Ammonia Oxidizing Archaea and Bacteria in East Asian Paddy Soils—A Mini Review
Abstract
:1. Introduction
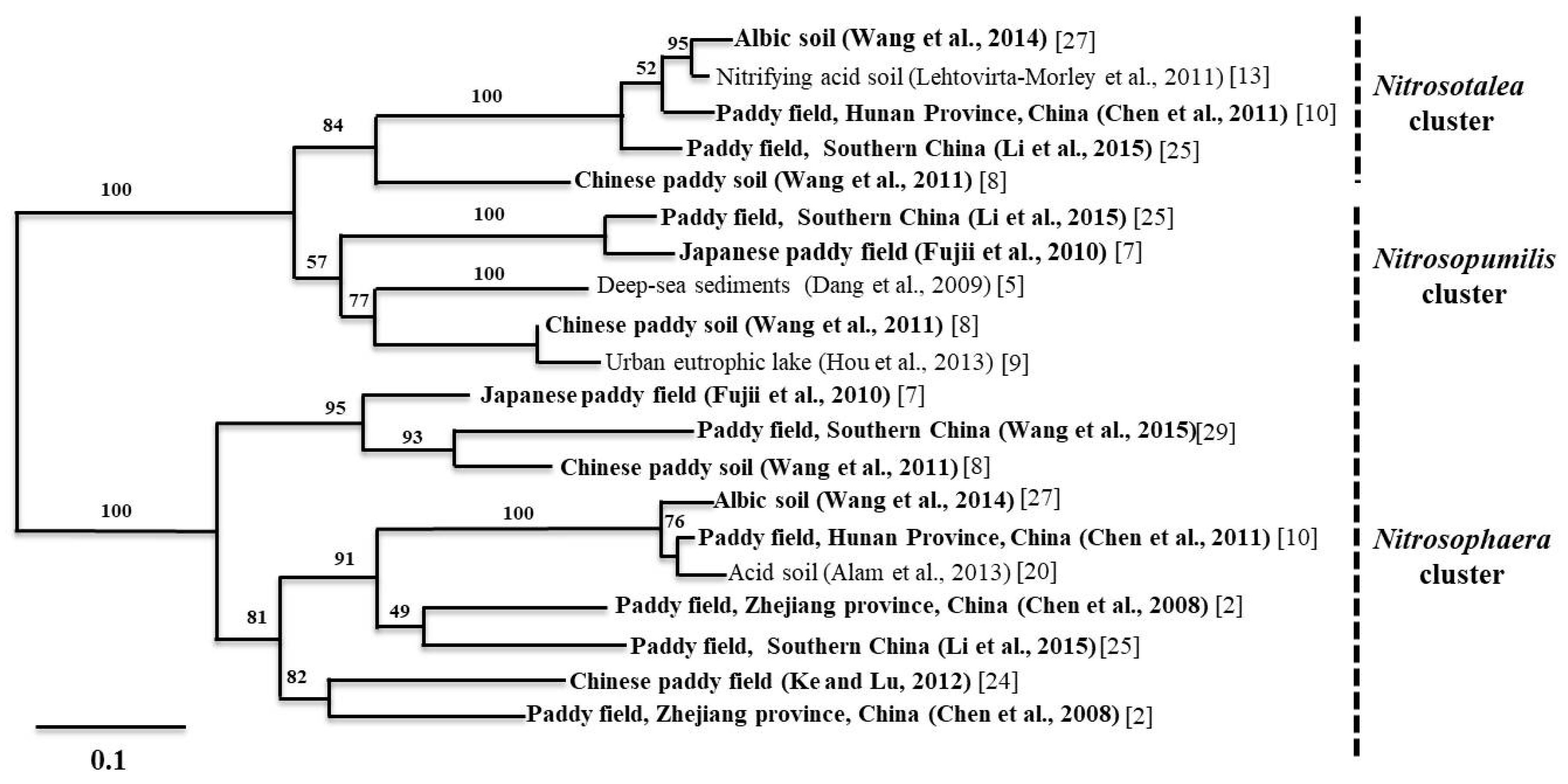
2. Abundance and Composition of AOA and AOB
3. AOA and AOB Contribution to Nitrification
4. AOA and AOB Response to Environmental Factors
4.1. Nitrogen Fertilizer and pH
4.2. Other Factors
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arth, I.; Frenzel, P.; Conrad, R. Denitrification coupled to nitrification in the rhizosphere of rice. Soil Biol. Biochem. 1998, 30, 509–515. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Xia, Y.; Shen, J.; He, J. Ammonia-oxidizing archaea: Important players in paddy rhizosphere soil? Environ. Microbiol. 2008, 10, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Q.; Liu, Y.; Jin, Z.; Zhang, A.; Pan, G.; Li, L.; Crowley, D.; Zhang, X.; Song, X.; Cui, L. Temporal dynamics of ammonia oxidizer (amoA) and denitrifier (nirK) communities in the rhizosphere of a rice ecosystem from Tai Lake region, China. Appl. Soil Ecol. 2011, 48, 210–218. [Google Scholar] [CrossRef]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Li, J.; Zhang, X.; Li, T.; Tian, F.; Jin, W. Diversity and spatial distribution of amoA-encoding archaea in the deep-sea sediments of the tropical West Pacific Continental Margin. J. Appl. Microbiol. 2009, 106, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-Y.; Zhang, L.-M.; Shen, J.-P.; Li, L.-H.; Yuan, C.-L.; He, J.-Z. Nitrogen loading levels affect abundance and composition of soil ammonia oxidizing prokaryotes in semiarid temperate grassland. J. Soils Sediments 2011, 11, 1243. [Google Scholar] [CrossRef]
- Fujii, C.; Nakagawa, T.; Onodera, Y.; Matsutani, N.; Sasada, K.; Takahashi, R.; Tokuyama, T. Succession and community composition of ammonia-oxidizing archaea and bacteria in bulk soil of a Japanese paddy field. Soil Sci. Plant Nutr. 2010, 56, 212–219. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Feng, X.; Zhai, L.; Zhu, G. Quantitative analyses of ammonia-oxidizing Archaea and bacteria in the sediments of four nitrogen-rich wetlands in China. Appl. Microbiol. Biotechnol. 2011, 90, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Cao, X.; Song, C.; Zhou, Y. Predominance of ammonia-oxidizing archaea and nirK-gene-bearing denitrifiers among ammonia-oxidizing and denitrifying populations in sediments of a large urban eutrophic lake (Lake Donghu). Can. J. Microbiol. 