1. Introduction
Soil treatment is a widely used technique to improve the engineering properties of natural soils not suitable for construction purposes by means of the addition of chemical agents, which react with soil particles. The use of traditional binders such as lime and cement has a strong impact on the physical and hydro-mechanical properties of soils as results of short and long term reactions which take place after the treatment [
1,
2,
3,
4,
5,
6,
7,
8,
9].
With reference to traditional binders, the mechanical improvement of soil is mainly due to pozzolanic activity developed after the addition of binder. Pozzolanic activity is connected to the availability of silica and alumina in a high pH environment promoting the formation of cementitious compounds responsible for mechanical improvement of treated soils [
10,
11,
12,
13].
The development and the use of environmentally friendly binders as an alternative solution to traditional binders in a low carbon agenda is of prime importance particularly in the construction sector.
The use of novel and efficient binders for geotechnical applications is a promising issue in terms of sustainability since it reduces the carbon footprint and allows reusing secondary by-products such as artificial pozzolans. These by-products can be involved in soil improvement as cementing agents if properly activated, inducing a mechanical improvement which allows the reuse of soils not suitable in their natural state as construction materials for earthworks. The recycling of waste materials such as by-products from industrial process to synthesize a new binder favours a closed loop of material use, which minimizes waste generation and reduces production costs [
14].
Differently from the use of lime and cement for soil improvement, experimental research about the use of alkali activated binders in soil improvement is still limited [
15]; nevertheless, recent studies highlight the relevant potential of novel binders for geotechnical purposes. Cristelo et al. (2011) [
16] researched the optimum fly ash—based alkaline activated binder for the improvement of soil to be used in rammed earth construction through a parametric analysis using laboratory tests. Rios et al. (2016) [
17] compared the mechanical behaviour of samples of sand improved by alkali activated fly ash binder and by cement, highlighting the effective increase of shear strength soil properties induced by alkali activated binders.
