy, D.O. Cognitive demand and blood glucose. Physiol. Behav. 2001, 73, 585–592. [Google Scholar] [CrossRef]
- Sünram-Lea, S.I.; Foster, J.K.; Durlach, P.; Perez, C. The effect of retrograde and anterograde glucose administration on memory performance in healthy young adults. Behav. Brain Res. 2002, 134, 505–516. [Google Scholar]
- Wesnes, K.A.; Pincock, C.; Richardson, D.; Helm, O.; Hails, S. Breakfast reduces declines in attention and memory over the morning in schoolchildren. Appetite 2003, 41, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Benton, D. The impact of increasing blood glucose on psychological functioning. Biol. Psychol. 1990, 30, 13–19. [Google Scholar] [CrossRef]
- Molden, D.C.; Hui, C.M.; Scholer, A.A.; Meier, B.P.; Noreen, E.E.; D’Agostino, P.R.; Martin, V. Motivational versus metabolic effects of carbohydrates on self-control. Psychol. Sci. 2012, 23, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.A.; Shirk, S.D.; Burgin, C.J.; Martin, L.L. The gargle effect rinsing the mouth with glucose enhances self-control. Psychol. Sci. 2012, 23, 1470–1472. [Google Scholar] [CrossRef] [PubMed]
- West, R.; Willis, N. Double-blind placebo controlled trial of dextrose tablets and nicotine patch in smoking cessation. Psychopharmacology 1998, 136, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.D.; Stabler, B.; Ross, S.L.; Morris, M.A.; Litton, J.C.; Surwit, R.S. Psychological predictors of glucose control in patients with IDDM. Diabetes Care 1988, 11, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Lustman, P.J.; Frank, B.L.; McGill, J.B. Relationship of personality characteristics to glucose regulation in adults with diabetes. Psychosom. Med. 1991, 53, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.C.; McCullough, M.E. Publication bias and the limited strength model of self-control: Has the evidence for ego depletion been overestimated? Front. Psychol. 2014, 5, 1–11. [Google Scholar]
- Chatzisarantis, N.L.; Hagger, M.S. Unsuccessful attempts to replicate effects of self control operations and glucose on ego-depletion pose an interesting research question that demands explanation. Appetite 2015, 84, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Kurzban, R. Does the brain consume additional glucose during self-control tasks? Evol. Psychol. 2010, 8, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Lange, F.; Eggert, F. Sweet delusion. Glucose drinks fail to counteract ego depletion. Appetite 2014, 75, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Lange, F.; Seer, C.; Rapior, M.; Rose, J.; Eggert, F. Turn it all you want: Still no effect of sugar consumption on ego depletion. J. Eur. Psychol. Stud. 2014, 5, 1–8. [Google Scholar]