2013, 59, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, L.-M.; Shen, J.-P.; Wei, W.-X.; He, J.-Z. Abundance and community structure of ammonia-oxidizing archaea and bacteria in an acid paddy soil. Biol. Fertil. Soils 2011, 47, 323–331. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Lu, Q.; Raza, W.; Huang, Q.; Shen, Q. Ammonia oxidizer abundance in paddy soil profile with different fertilizer regimes. Appl. Soil Ecol. 2014, 84, 38–44. [Google Scholar] [CrossRef]
- Yang, X.-R.; Li, H.; Nie, S.-A.; Su, J.-Q.; Weng, B.-S.; Zhu, G.-B.; Yao, H.-Y.; Gilbert, J.A.; Zhu, Y.-G. Potential contribution of anammox to nitrogen loss from paddy soils in Southern China. Appl. Environ. Microbiol. 2015, 81, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Lehtovirta-Morley, L.E.; Stoecker, K.; Vilcinskas, A.; Prosser, J.I.; Nicol, G.W. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc. Natl. Acad. Sci. USA 2011, 108, 15892–15897. [Google Scholar] [CrossRef] [PubMed]
- Erguder, T.H.; Boon, N.; Wittebolle, L.; Marzorati, M.; Verstraete, W. Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiol. Rev. 2009, 33, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ke, X.; Wu, L.; Lu, Y. Community composition of ammonia-oxidizing bacteria and archaea in rice field soil as affected by nitrogen fertilization. Syst. Appl. Microbiol. 2009, 32, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lu, L.; Wang, B.; Lin, X.; Zhu, J.; Cai, Z.; Yan, X.; Jia, Z. Long-term field fertilization significantly alters community structure of ammonia-oxidizing bacteria rather than archaea in a paddy soil. Soil Sci. Soc. Am. J. 2011, 75, 1431–1439. [Google Scholar] [CrossRef]
- He, J.; Shen, J.; Zhang, L.; Zhu, Y.; Zheng, Y.; Xu, M.; Di, H. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol. 2007, 9, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Avrahami, S.; Liesack, W.; Conrad, R. Effects of temperature and fertilizer on activity and community structure of soil ammonia oxidizers. Environ. Microbiol. 2003, 5, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Zhang, X.; Xie, S. Depth-related changes of sediment ammonia-oxidizing microorganisms in a high-altitude freshwater wetland. Appl. Microbiol. Biotechnol. 2014, 98, 5697–5707. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Ren, G.; Lu, L.; Zheng, Y.; Peng, X.; Jia, Z. Ecosystem-specific selection of microbial ammonia oxidizers in an acid soil. Biogeosci. Discuss. 2013, 10. [Google Scholar]