Alkali activation is the consequence of a chemical reaction between of an aluminosilicate source with an alkaline solution (i.e., sodium hydroxide, sodium silicate). The aluminosilicate source is formed by precursor materials like natural pozzolans (e.g., pyroclastic soils) or artificial pozzolans (e.g., fly ash, silica fume, steel sludge). After the activation the chemo-physical evolution is characterized by formation of cementitious compounds [
18,
19]. The reaction mechanism is promoted by the alkaline solution, which enables the dissolution of the aluminosilicate source (precursor) and the subsequent precipitation of gel phases, which condense in a three-dimensional aluminosilicate network [
20,
21,
22,
23].
In the present study, an insight into the mechanical improvement induced by alkali-activated binders based on the activation of two different type of fly ashes on a clayey soil has been presented. One-dimensional compression tests have been performed and interpreted taking into account the chemo-physical evolution of the system. The effects of type of binder, binder content and curing time and have been considered. Mineralogical changes induced by alkali-activated binders have been monitored over time by means of X ray diffraction (XRD). The efficiency of treatment has been also investigated by comparing the mechanical performance of soil treated with alkali-activated binders and with ordinary Portland cement.
2. Materials and Methods
2.1. Materials
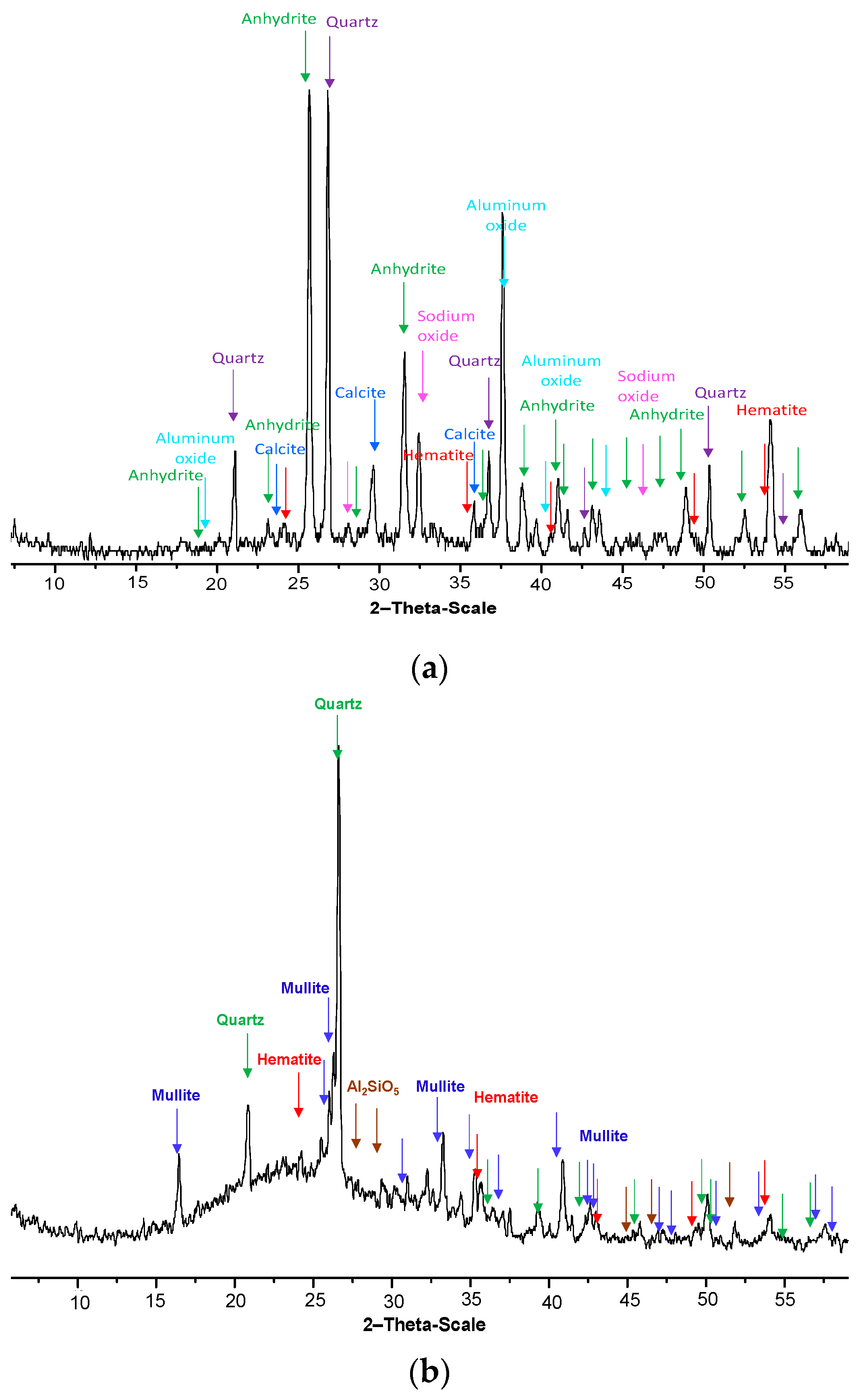
Two fly ashes of different mineralogy have been considered for this study, namely fly ash B (FAB) and fly ash R (FAR). The X-ray diffraction patterns of two fly ashes are reported in
Figure 1. FAB is a fluidal bed combustion fly ash supplied by a power plant located in Italy. The raw material (
Figure 1a) is composed by vitreous and crystalline phases which include calcium-containing minerals: anhydrite, calcite and other minerals: quartz and hematite. The chemical composition of the FAB is given in
Table 1. FAR is a fly ash produced by a German thermoelectric power plant at higher combustion temperature. It is mainly composed (
Figure 1b) of alumino-silicate glasses with varying amounts of crystalline phases such as quartz, mullite and hematite. The halo between 17° and 30° clearly identify the vitreous phases of the ash. The chemical composition of the FAR is given in
Table 2. Both fly ashes are characterized by low free calcium content.
The sodium silicate solution was supplied by Woellner (Ludwigshafen, Germany) with a SiO2/Na2O mass ratio equal to 1.7.
