Decomposition of Leaves, Stems and Roots of Transgenic Aspen with the Xyloglucanase (sp-Xeg) Gene under Laboratory Microcosm Conditions
Abstract
:1. Introduction
2. Materials and Methods
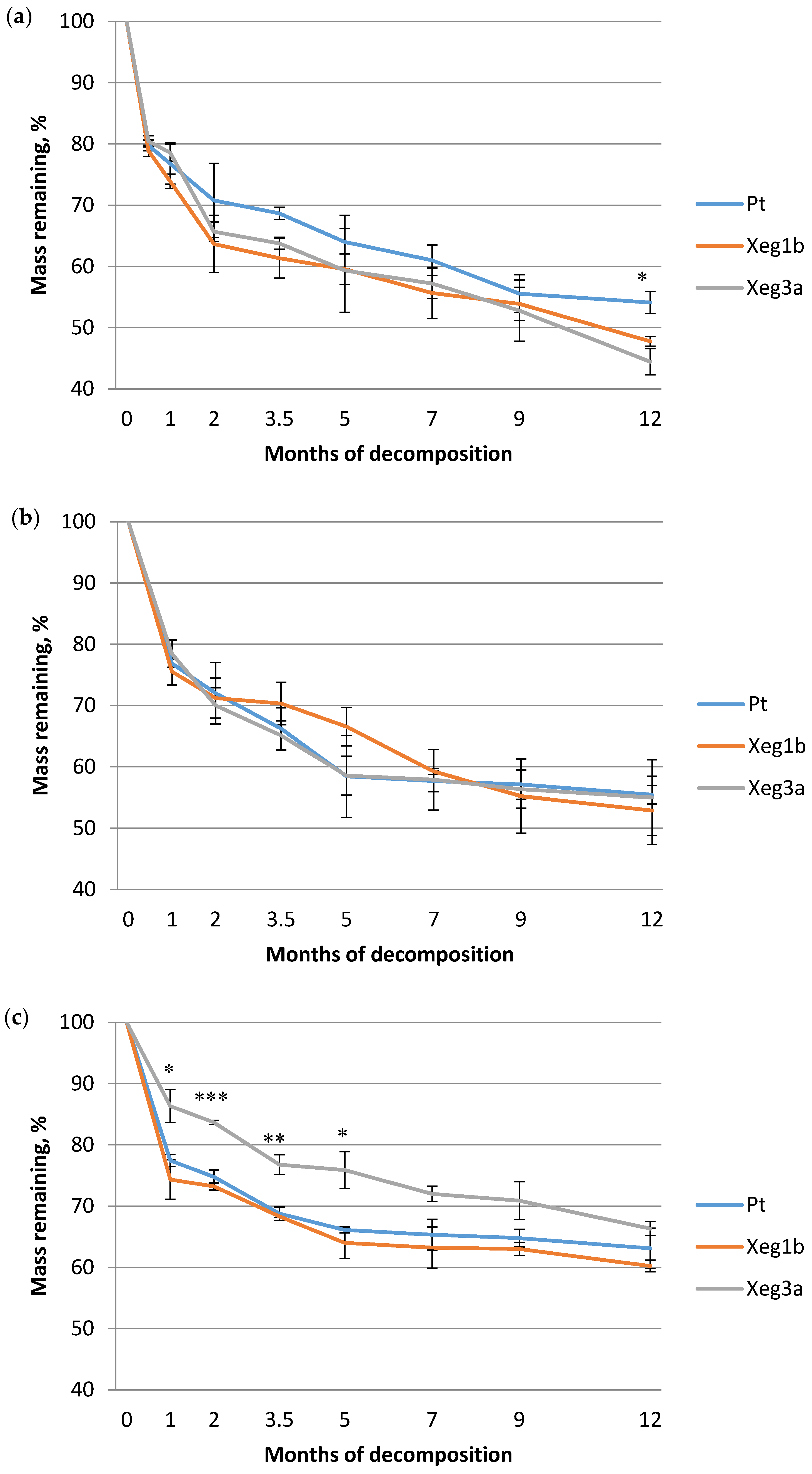
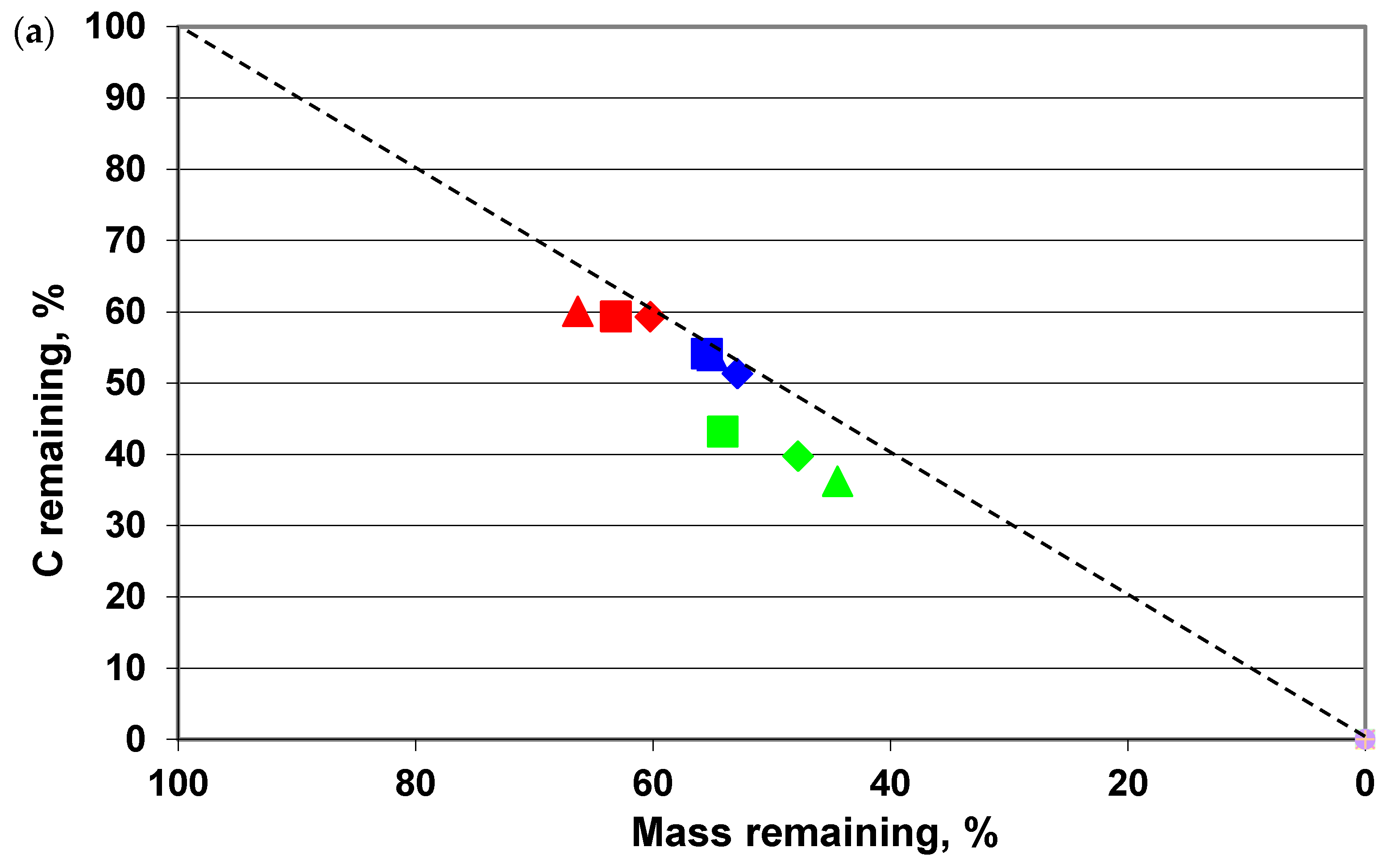
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). The Global Outlook for Future Wood Supply from Forest Plantations; Working Paper GFPOS/WP/03; FAO: Rome, Italy, 2000. [Google Scholar]
- York, W.S.; O’Neill, M.A. Biochemical control of xylan biosynthesis—Which end is up? Curr. Opin. Plant Biol. 2008, 11, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.A.C.; Dupree, P.; Shewry, P.R. A novel bioinformatics approach identifies candidate genes for the synthesis and feruloylation of arabinoxylan. Plant Physiol. 2007, 144, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Baba, K.; Furuta, Y.; Iida, I.; Sameshima, K.; Arai, M.; Hayashi, T. Enhancement of growth and cellulose accumulation by overexpression of xyloglucanase in poplar. FEBS Lett. 2004, 564, 183–187. [Google Scholar] [CrossRef]
- Hartati, N.S.; Rahayuningsih, L.; Kaida, R.; Sudarmonowati, E.; Hayashi, T. Overexpression of xyloglucanase gene in sengon (Paraserianthes falcataria) for growth acceleration. J. Biotech. Res. Trop. Reg. 2009, 2, 1–4. [Google Scholar]
- Kaku, T.; Kaida, R.; Baba, K.; Hartati, S.; Sudarmonowati, E.; Hayashi, T. Improvement of fermentable sugar yields of mangium through transgenic overexpression of xyloglucanase. J. Wood Sci. 2011, 57, 545–548. [Google Scholar] [CrossRef]
- Taniguchi, T.; Ohmiya, Y.; Kurita, M.; Tsubomura, M.; Kondo, T.; Park, Y.W.; Baba, K.; Hayashi, T. Biosafety assessment of transgenic poplars overexpressing xyloglucanase (AaXEG2) prior to field trials. J. Wood Sci. 2008, 54, 408–413. [Google Scholar] [CrossRef]