- Ouyang, Y.; Norton, J.M.; Stark, J.M. Ammonium availability and temperature control contributions of ammonia oxidizing bacteria and archaea to nitrification in an agricultural soil. Soil Biol. Biochem. 2017, 113, 161–172. [Google Scholar] [CrossRef]
- Tago, K.; Okubo, T.; Shimomura, Y.; Kikuchi, Y.; Hori, T.; Nagayama, A.; Hayatsu, M. Environmental factors shaping the community structure of ammonia-oxidizing bacteria and archaea in sugarcane field soil. Microbes Environ. 2015, 30, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, L.-M.; Shen, J.-P.; Xu, Z.; He, J.-Z. Soil type determines the abundance and community structure of ammonia-oxidizing bacteria and archaea in flooded paddy soils. J. Soils Sediments 2010, 10, 1510–1516. [Google Scholar] [CrossRef]
- Ke, X.; Lu, Y. Adaptation of ammonia-oxidizing microorganisms to environment shift of paddy field soil. FEMS Microbiol. Ecol. 2012, 80, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Weng, B.-S.; Huang, F.-Y.; Su, J.-Q.; Yang, X.-R. pH regulates ammonia-oxidizing bacteria and archaea in paddy soils in Southern China. Appl. Microbiol. Biotechnol. 2015, 99, 6113–6123. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-P.; Zhang, L.-M.; Di, H.J.; He, J.-Z. A review of ammonia-oxidizing bacteria and archaea in Chinese soils. Front. Microbiol. 2012, 3, 296. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Gu, J.-D. Community structure and abundance of ammonia-oxidizing archaea and bacteria after conversion from soybean to rice paddy in albic soils of Northeast China. Appl. Microbiol. Biotechnol. 2014, 98, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Dong, H.; Wang, S.; Huang, Q.; Jiang, H. Diversity and abundance of ammonia-oxidizing archaea and bacteria in diverse Chinese paddy soils. Geomicrobiol. J. 2014, 31, 12–22. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Guo, Z.; Ma, J.; Xu, H.; Jia, Z. Differential contributions of ammonia oxidizers and nitrite oxidizers to nitrification in four paddy soils. ISME J. 2015, 9, 1062. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-H.; Song, J.; Kim, B.-Y.; Kim, M.-S.; Joa, J.-H.; Weon, H.-Y. Characterization of the bacterial and archaeal communities in rice field soils subjected to long-term fertilization practices. J. Microbiol. 2012, 50, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, H.; Wang, J.; Liu, X.; Cheng, K.; Li, L.; Zheng, J.; Zhang, X.; Zheng, J.; Pan, G. Short-term response of nitrifier communities and potential nitrification activity to elevated CO2 and temperature interaction in a Chinese paddy field. Appl. Soil Ecol. 2015, 96, 88–98. [Google Scholar] [CrossRef]
- Baolan, H.; Shuai, L.; Lidong, S.; Ping, Z.; Xiangyang, X.; Liping, L. Effect of different ammonia concentrations on community succession of ammonia-oxidizing microorganisms in a simulated paddy soil column. PLoS ONE 2012, 7, e44122. [Google Scholar] [CrossRef] [PubMed]
- Bannert, A.; Kleineidam, K.; Wissing, L.; Mueller-Niggemann, C.; Vogelsang, V.; Welzl, G.; Cao, Z.; Schloter, M. Changes in diversity and functional gene abundances of microbial communities involved in nitrogen fixation, nitrification, and denitrification in a tidal wetland versus paddy soils cultivated for different time periods. Appl. Environ. Microbiol. 2011, 77, 6109–6116. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hou, X.; Zhou, X.; Xin, X.; Wright, A.; Jia, Z. pH regulates key players of nitrification in paddy soils. Soil Biol. Biochem. 2015, 81, 9–16. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Song, L.; Wang, S.; Yin, C. Manure fertilization alters the population of ammonia-oxidizing bacteria rather than ammonia-oxidizing archaea in a paddy soil. J. Basic Microbiol. 2014, 54, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Angel, R.; Lu, Y.; Conrad, R. Niche differentiation of ammonia oxidizers and nitrite oxidizers in rice paddy soil. Environ. Microbiol. 2013, 15, 2275–2292. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ding, W.; Liu, D.; He, T.; Yoo, G.; Yuan, J.; Chen, Z.; Fan, J. Wheat straw-derived biochar amendment stimulated N2O emissions from rice paddy soils by regulating the amoA genes of ammonia-oxidizing bacteria. Soil Biol. Biochem. 2017, 113, 89–98. [Google Scholar] [CrossRef]