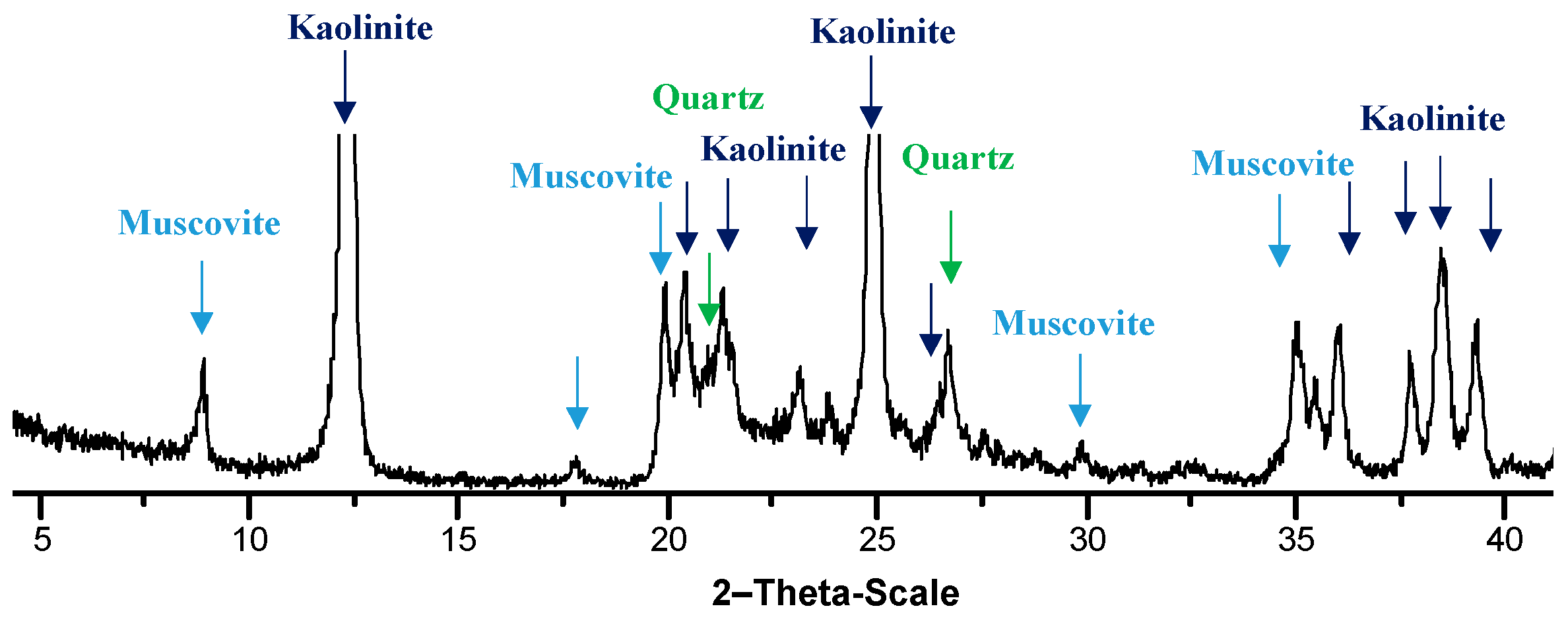
The soil considered for the experimental investigation is Speswhite kaolin from deposits in the South West of England. The soil is manly composed of kaolinite clay minerals with small amounts of quartz and muscovite (
Figure 2). The chemical composition of the soil is given in
Table 3. Specific gravity G
s is 2.6 and surface area, determined by BET, is 14 m
2/g. The pH value is 4.6 and plastic limit and liquid limit are respectively 32% and 70%, with a plasticity index IP equal to 38%.
2.2. Sample Preparation
Alkali activated binders (Activated fly ash B (AFAB) and activated fly ash R (AFAR)) in the following) were prepared by mixing fly ash, alkaline solution and water in fixed proportions. Alkaline solution/fly ash mass ratio was kept constant and equal to 0.5. Additional deionised water was provided to the system in order to guarantee workability during mixing and a sufficient liquid phase amount for dissolving the aluminosilicate source. The deionised water/solid ratio by mass was selected equal to 0.5.
Soil samples treated with alkali-activated binders were prepared by adding increasing fly ash percentages, namely 20% (sample KFA20%) and 40% (sample KFA40%) by dry weight of solids (soil + fly ash), maintaining the ratio between alkaline solution and fly ash equal to 0.5. The initial water content added to the mixtures was equal to the liquid limits of the treated samples.
Reconstituted samples for oedometer tests were prepared by hand mixing solids (fly ash + soil), alkaline solution and water at their liquid limit (i.e., wL = 70% for kaolin, wL = 61% for kaolin treated with 20% of activated fly ash R (KFAR20%), wL = 57% for kaolin treated with 40% of activated fly ash R (KFAR40%), wL = 66% for kaolin treated with 20% of activated fly ash B (KFAB20%), wL = 61% for kaolin treated with 40% of activated fly ash B (KFAB40%)). Samples were poured in the mould and placed in oedometer cell without compaction. The dimension of the specimens was 50 × 20 mm. The treated samples were then sealed in plastic bags and cured at increasing curing times of 24 h, 7, 28 and 60 days before performing mechanical tests.