- Kaku, T.; Baba, K.; Taniguchi, T.; Kurita, M.; Konagaya, K.; Ishii, K.; Kondo, T.; Serada, S.; Iizuka, H.; Kaida, R.; et al. Analyses of leaves from open field-grown transgenic poplars overexpressing xyloglucanase. J. Wood Sci. 2012, 58, 281–289. [Google Scholar] [CrossRef]
- Funahashi, F.; Ohta, S.; Taniguchi, T.; Kurita, M.; Konagaya, K.; Hayashi, T. Architectural and physiological characteristics related to the depressed growth of poplars overexpressing xyloglucanase in a field study. Trees 2014, 28, 65–76. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L.; Gonzalez-Arzac, A.; Perez, L.I. There’s no place like home? An exploration of the mechanisms behind plant litter–decomposer affinity in terrestrial ecosystems. New Phytol. 2014, 204, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Sayad, E.; Hosseini, V.; Gholami, S.; Salehe-Shooshtari, M.H. Different predictors determining litter decomposition rate in functional groups of the tree plantations in a common garden. Trees 2015, 29, 1883–1891. [Google Scholar] [CrossRef]
- Vauramo, S.; Pasonen, H.-L.; Pappinen, A.; Setala, H. Decomposition of leaf litter from chitinase transgenic silver birch (Betula pendula) and effects on decomposer populations in a field trial. Appl. Soil Ecol. 2006, 32, 338–349. [Google Scholar] [CrossRef]
- Seppanen, S.-K.; Pasonen, H.-L.; Vauramo, S.; Vahala, J.; Toikka, M.; Kilpelainen, I.; Setala, H.; Teeri, T.H.; Timonen, S.; Pappinen, A. Decomposition of the leaf litter and mycorrhiza forming ability of silver birch with a genetically modified lignin biosynthesis pathway. Appl. Soil Ecol. 2007, 36, 100–106. [Google Scholar] [CrossRef]
