Non-Invasive Brain Stimulation for Children with Autism Spectrum Disorders: A Short-Term Outcome Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Selection and Group Distribution
2.3. Clinical Evaluation
2.4. Neurophysiological Evaluation
2.4.1. Functional Brain Connectivity
2.4.2. Event-Related Potentials
2.5. Intervention
2.5.1. Transcranial Direct Current Stimulation (tDCS)
2.5.2. Repetitive Transcranial Magnetic Stimulation (rTMS)
2.6. Ethical Considerations
3. Results
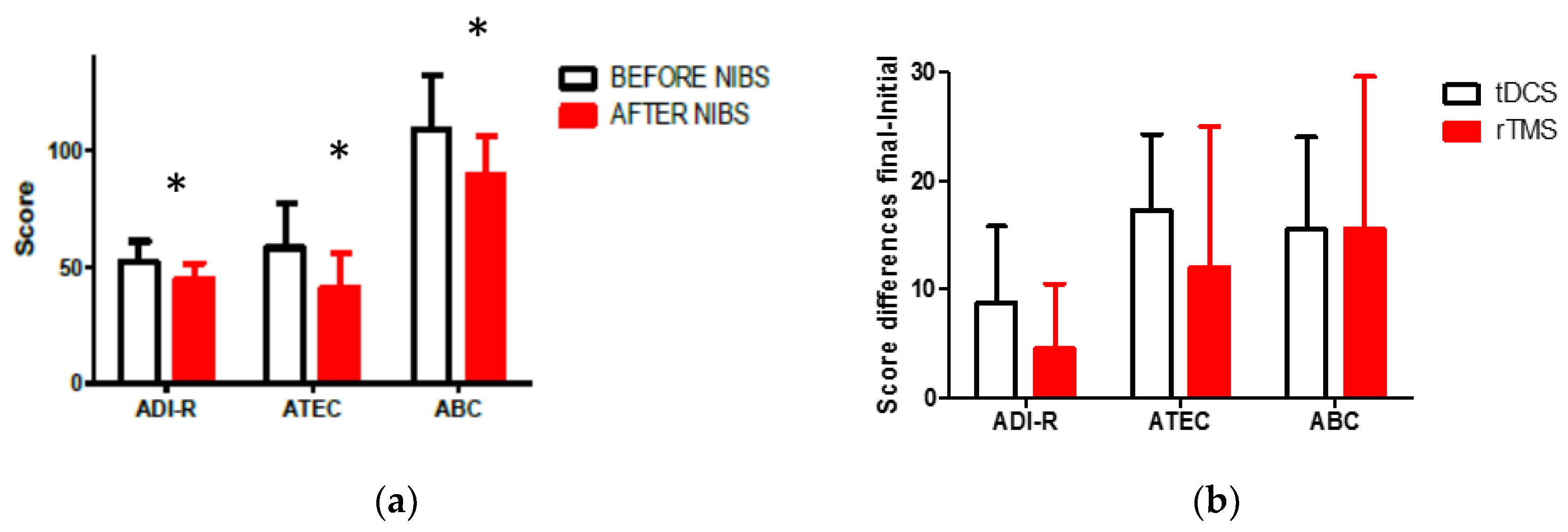
3.1. Change in Clinical Scales One Month after the Intervention
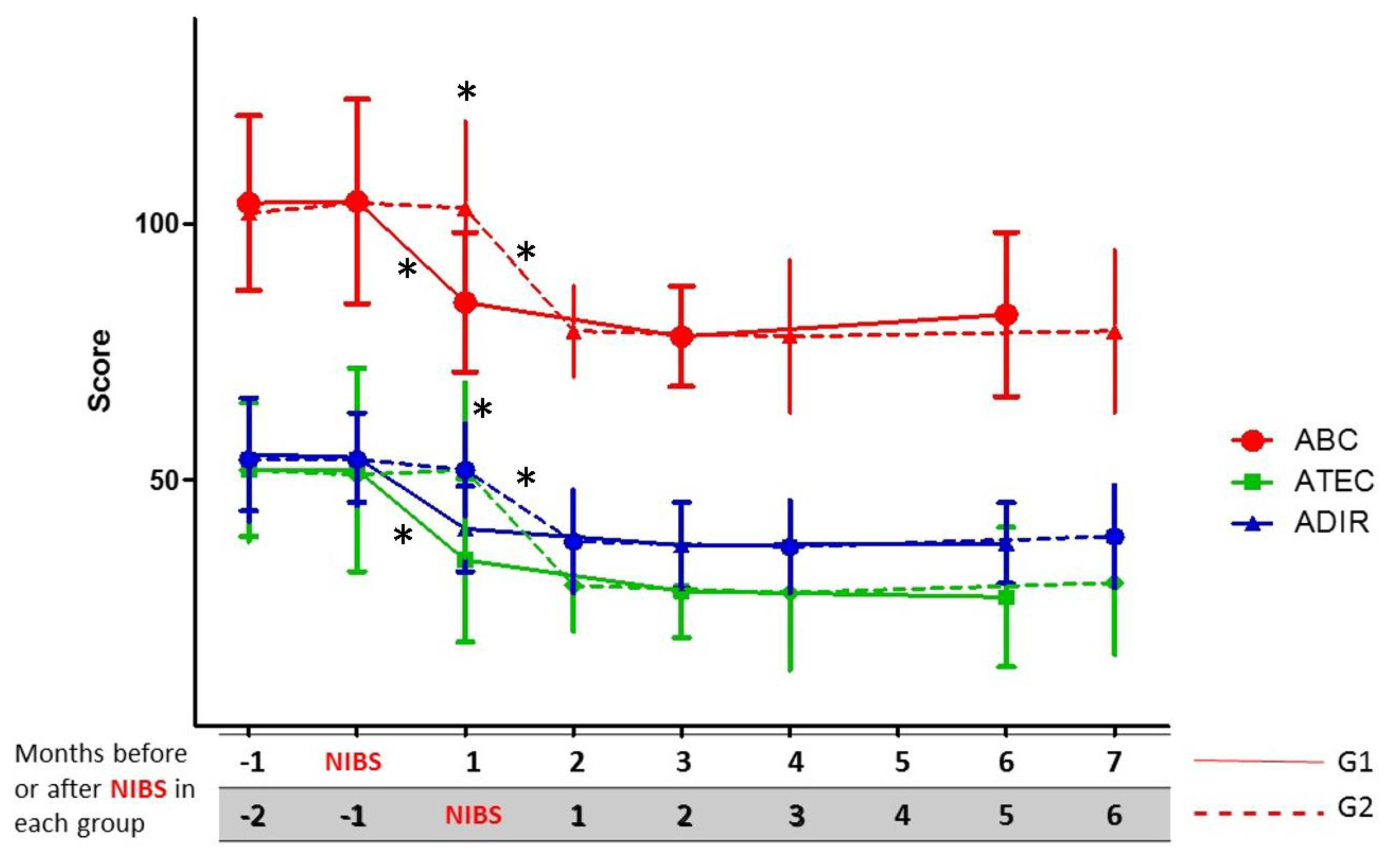
3.2. Change in Clinical Scales during the First Six Months after the Intervention
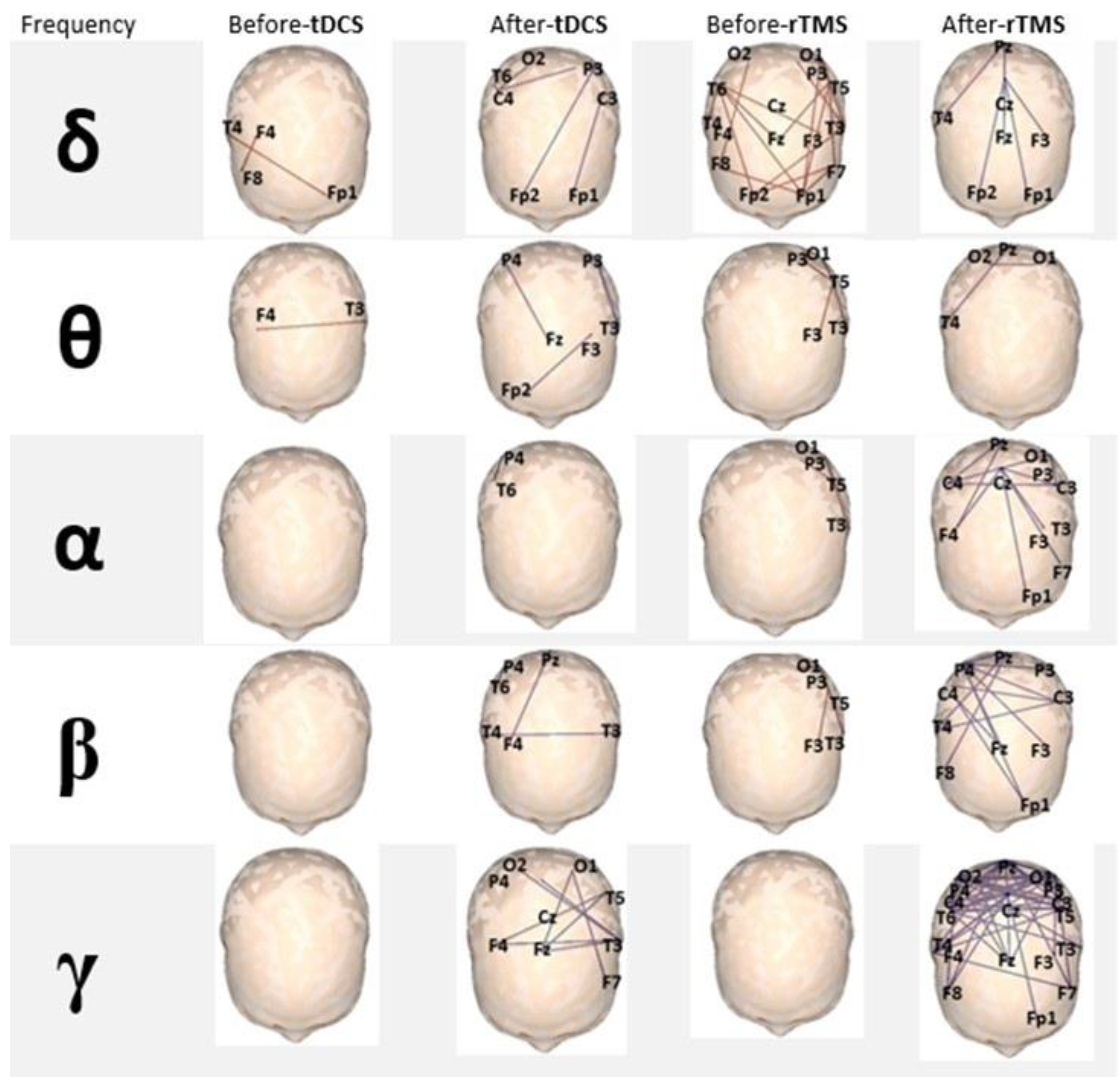
3.3. EEG-Based Brain Functional Connectivity Analysis
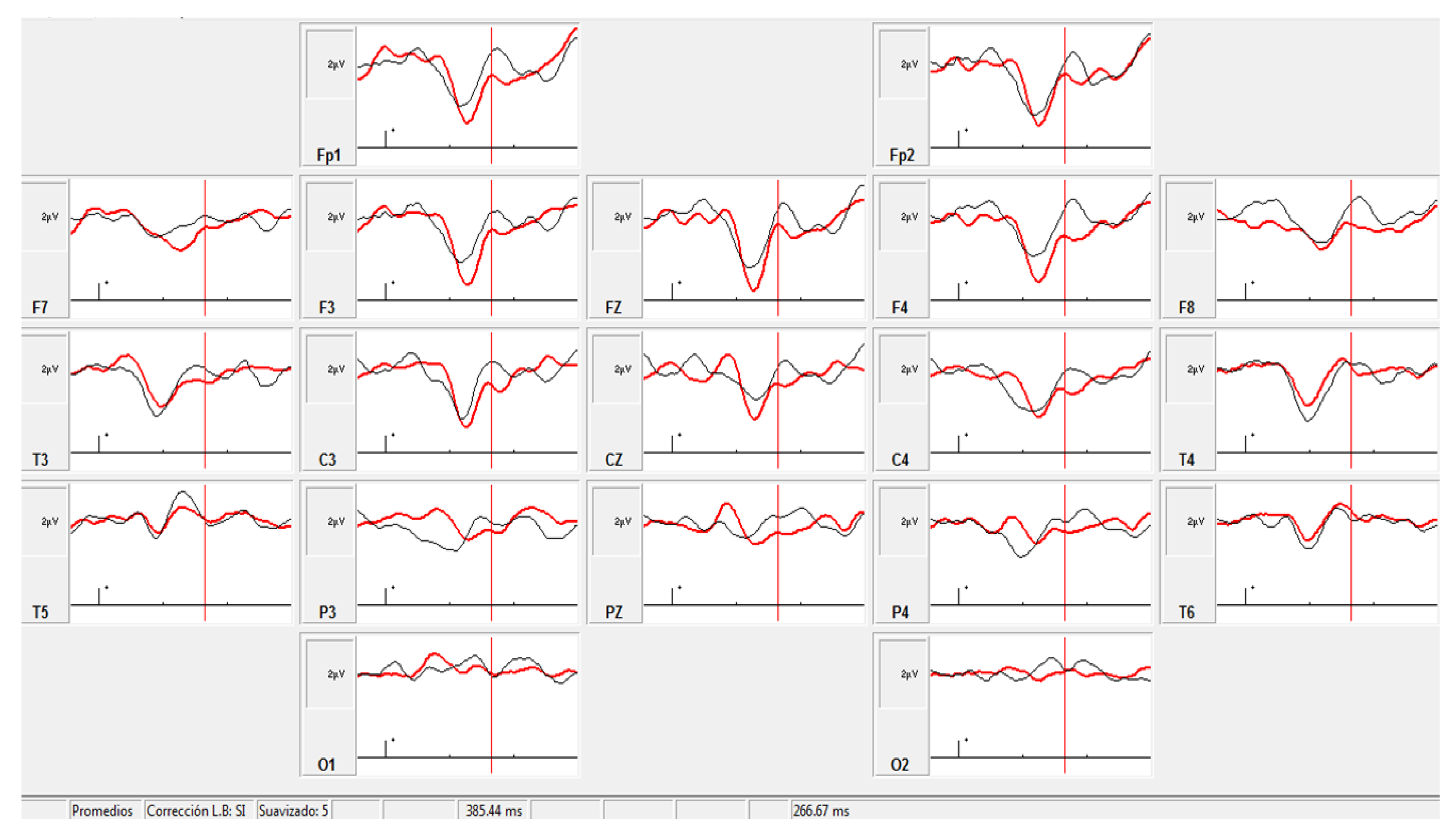
3.4. ERP Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Casanova, M.F.; Buxhoeveden, D.P.; Switala, A.E.; Roy, E. Minicolumnar pathology in autism. Neurology 2002, 58, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Freitag, C.M.; Luders, E.; Hulst, H.E.; Narr, K.L.; Thompson, P.M.; Toga, A.W.; Krick, C.; Konrad, C. Total brain volume and corpus callosum size in medication-naive adolescents and young adults with autism spectrum disorder. Biol. Psychiatry 2009, 66, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sundaram, S.K.; Sivaswamy, L.; Behen, M.E.; Makki, M.I.; Ager, J.; Janisse, J.; Chugani, H.T.; Chugani, D.C. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cereb. Cortex 2010, 20, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.F.; El-Baz, A.; Vanbogaert, E.; Narahari, P.; Switala, A. A topographic study of minicolumnar core width by lamina comparison between autistic subjects and controls: Possible minicolumnar disruption due to an anatomical element in-common to multiple laminae. Brain Pathol. 