2.3. Experimental Procedures
2.3.1. X-ray Diffraction Analysis (XRD)
The mineralogical composition of samples was investigated by X-ray diffraction analysis performed on randomly oriented powder using a Brucker AXS D8 Advance Diffractometer with CuKα (
λ = 0.154 nm) radiation and a step size of 0.017°. Samples were dehydrated before testing by freeze-drying technique [
24].
2.3.2. One Dimensional Compression Tests
One dimensional compression tests have been performed on raw samples and alkali activated binder treated samples cured for 24 h, 7, 28 and 60 days. Tests have been performed in standard oedometer cells, where vertical stress was conventionally applied in successive steps (Δσv/σv = 1) within the stress interval 10 ÷ 2400 kPa. Micrometre dial gauges with an accuracy of 0.001 mm have been used to measure vertical displacements.
3. Results
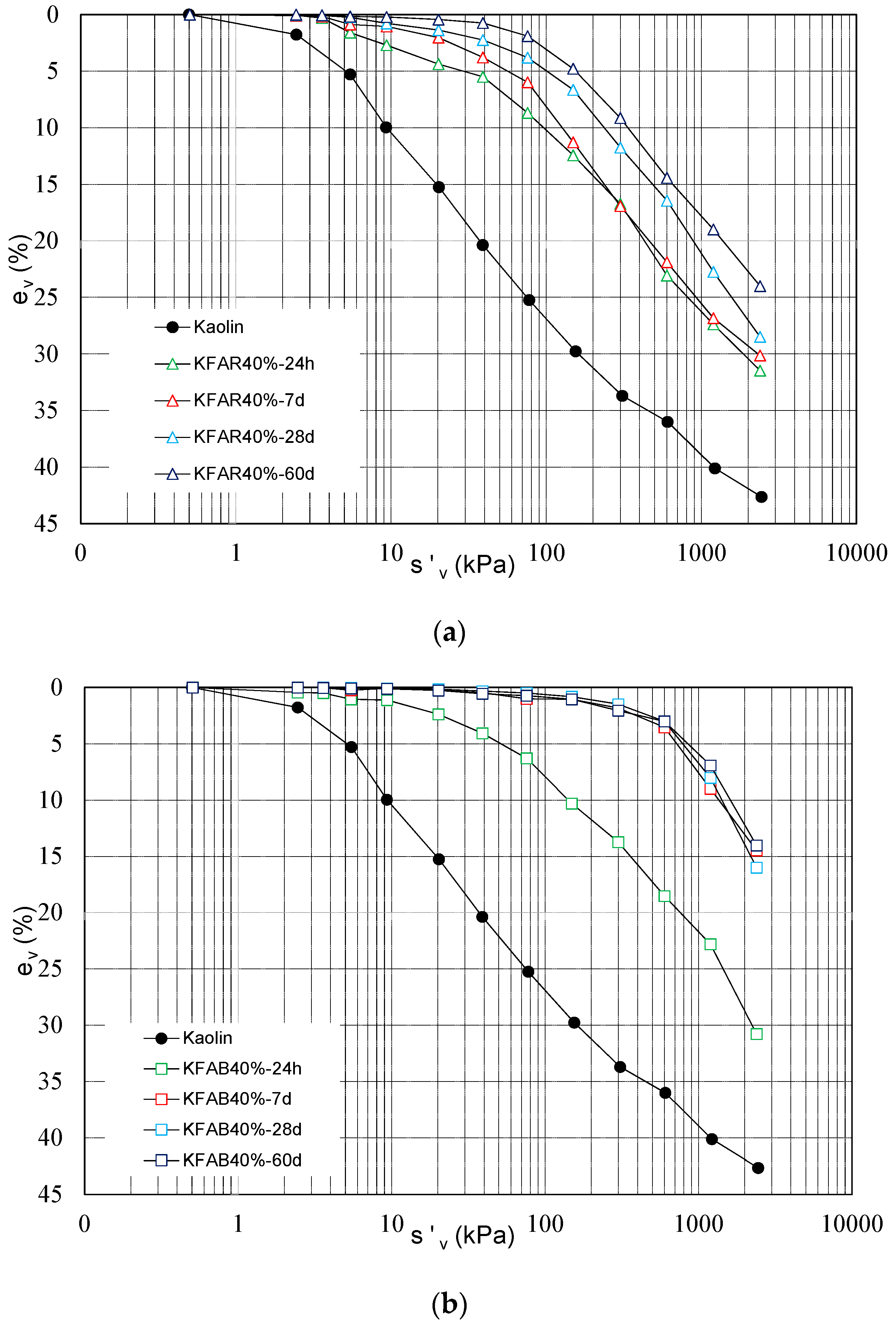
1D compressibility curves of not treated and alkali activated binder treated kaolin (KFAR40% and KFAB40%) at increasing curing times, namely 24 h, 7, 28 and 60 days are reported in
Figure 3a,b. Addition of alkali activated binders induces an overall reduction of compressibility of the treated samples, with reduced volume strains for reference vertical stresses since the short term (i.e., 24 h of curing). With reference to KFAR40% (
Figure 3a), the reduction is more relevant for increasing curing times, showing a stiffer behaviour of the treated sample. Conversely, the behaviour of KFAB40% treated samples does not evolve with time after 7 days of curing, as shown in
Figure 3b.