- Freschet, G.T.; Cornwell, W.K.; Wardle, D.A.; Elumeeva, T.G.; Liu, W.; Jackson, B.G.; Onipchenko, V.G.; Soudzilovskaia, N.A.; Tao, J.; Johannes, H.C. Cornelissen. Linking litter decomposition of above- and below-ground organs to plant–soil feedbacks worldwide. J. Ecol. 2013, 101, 943–952. [Google Scholar] [CrossRef]
- Zhang, D.; Hui, D.; Luo, Y.; Zhou, G. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef]
- Freschet, G.T.; Aerts, R.; Cornelissen, J.H.C. A plant economics spectrum of litter decomposability. Funct. Ecol. 2012, 26, 56–65. [Google Scholar] [CrossRef]
- Berg, B. Decomposition patterns for foliar litter: A theory for influencing factors. Soil Biol. Biochem. 2014, 78, 222–232. [Google Scholar] [CrossRef]
- Preston, C.M.; Nault, J.R.; Trofymow, J.A.; Smyth, C.; CIDET Working Group. Chemical changes during 6 years of decomposition of 11 litters in some Canadian forest sites. Part 1. Elemental composition, tannins, phenolics, and proximate fractions. Ecosystems 2009, 12, 1053–1077. [Google Scholar] [CrossRef]
- Berg, B. Litter decomposition and organic matter turnover in northern forest soils. For. Ecol. Manag. 2000, 133, 13–22. [Google Scholar] [CrossRef]
- Shestibratov, K.A.; Podresov, A.S.; Salmova, M.A.; Kovalitskaya, Y.A.; Vidyagina, E.O.; Loginov, D.S.; Koroleva, O.V.; Miroshnikov, A.I. Phenotypic manifestation of gene expression encoding xyloglucanase from Penicillium canescens in transgenic aspen plants. Russ. J. Plant Physiol. 2012, 59, 618–625. [Google Scholar] [CrossRef]
- Huntley, S.K.; Ellis, D.; Gilbert, M.; Chapple, C.; Mansfield, S.D. Significant increases in pulping efficiency in C4H-F5H-transformed poplars: Improved chemical savings and reduced environmental toxins. J. Agric. Food Chem. 2003, 51, 6178–6183. [Google Scholar] [CrossRef] [PubMed]
- Kurschner, K.; Hanak, A. Determination of cellulose. Z. Untersuch. Lebensm. 1930, 59, 448–485. [Google Scholar]