2010, 20, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Reutiman, T.J.; Folsom, T.D.; Rustan, O.G.; Rooney, R.J.; Thuras, P.D. Downregulation of GABAA receptor protein subunits alpha6, beta2, delta, epsilon, gamma2, theta, and rho2 in superior frontal cortex of subjects with autism. J. Autism Dev. Disord. 2014, 44, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Enticott, P.G.; Kennedy, H.A.; Rinehart, N.J.; Tonge, B.J.; Bradshaw, J.L.; Fitzgerald, P.B. GABAergic activity in autism spectrum disorders: An investigation of cortical inhibition via transcranial magnetic stimulation. Neuropharmacology 2013, 68, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Oberman, L.M.; Pascual-Leone, A.; Rotenberg, A. Modulation of corticospinal excitability by transcranial magnetic stimulation in children and adolescents with autism spectrum disorder. Front. Hum Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baruth, J.M.; Casanova, M.F.; El-Baz, A.; Horrell, T.; Mathai, G.; Sears, L.; Sokhadze, E. Low-frequency repetitive transcranial magnetic stimulation (rTMS) modulates evoked-gamma frequency oscillations in autism spectrum disorder (ASD). J. Neurother. 2010, 14, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.F.; Baruth, J.M.; El-Baz, A.; Tasman, A.; Sears, L.; Sokhadze, E. Repetitive transcranial magnetic stimulation (rTMS) modulates event-related potential (ERP) indices of attention in autism. Transl. Neurosci. 2012, 3, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Amatachaya, A.; Auvichayapat, N.; Patjanasoontorn, N.; Suphakunpinyo, C.; Ngernyam, N.; Aree-uea, B.; Keeratitanont, K.; Auvichayapat, P. Effect of anodal transcranial direct current stimulation on autism: A randomized double-blind crossover trial. Behav. Neurol. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Bruzzese, D.; Ferrucci, R.; Priori, A.; Pascotto, A.; Galderisi, S.; Altamura, A.C.; Bravaccio, C. Transcranial direct current stimulation for hyperactivity and noncompliance in autistic disorder. World J. Biol. Psychiatry 2015, 16, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Enticott, P.G.; Fitzgibbon, B.M.; Kennedy, H.A.; Arnold, S.L.; Elliot, D.; Peachey, A.; Zangen, A.; Fitzgerald, P.B. A double-blind, randomized trial of deep repetitive transcranial magnetic stimulation (rTMS) for autism spectrum disorder. Brain Stimul. 2014, 7, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.D.; Hopp, J.P. The use of the Bilingual Aphasia Test for assessment and transcranial direct current stimulation to modulate language acquisition in minimally verbal children with autism. Clin. Linguist. Phon. 2011, 25, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Downar, J.; Daskalakis, Z.J. New targets for rTMS in depression: A review of convergent evidence. Brain Stimul. 2013, 6, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Gómez, L.; Denis, M.; Marín, T.; Vidal, B.; Maragoto, C.; Vera, C.; Serguera, M.; Morales, L.; Báez, M.; Sánchez, A.; et al. Estudio piloto sobre el efecto de la Estimulación Cerebral No Invasiva en el Trastorno del Espectro Autista. Rev. Mex. Neurocienc. 2016, 17, 51. [Google Scholar]
- Gómez, L.; Denis, M.; Marín, T.; Vidal, B.; Maragoto, C.; Vera, H.; Serguera, M.; Morales, L.; Báez, M.; Sánchez, A.; et al. Non invasive brain stimulation in children with autism spectrum disorder. Brain Stimul. 2017, 10, 347. [Google Scholar] [CrossRef]