Coupled with the reduction of compressibility, all the samples show an increase of yield stress which characterizes the transition from a reversible behaviour to an irreversible one. For KFAR40% treated samples yield stress increases with curing time. For KFAB40% treated samples, yield stress is shifted to the highest level within 7 days of curing and then does not change for increasing curing times.
For stress levels higher than yield stress, treated samples show a higher compressibility coefficient (i.e., slope of the compressibility curve) with respect to not treated samples, depending on the de-structuration stage induced by load increase, more evident for KFAB40% samples (
Figure 3b). Stress levels achieved during the tests were not high enough to induce the complete de-structuration of the treated samples.
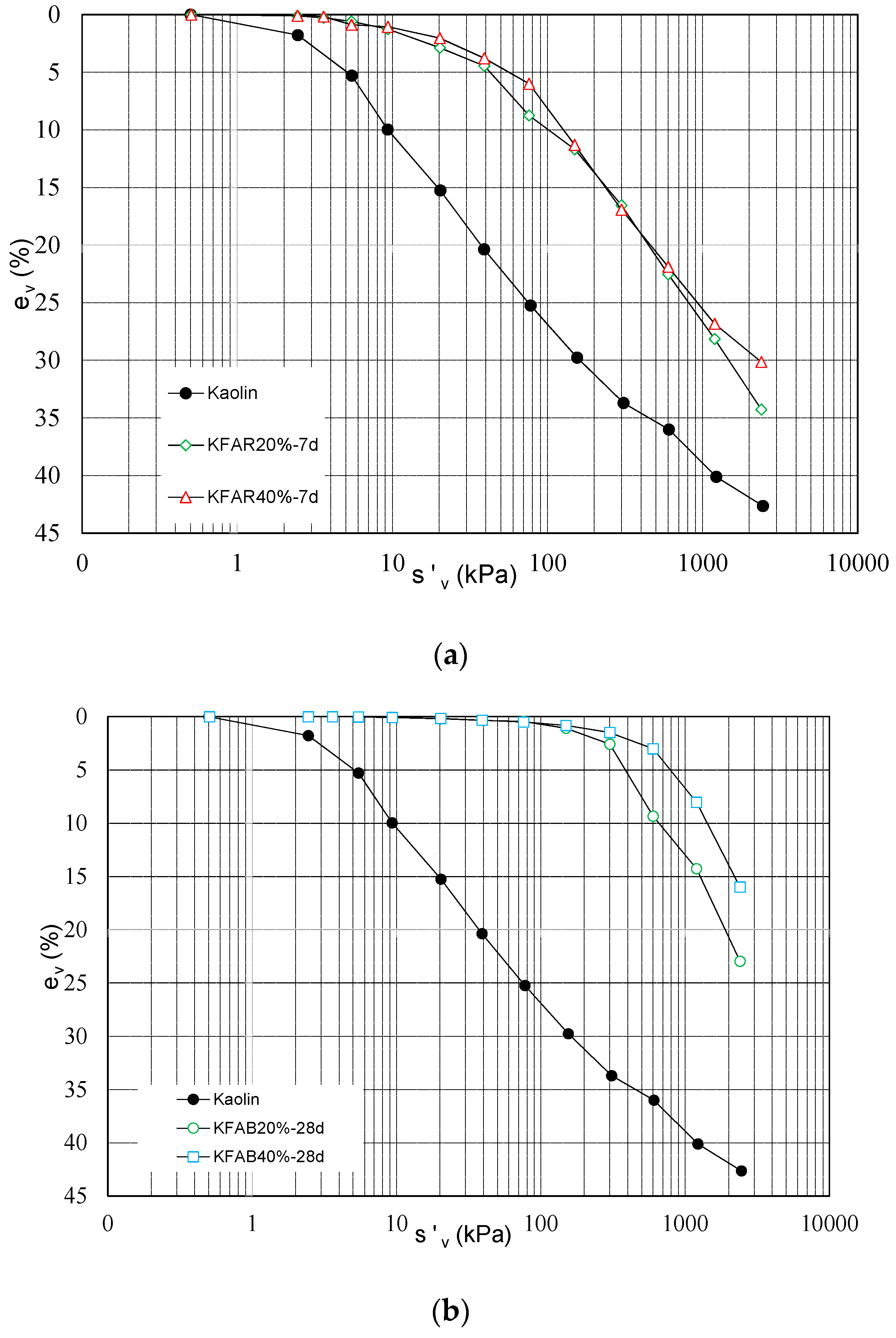
In
Figure 4, the compressibility curves of treated samples are reported as function of binder content. KFAR samples prepared at 20% and 40% of alkali activated fly ash content (KFAR20% vs. KFAR40%) and cured for 7 days before testing (
Figure 4a) show no relevant changes in the compressibility curves. A more pronounced structure-induced behaviour [
25] is observed for KFAB treated samples after 28 days of curing increasing the amount of binder; both reduction of compressibility and yield stress increase depend on binder content.
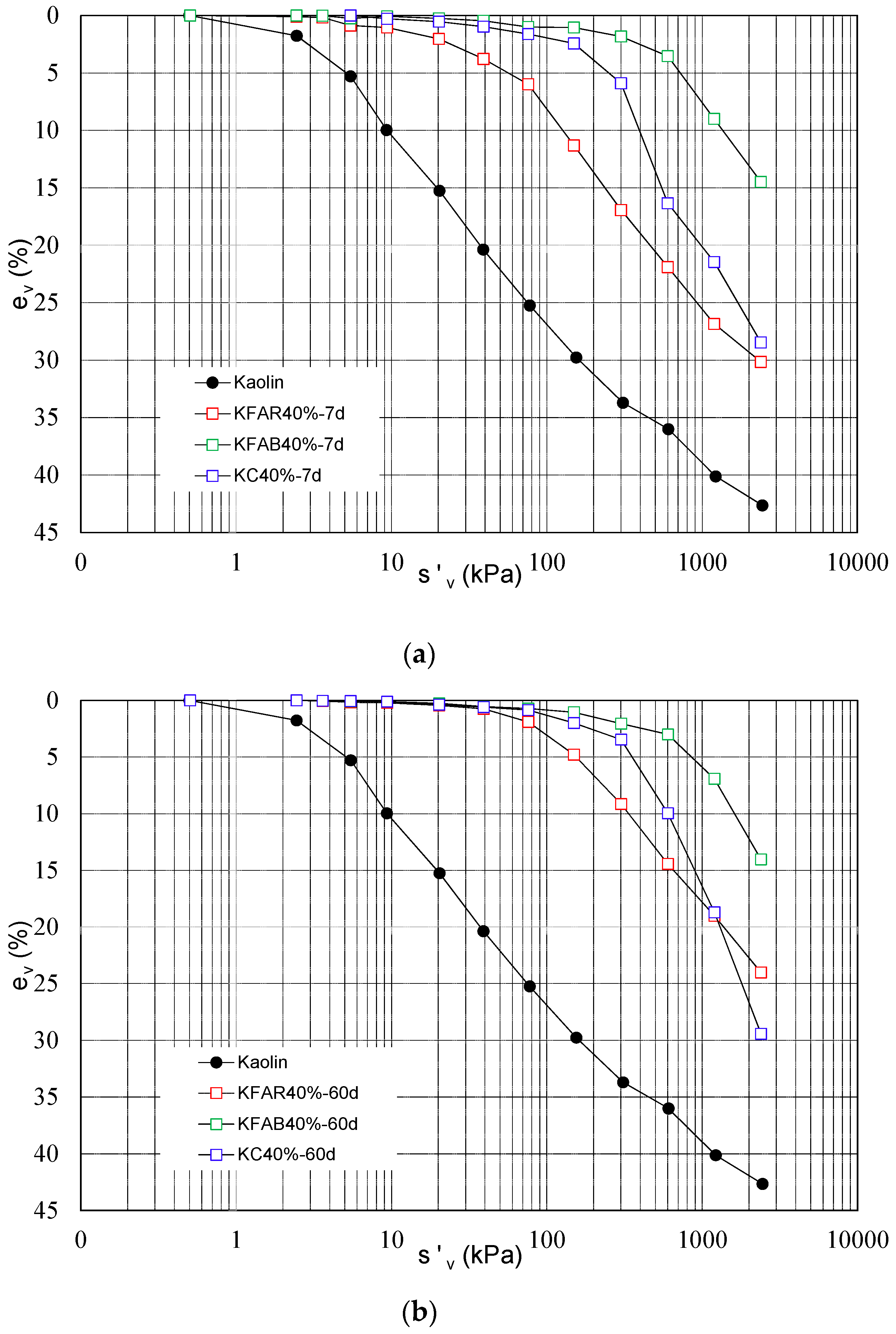
A comparison between the mechanical performance induced by alkali activated binders (KFAR40%; KFAB40%) and by ordinary Portland cement (KC40%—kaolin treated with 40% of cement) has been shown in