- Fraser, J.R.; Branden-Bravo, M.; Holmes, O.C. The proximate analysis of wheat flour carbohydrates. I.—Methods and scheme of analysis. J. Sci. Food Agric. 1956, 7, 577–589. [Google Scholar] [CrossRef]
- Perez-Corona, M.E.; Perez Hernandez, M.C.; Bermudez de Castro, F. Decomposition of alder, ash, and poplar litter in a Mediterranean riverine area. Commun. Soil Sci. Plant Anal. 2006, 37, 1111–1125. [Google Scholar] [CrossRef]
- Laganière, J.; Paré, D.; Bradley, R.L. How does a tree species influence litter decomposition? Separating the relative contribution of litter quality, litter mixing, and forest floor conditions. Can. J. For. Res. 2010, 40, 465–475. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; De Santo, A.V.; Alfani, A.; Bartoli, G.; De Cristofaro, A. Effects of urban heavy metal pollution on organic matter decomposition in Quercus ilex L. woods. Environ. Pollut. 1995, 89, 81–87. [Google Scholar] [CrossRef]
- Fioretto, A.; Papa, S.; Pellegrino, A.; Fuggi, A. Decomposition dynamics of Myrtus communis and Quercus ilex leaf litter: Mass loss, microbial activity and quality change. Appl. Soil Ecol. 2007, 36, 32–40. [Google Scholar] [CrossRef]
- Weedon, J.T.; Cornwell, W.K.; Cornelissen, J.H.; Zanne, A.E.; Wirth, C.; Coomes, D.A. Global meta-analysis of wood decomposition rates: A role for trait variation among tree species? Ecol. Lett. 2009, 12, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Parsons, W.J.F.; Taylor, B.R.; Parkinson, D. Decomposition of aspen (Populus tremuloides) leaf litter modified by leaching. Can. J. For. Res. 1990, 20, 943–951. [Google Scholar] [CrossRef]
- Prescott, C.E.; Zabek, L.M.; Staley, C.L.; Kabzems, R. Decomposition of broadleaf and needle litter in forests of British Columbia: Influences of litter type, forest type, and litter mixtures. Can. J. For. Res. 2000, 30, 1742–1750. [Google Scholar] [CrossRef]