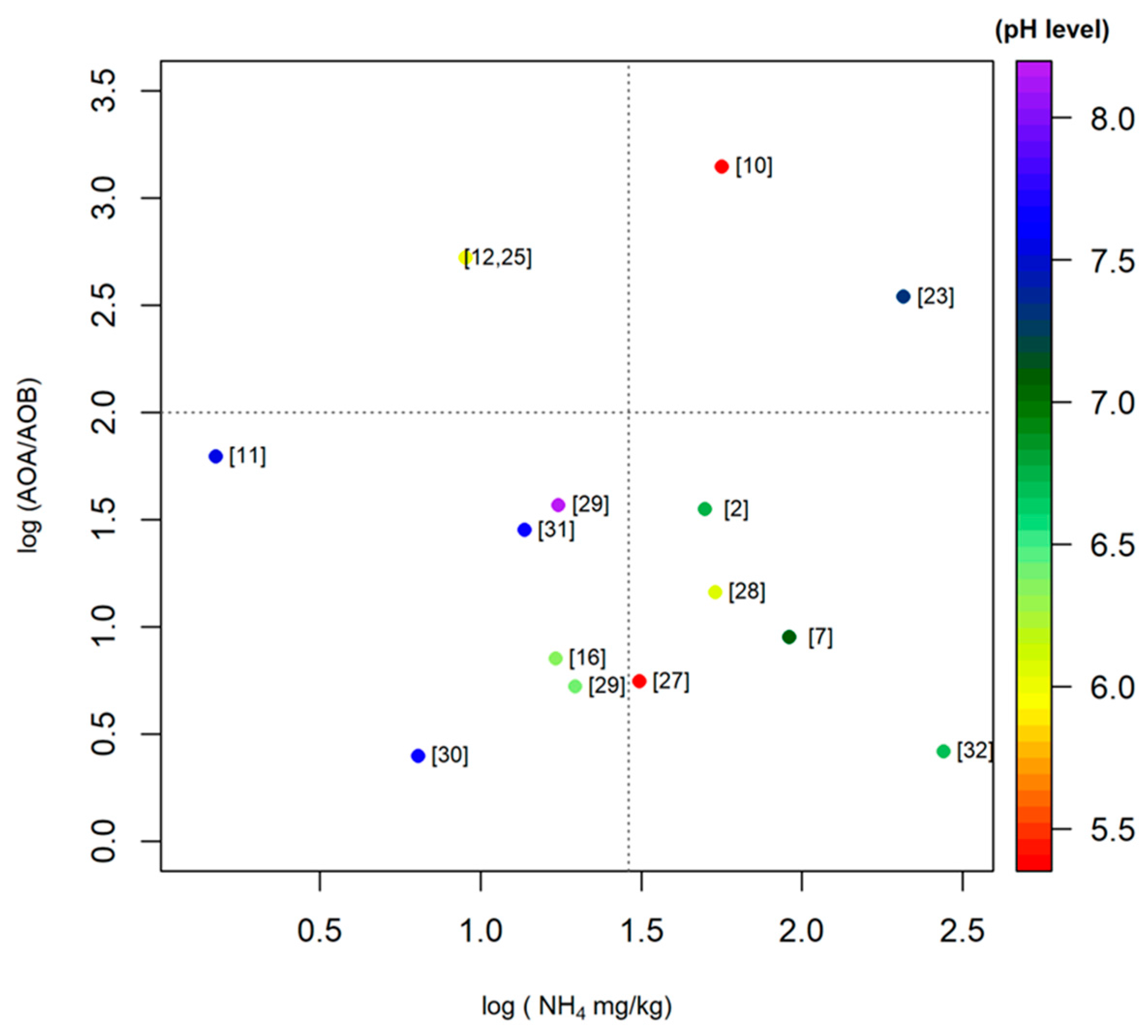
| Paddy Fields Location | pH | OM | NH4+ | NO3− | AOA/AOB Ratio | References |
|---|---|---|---|---|---|---|
| mg/kg * g/kg | mg/kg | mg/kg | ||||
| Zhejiang province, China | 6.8 | 9.32 * | 49.9 3 | --------- | 1.2–69.3 | [2] |
| Flooded paddy soils, China | 7.0–7.7 | --------- | 45.1–370.0 | 0.5–1.0 | 22.9–667 | [23] |
| Taoyuan, Hunan Province, China | 5.3–5.4 | 29.2–41.6 | 27.1–85.9 | 0.6–0.9 | 417–2383 | [10] |
| Changshu Ecological Experimental Station, China | 6.3–6.4 | 20.8–30.7 * | 13.7–21.4 | 1.8–2.7 | 0.39–12.3 | [16] |
| Eastern China | 5.2–7.6 | 9.6–43.1 * | 9.6–97.9 | 0.1–6.2 | 9–20 1 | [28] |
| Albic soil, northeast China | 4.4–6.4 | --------- | 10.3–52.2 | 0.2–7.1 | 0.65–10.48 | [27] |
| Changshu, Jiangsu province, China | 6.8–8.0 | 6.0–19.3 * | 0.5–2.5 | 0.9–8.9 | 2.6–121.9 | [11] |
| Southern China | 8.2 | 30.4 * | 17.5 | 6.8 | 37.0 | [29] |
| Southern China | 6.0 – 6.8 | 10.2 – 35.4 * | 4.6–33.9 | 1.4–11.3 | 1.9–10.5 | [29] |
| Southern China | 5.1–7.3 | 13–46 * | 3–15 | 0.3–5 | 1.91–1048 | [12,25] |
| Experimental rice field, Suwon, Korea | 5.7–7.4 2 | 19–41 2 | --------- | --------- | 0.03–0.05 2 | [30] |
| Japanese paddy field | --------- | --------- | 10–140 2 | 0–135 2 | 4–346 2 | [7] |
| Jiangsu province, China. | 7.2–8.2 | --------- | 2.7–10.1 | 6.4–17.7 | 1.5–3.5 | [31] |
| Zhejiang Province, China | 7.0–7.2 | --------- | 39.9–143 | --------- | 1.0–16.9 | [32] |
| Zhejiang Province, China, | 7.3–8.1 | 5.8–31.0 2 | 0.42–27 | 2.1–16 | 0.97–55.5 2 | [33] |
| Jiangsu Province, China | 6.7 | 20.2 | 276.1 4 | --------- | 1.46–3.8 | [3] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhtar, H.; Lin, Y.-P.; Anthony, J. Ammonia Oxidizing Archaea and Bacteria in East Asian Paddy Soils—A Mini Review. Environments 2017, 4, 84. https://doi.org/10.3390/environments4040084
Mukhtar H, Lin Y-P, Anthony J. Ammonia Oxidizing Archaea and Bacteria in East Asian Paddy Soils—A Mini Review. Environments. 2017; 4(4):84. https://doi.org/10.3390/environments4040084
Chicago/Turabian StyleMukhtar, Hussnain, Yu-Pin Lin, and Johnathen Anthony. 2017. "Ammonia Oxidizing Archaea and Bacteria in East Asian Paddy Soils—A Mini Review" Environments 4, no. 4: 84. https://doi.org/10.3390/environments4040084