Figure 5 for samples prepared with the same binder content and cured for 7 and 60 days, in order to highlight the role of type of binder on the mechanical improvement of treated soil. Results show a lower compressibility reduction and yield stress increase for KFAR treated samples compared to cement treated samples, whereas the compressibility curves of KFAB treated samples show a lower compressibility and higher yield stress than those pertaining to cemented samples (KC40%). The post-yield behaviour induced by ordinary Portland cement shows the highest slope of the compressibility curve, highlighting a more evident de-structuration stage for cement treated samples at increasing vertical stresses.
4. Discussion
The high reactivity of alkali activated binders favours an advantageous behaviour of the improved material for geotechnical purposes. Since the very short term, the mechanical improvement of the treated soil is characterized by an overall reduction of compressibility and a remarkable extension of the stress interval for which the improved soil shows a reversible behaviour (i.e., increase of yield stress).
The macroscopic behaviour of treated samples and its improvement has been interpreted taking account of the chemo-physical evolution of the alkali activated fly ashes. Mineralogical changes induced by alkali-activation process have been monitored over time by means of X-Ray diffraction.
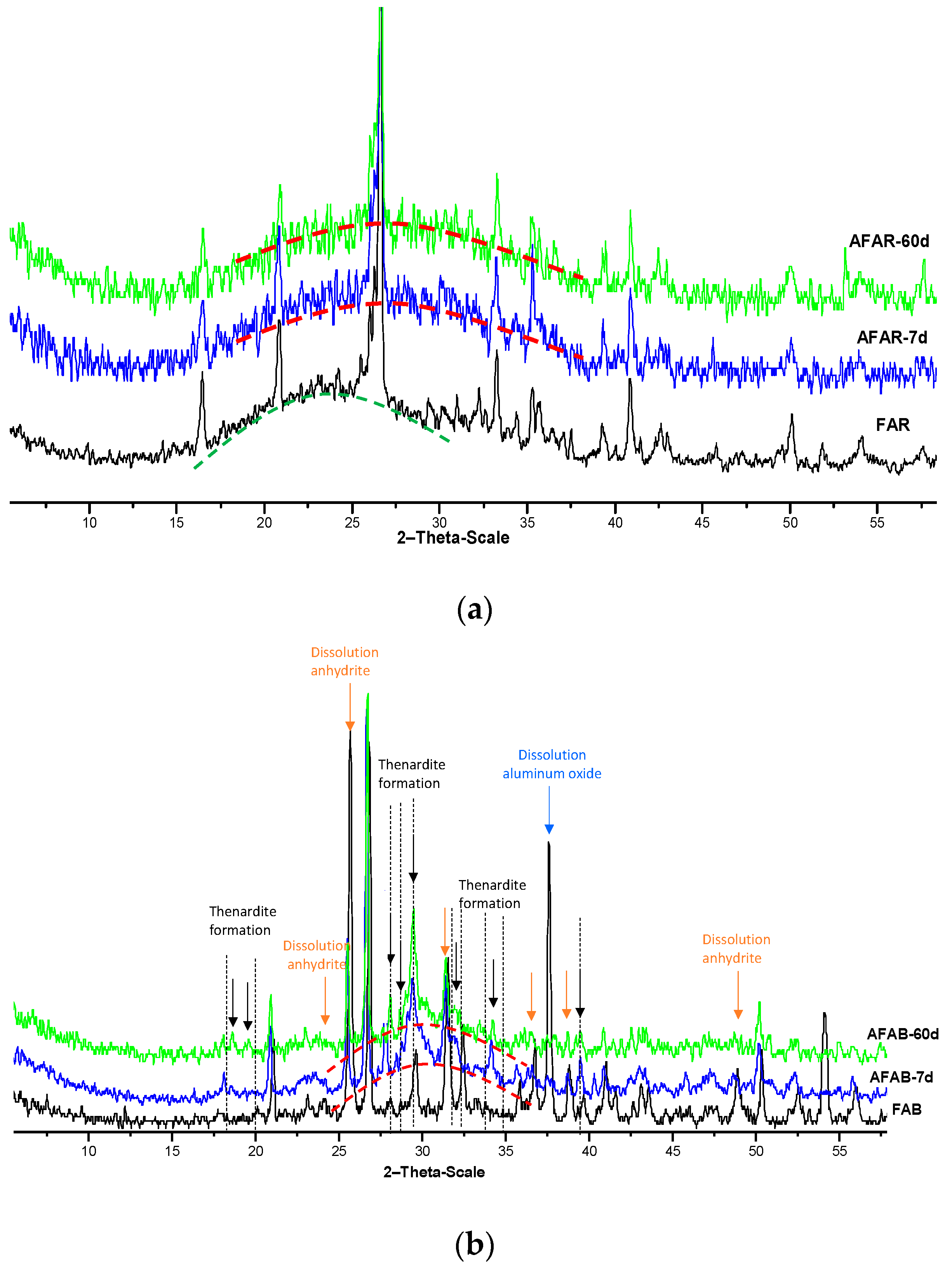
X-ray diffraction patterns of raw and alkali activated fly ash binders (AFAR and AFAB) are shown in