- Jacob, M.; Viedenz, K.; Polle, A.; Thomas, F.M. Leaf litter decomposition in temperate deciduous forest stands with a decreasing fraction of beech (Fagus sylvatica). Oecologia 2010, 164, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Püttsepp, Ü.; Lõhmus, K.; Koppel, A. Decomposition of fine roots and α-cellulose in a short rotation willow (Salix spp.) plantation on abandoned agricultural land. Silva Fenn. 2007, 41, 247–258. [Google Scholar] [CrossRef]
- Fujii, S.; Makita, N.; Mori, A.S.; Takeda, H. A stronger coordination of litter decomposability between leaves and fine roots for woody species in a warmer region. Trees 2016, 30, 395–404. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Antignani, V.; Capodilupo, M.; Mazzoleni, S. Decomposition and nutrient dynamics in mixed litter of Mediterranean species. Plant Soil 2010, 331, 481–496. [Google Scholar] [CrossRef]
- Silfver, T.; Mikola, J.; Rousi, M.; Roininen, H.; Oksanen, E. Leaf litter decomposition divers among genotypes in a local Betula pendula population. Oecologia 2007, 152, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Ineson, P. Effects of enhanced atmospheric CO2 and nutrient supply on the quality and subsequent decomposition of fine roots of Betula pendula Roth. and Picea sitchensis (Bong.) Carr. Plant Soil 1995, 170, 267–277. [Google Scholar] [CrossRef]
- Berg, B.; Staaf, H.; Wessen, B. Decomposition and nutrient release in needle litter from nitrogen-fertilized Scots pine (Pinus silvestris) stands. Scand. J. For. Res. 1987, 2, 399–415. [Google Scholar] [CrossRef]