- Schopler, E.; Reichler, R.; DeVellis, R.; Daly, K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J. Autism Dev. Disord. 1980, 10, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Krug, D.; Arisk, J.; Almond, P. Behavior checklist for identifying severely handicapped individuals with high levels of autistic behavior. J. Child Psychol. Psychiatry 1980, 21, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Magiati, I.; Moss, J.; Yates, R.; Charman, T.; Howlin, P. Is the Autism Treatment Evaluation Checklist a useful tool for monitoring progress in children with autism spectrum disorders? J. Intellect. Disabil. Res. 2011, 55, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Busner, J.; Targum, S. The clinical global impressions scale: Applying a research tool in clinical practice. Psychiatry 2007, 4, 28–37. [Google Scholar] [PubMed]
- Stam, J.C.; van Dijk, B.W. Synchronization likelihood: An unbiased measure of generalized synchronization in multivariate data sets. Phys. D Nonlinear Phenom. 2002, 163, 236–251. [Google Scholar] [CrossRef]
- Näätänen, R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behav. Brain Sci. 1990, 13, 201–233. [Google Scholar] [CrossRef]
- Rossi, S.; Hallet, M.; Rossini, P.M.; Pascual-Leone, A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [PubMed]
- Catani, M.; Dell’Acqua, F.; Budisavljevic, S.; Howells, H.; Thiebaut de Schotten, M.; Froudist-Walsh, S.; D`Anna, L.; Thompson, A.; Sandrone, S.; Bullmore, E.; et al. Frontal networks in adults with autism spectrum disorder. Brain 2016, 139, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Baruth, J.M.; Wall, C.A.; Patterson, M.C.; Port, J.D. Proton magnetic resonance spectroscopy as a probe into the pathophysiology of autism spectrum disorders (ASD): A review. Autism Res. 2013, 6, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Andre-Obadia, N.; Poulet, E.; Devanne, H.; Haffen, E.; Londero, A.; Cretin, B.; Leroi, A.M.; Radtchenko, A.; Saba, G.; et al. French guidelines on the use of repetitive transcranial magnetic stimulation (rTMS): Safety and therapeutic indications. Neurophysiol. Clin. 2011, 41, 221–295. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.; George, M.; Grammer, G.; Janicak, P.; Pascual-Leone, A.; Wirecki, T. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016, 9, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.Q.; Dhamne, S.C.; Gersner, R.; Kaye, H.L.; Oberman, L.M.; Pascual-Leone, A.; Rotenberg, A. Transcranial magnetic and direct current stimulation in children. Curr. Neurol. Neurosci. Rep. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, C.; Santos, L.; Peterson, M.; Ehinger, M. Safety of noninvasive brain stimulation in children and adolescents. Brain Stimul. 2015, 8, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.; Koslowsky, M.; Lavidor, M. tDCS polarity effects in motor and cognitive domains: A meta-analytical review. Exp. Brain Res. 2012, 216, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Joos, K.; De, R.D.; Van de Heyning, P.; Vanneste, S. Polarity specific suppression effects of transcranial direct current stimulation for tinnitus. Neural Plast. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, A.; Fregni, F.; Campanha, C.; Valasek, C.A.; De, R.D.; Brunoni, A.R.; Boggio, P.S. Polarity-dependent transcranial direct current stimulation effects on central auditory processing. PLoS ONE 2011, 6, e25399. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.K.; Jee, S.J.; Kim, Y.W. Effect of transcranial direct current stimulation on postural stability and lower extremity strength in hemiplegic stroke patients. Ann. Rehabil. Med. 2013, 37, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Best, J.G.; Stephenson, M.C.; O’Shea, J.; Wylezinska, M.; Kincses, Z.T.; Morris, P.G.; Matthews, P.M.; Johansen-Berg, H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 2009, 29, 5202–5206. