Diffusive Uptake Rates of Volatile Organic Compounds on Standard ATD Tubes for Environmental and Workplace Applications
Abstract
:1. Introduction
2. Methods
2.1. The Passive Diffusion Theory
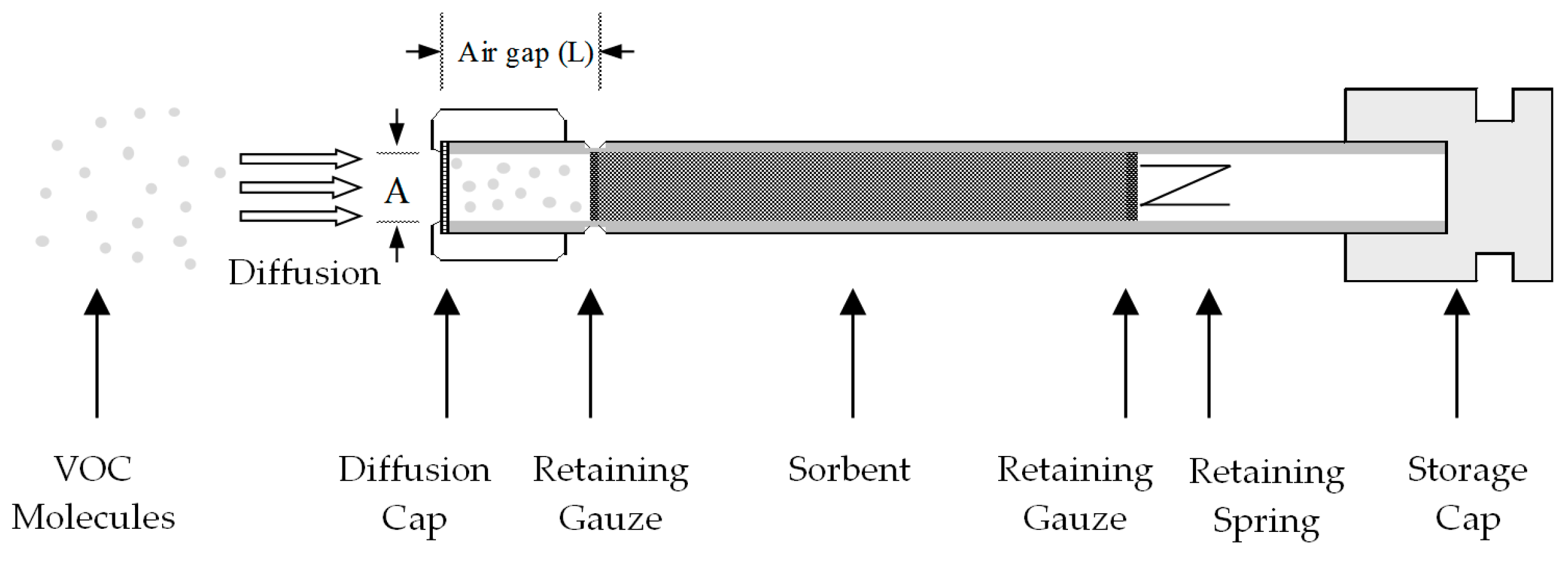
2.2. Standard ATD Tubes
- (1)
- The air gap (L) is 15 mm between the sampling end and sorbent surface when a diffusion cap is fitted. The UR changes with or without the diffusion cap, as it changes the air gap [28]. It is recommended to use the diffusion cap during passive sampling, as it prevents the convective transport of chemical molecules.
- (2)
- The cross-sectional diffusion area (A) is 0.196 cm2. The retaining gaze has a pore size of 80 mesh and the wire is 0.21 mm in diameter, giving an effective area of 46% of the full area by calculation. Previous studies [15,18] assumed full cross-sectional diffusion area without considering the effective area on the retaining gauze. This assumption is questionable; however, it does not impact the determination of UReff, since UReff is not determined directly from the tube geometry, rather, by experiments or modelling as presented later.
- (3)
- The sorbent tube is packed with only one sorbent for the passive sampling purpose. The multisorbent configuration is not necessary, as the later sorbent layers do not contact compounds. Tenax TA is the most common sorbent due to its high thermal stability, low inherent artifacts and degradation, hydrophobicity and accommodation for wide volatility [29,30]. It also allows efficient desorption and displays optimal GC performance when used as a chromatographic stationary phase. An ATD tube is typically packed with 150–250 mg of Tenax TA, allowing both active and passive sampling.