- Trofymow, J.A.; Preston, C.M.; Prescott, C.E. Litter quality and its potential effect on decay rates of materials from Canadian forests. Water Air Soil Pollut. 1995, 82, 215–226. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Winder, R.S.; Lamarche, J.; Constabel, C.P.; Hamelin, R.C. The effects of high-tannin leaf litter from transgenic poplars on microbial communities in microcosm soils. Front. Microbiol. 2013, 4, 290. [Google Scholar] [CrossRef] [PubMed]

| Genotype | Wood Composition, mg/g | ||
|---|---|---|---|
| Lignin 1 | Cellulose 2 | Pentosans 3 | |
| Pt | 209.0 | 374.3 | 148.3 a 4 |
| Xeg1b | 213.0 | 397.6 | 115.6 c |
| Xeg3a | 211.9 | 373.4 | 137.8 b |
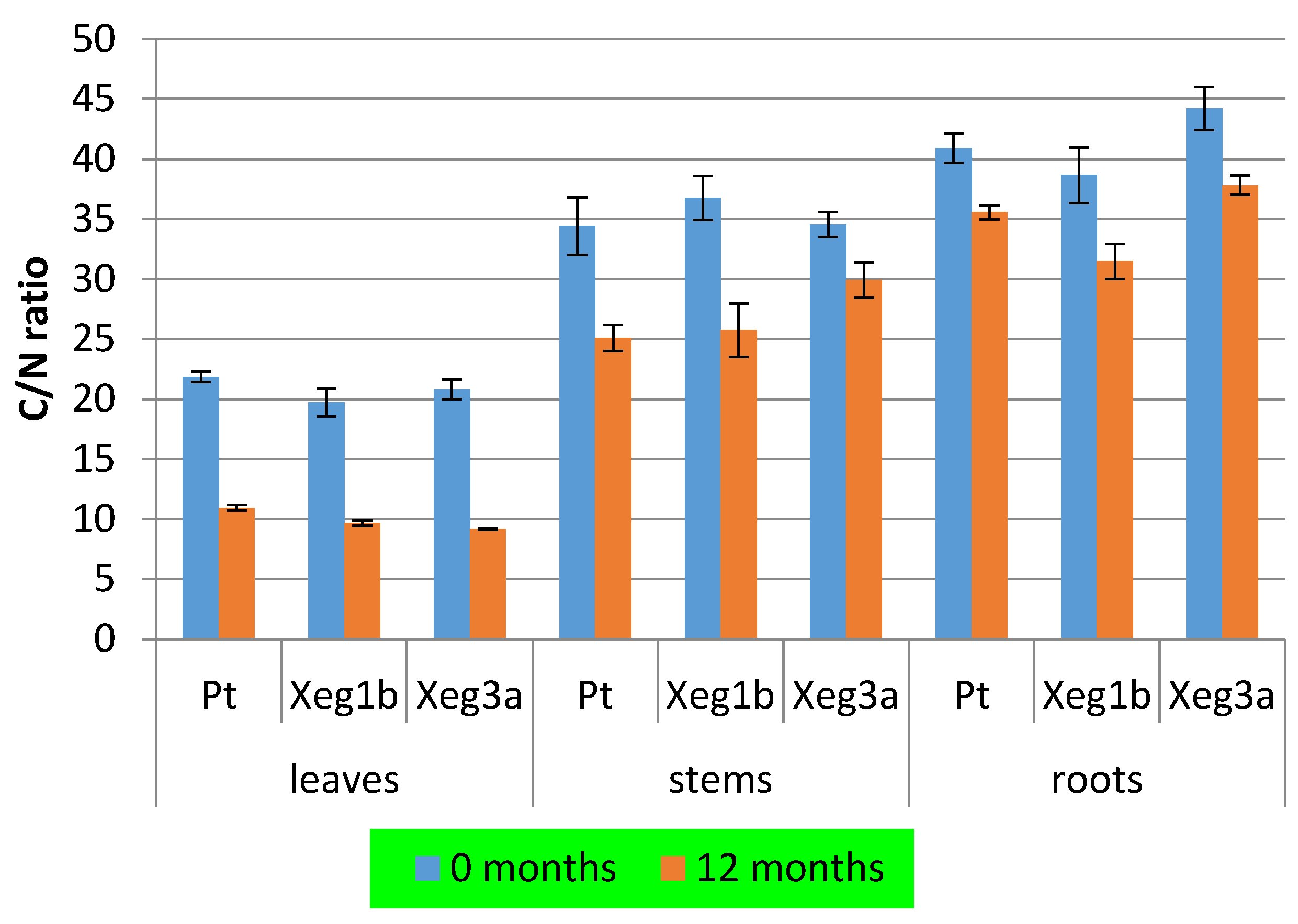
| Organ | Genotype | N, % | C, % | C/N Ratio |
|---|---|---|---|---|
| leaves | Pt | 1.90 | 41.5 | 21.8 |
| Xeg1b | 2.10 | 41.4 | 19.7 | |
| Xeg3a | 2.00 | 41.6 | 20.8 | |
| stems | Pt | 1.32 | 45.4 | 34.4 |
| Xeg1b | 1.26 | 46.3 | 36.7 | |
| Xeg3a | 1.32 | 45.6 | 34.5 | |
| roots | Pt | 1.12 ab 1 | 45.8 | 40.9 ab |
| Xeg1b | 1.19 a | 46.0 | 38.7 a | |
| Xeg3a | 1.05 b | 46.4 | 44.2 b |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedev, V.G.; Vidyagina, E.O.; Larionova, A.A.; Shestibratov, K.A. Decomposition of Leaves, Stems and Roots of Transgenic Aspen with the Xyloglucanase (sp-Xeg) Gene under Laboratory Microcosm Conditions. Environments 2017, 4, 4. https://doi.org/10.3390/environments4010004
Lebedev VG, Vidyagina EO, Larionova AA, Shestibratov KA. Decomposition of Leaves, Stems and Roots of Transgenic Aspen with the Xyloglucanase (sp-Xeg) Gene under Laboratory Microcosm Conditions. Environments. 2017; 4(1):4. https://doi.org/10.3390/environments4010004
Chicago/Turabian StyleLebedev, Vadim G., Elena O. Vidyagina, Alla A. Larionova, and Konstantin A. Shestibratov. 2017. "Decomposition of Leaves, Stems and Roots of Transgenic Aspen with the Xyloglucanase (sp-Xeg) Gene under Laboratory Microcosm Conditions" Environments 4, no. 1: 4. https://doi.org/10.3390/environments4010004