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Brunoni, A.R.; Anastasia, A.; Micillo, M.; Mantovani, A. Polarity-dependent effects of transcranial direct current stimulation in obsessive-compulsive disorder. Neurocase 2016, 22, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Van Steenburgh, J.J.; Varvaris, M.; Schretlen, J.; Vannorsdall, T.J.; Gordon, B. Balanced bifrontal transcranial direct current stimulation enhaces working memory in adults with high-functioning autism: A sham-controlled crossover study. Mol. Autism 2017, 8, 40. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, L.; Vidal, B.; Maragoto, C.; Morales, L.M.; Berrillo, S.; Vera Cuesta, H.; Baez, M.; Denis, M.; Marín, T.; Cabrera, Y.; et al. Non-Invasive Brain Stimulation for Children with Autism Spectrum Disorders: A Short-Term Outcome Study. Behav. Sci. 2017, 7, 63. https://doi.org/10.3390/bs7030063
Gómez L, Vidal B, Maragoto C, Morales LM, Berrillo S, Vera Cuesta H, Baez M, Denis M, Marín T, Cabrera Y, et al. Non-Invasive Brain Stimulation for Children with Autism Spectrum Disorders: A Short-Term Outcome Study. Behavioral Sciences. 2017; 7(3):63. https://doi.org/10.3390/bs7030063
Chicago/Turabian StyleGómez, Lázaro, Belkis Vidal, Carlos Maragoto, Lilia Maria Morales, Sheyla Berrillo, Héctor Vera Cuesta, Margarita Baez, Marlén Denis, Tairí Marín, Yaumara Cabrera, and et al. 2017. "Non-Invasive Brain Stimulation for Children with Autism Spectrum Disorders: A Short-Term Outcome Study" Behavioral Sciences 7, no. 3: 63. https://doi.org/10.3390/bs7030063