- Schimmack, U. The ironic effect of significant results on the credibility of multiple-study articles. Psychol. Methods 2012, 17, 551–566. [Google Scholar] [CrossRef] [PubMed]
- De Petrillo, F.; Micucci, A.; Gori, E.; Truppa, V.; Ariely, D.; Addessi, E. Self-control depletion in tufted capuchin monkeys (Sapajus spp.): Does delay of gratification rely on a limited resource? Front. Psychol. 2015, 6, 1193. [Google Scholar] [CrossRef] [PubMed]
- Beran, M.J. Maintenance of self-imposed delay of gratification by four chimpanzees (Pan troglodytes) and an orangutan (Pongo pygmaeus). J. Gen. Psychol. 2002, 129, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.C.; Pattison, K.F.; DeWall, C.N.; Rayburn-Reeves, R.; Zentall, T.R. Self-control without a “self?” Common self-control processes in humans and dogs. Psychol. Sci. 2010, 21, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.C.; Bender, C. The breakfast effect: Dogs (Canis familiaris) search more accurately when they are less hungry. Behav. Process. 2012, 91, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Mayack, C.; Naug, D. Starving honeybees lose self-control. Biol. Lett. 2015. [Google Scholar] [CrossRef] [PubMed]
- Addessi, E.; Paglieri, F.; Beran, M.J.; Evans, T.A.; Macchitella, L.; De Petrillo, F.; Focaroli, V. Delay choice versus delay maintenance: Different measures of delayed gratification in capuchin monkeys (Cebus apella). J. Comp. Psychol. 2013, 127, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.A.; Beran, M.J.; Paglieri, F.; Addessi, E. Delaying gratification for food and tokens in capuchin monkeys (Cebus apella) and chimpanzees (Pan troglodytes): When quantity is salient, symbolic stimuli do not improve performance. Anim. Cog. 2012, 15, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Paglieri, F.; Focaroli, V.; Bramlett, J.; Tierno, V.; McIntyre, J.M.; Addessi, E.; Beran, M.J. The hybrid delay task: Can capuchin monkeys (Cebus apella) sustain a delay after an initial choice to do so? Behav. Proc. 2013, 94, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, J.W.; Silva, J.D.; Rylands, A.B. How different are robust and gracile capuchin monkeys? An argument for the use of Sapajus and Cebus. Am. J. Primatol. 2012, 74, 273–286. [Google Scholar] [PubMed]
- Alfaro, J.W.; Boubli, J.P.; Olson, L.E.; Di Fiore, A.; Wilson, B.; Gutiérrez-Espeleta, G.A.; Schwochow, D. Explosive Pleistocene range expansion leads to widespread Amazonian sympatry between robust and gracile capuchin monkeys. J. Biogeogr. 2012, 39, 272–288. [Google Scholar] [CrossRef]
- Muraven, M.; Slessareva, E. Mechanisms of self-control failure: Motivation and limited resources. Personal. Soc. Psychol. Bull. 2003, 29, 894–906. [Google Scholar] [CrossRef] [PubMed]