Figure 6. The mineralogical composition of fly ashes is modified by alkaline activation process. With reference to alkali activated FAR (
Figure 6a), the precipitation of new compounds in gel like state is evidenced by the broad bending detected between 25° and 35° resulting from the dissolution of initial amorphous phases of the ash (halo between 17° and 30°). In
Figure 6b, consumption of crystalline phases of alkali activated FAB, such as anhydrite and aluminium oxide is evidenced by the disappearance of their characteristic peaks after 7 days of curing. The precipitation of new crystalline phases such as thenardite (Na
2SO
4) seems to be consistent with the release of sulphate from anhydrite dissolution and its subsequent reaction with sodium provided by silicate solution. A broad reflection corresponding to new poorly crystalline compounds resulting from alkaline-activation reactions was observed in the treated samples starting from 7 days of curing time.
The high reactivity of the alkali activated fly ashes as alumino-silicate source for promoting precipitation of new mineralogical phases forming chains or networks with cementitious properties is responsible for the mechanical improvement of the treated soil.
The effects of curing time and binder content have been also considered. The reactions kinetic controlling the chemo-physical evolution of alkali activated binders and therefore the macroscopic evolution of soil properties depends on fly ashes mineralogy, as evidenced by the mechanical test results.
The efficiency of treatment has been highlighted by comparing the mechanical performance induced by alkali-activated binders with the one promoted by ordinary Portland cement. The addition of AFAR binder reduces the compressibility of treated soil although its extent is lower compared cement treated samples. A marked improvement of the mechanical behaviour of soil is induced by AFAB binder, representing a viable sustainable alternative to the use of ordinary stabilizing agent for soil improvement.
5. Conclusions
An insight into the mechanical improvement induced by alkali-activated binders based on the activation of two different type of fly ashes on a clayey soil has been presented. One-dimensional compression tests performed on treated sample highlighted the effectiveness of alkali activated binders to promote an improvement of the mechanical behaviour of treated soil. A reduction of compressibility an increase of the yield stress soil was observed since the very short term.
The macroscopic behaviour of treated soil has been linked to the experimental evidences at microscale. Microstructural analyses show a high reactivity of the alkali activated fly ashes as alumino-silicate source promoting the precipitation of new mineralogical phases forming chains and networks with cementitious properties.
The reactions kinetic controlling the chemo-physical evolution of alkali activated binders and therefore the macroscopic evolution of soil properties depends on fly ashes mineralogy, as evidenced by the compressive behaviour of the treated samples as function of curing times and binder contents. The efficiency of treatment has been highlighted by comparing the mechanical performance induced by alkali-activated binder and ordinary Portland cement.