| High-Glucose Condition | Low-Glucose Condition | |||||||
|---|---|---|---|---|---|---|---|---|
| Monkey | Weight (kg) | Honey Peanuts (g) | Honey (g) | Sugars (g) | Calories | Unsalted Peanuts (g) | Sugars (g) | Calories |
| Gabe | 3.6 | 3 | 9 | 7.29 | 41.8 | 8 | 0.43 | 42.9 |
| Gambit | 2.61 | 3 | 7 | 5.76 | 36.1 | 7 | 0.39 | 37.5 |
| Griffin | 5 | 3 | 12 | 9.57 | 50.4 | 9 | 0.47 | 48.2 |
| Liam | 3.82 | 3 | 9 | 7.29 | 41.8 | 7.5 | 0.41 | 40.2 |
| Lily | 3.51 | 3 | 8 | 6.53 | 38.9 | 7 | 0.40 | 37.5 |
| Logan | 4.1 | 3 | 10 | 8.05 | 44.6 | 8.5 | 0.45 | 45.5 |
| Nala | 3.17 | 3 | 7 | 5.76 | 36.1 | 7 | 0.40 | 37.5 |
| Nkima | 3.67 | 3 | 9 | 7.29 | 41.8 | 7.5 | 0.41 | 40.2 |
| Wren | 2.33 | 3 | 5 | 4.24 | 30.4 | 5.5 | 0.34 | 29.5 |
| Monkey | Mean Number of Accumulated Raisins | T-Test Results | |
|---|---|---|---|
| Low Glucose | High Glucose | ||
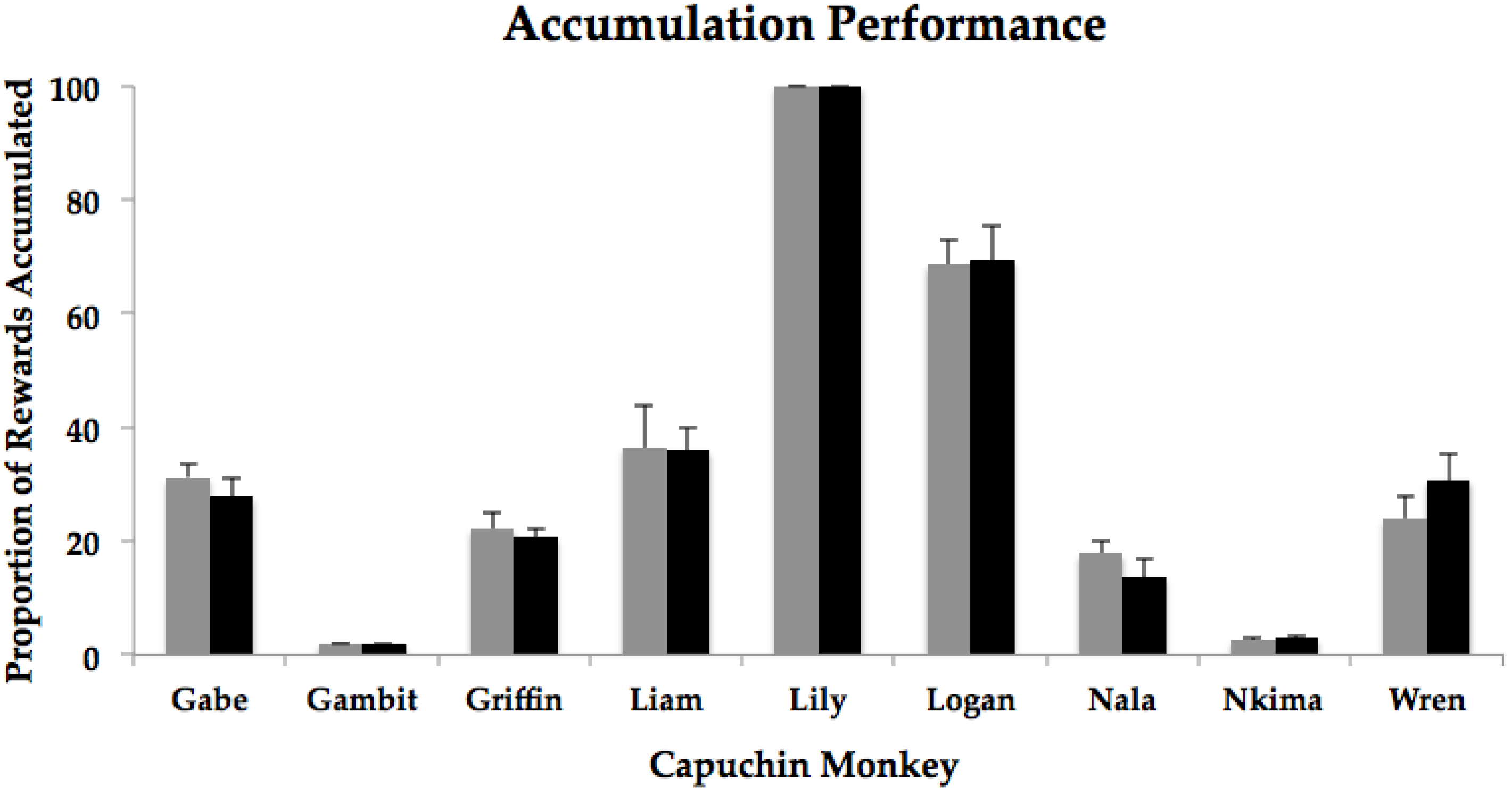
| Gabe | 15.6 | 13.9 | t(9) = −1.48, p = 0.17 |
| Gambit | 1 | 1 | NA * |
| Griffin | 11.1 | 10.3 | t(9) = −0.44, p = 0.67 |
| Liam | 18.2 | 18 | t(9) = −0.10, p = 0.92 |
| Lily | 50 | 49.9 | t(9) = −1.00, p = 0.34 |
| Logan | 34.3 | 34.7 | t(9) = 0.09, p = 0.93 |
| Nala | 8.9 | 6.9 | t(9) = −1.55, p = 0.16 |
| Nkima | 1.3 | 1.5 | t(9) = 1.00, p = 0.34 |
| Wren | 12 | 15.4 | t(9) = 1.54, p = 0.16 |
| Monkey | Correlation Results | |
|---|---|---|
| Low Glucose | High Glucose | |
| Gabe | r(10) = 0.42, p = 0.23 | r(10) = 0.53, p = 0.12 |
| Gambit | NA * | NA * |
| Griffin | r(10) = −0.43, p = 0.22 | r(10) = 0.24, p = 0.51 |
| Liam | r(10) = −0.67, p = 0.03 | r(10) = −0.40, p = 0.25 |
| Lily | NA * | r(10) = 0.17, p = 0.63 |
| Logan | r(10) = −0.28, p = 0.43 | r(10) = −0.33, p = 0.35 |
| Nala | r(10) = −0.41, p = 0.24 | r(10) = −0.29, p = 0.42 |
| Nkima | r(10) = 0.27, p = 0.46 | r(10) = 0.87, p = 0.001 |
| Wren | r(10) = −0.42, p = 0.23 | r(10) = −0.80, p = 0.006 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parrish, A.E.; Emerson, I.D.; Rossettie, M.S.; Beran, M.J. Testing the Glucose Hypothesis among Capuchin Monkeys: Does Glucose Boost Self-Control? Behav. Sci. 2016, 6, 16. https://doi.org/10.3390/bs6030016
Parrish AE, Emerson ID, Rossettie MS, Beran MJ. Testing the Glucose Hypothesis among Capuchin Monkeys: Does Glucose Boost Self-Control? Behavioral Sciences. 2016; 6(3):16. https://doi.org/10.3390/bs6030016
Chicago/Turabian StyleParrish, Audrey E., Ishara D. Emerson, Mattea S. Rossettie, and Michael J. Beran. 2016. "Testing the Glucose Hypothesis among Capuchin Monkeys: Does Glucose Boost Self-Control?" Behavioral Sciences 6, no. 3: 16. https://doi.org/10.3390/bs6030016