2.3. Diffusion Coefficients
2.4. Modeling Short-Term Effective Uptake Rates
2.5. Modeling Long-Term Effective Uptake Rates
2.6. Determination of MDLs
3. Results
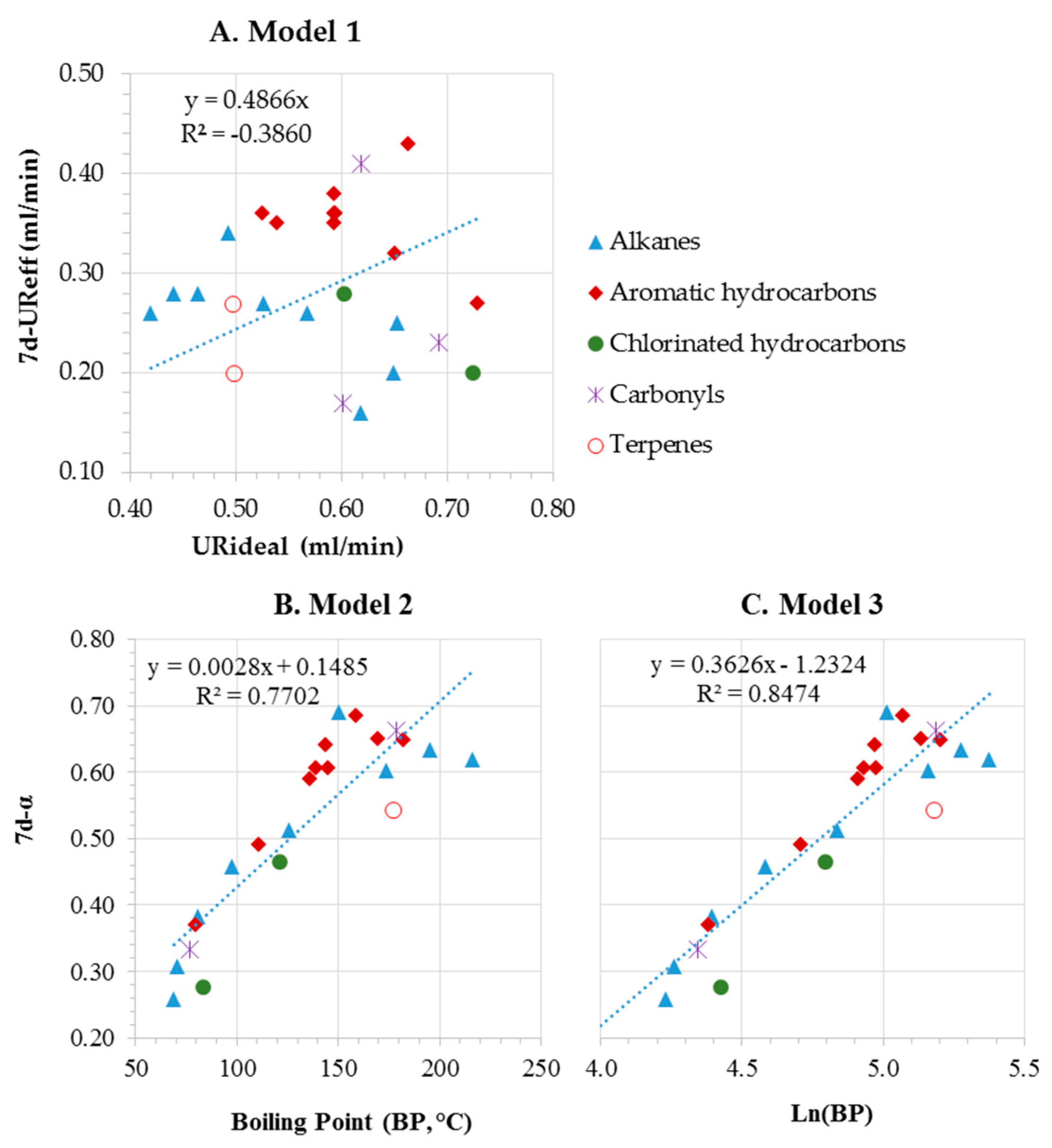
3.1. Effective Uptake Rates (UReff) for Short-Term Workplace Sampling
3.2. Effective Uptake Rates for Long-Term Environmental Sampling
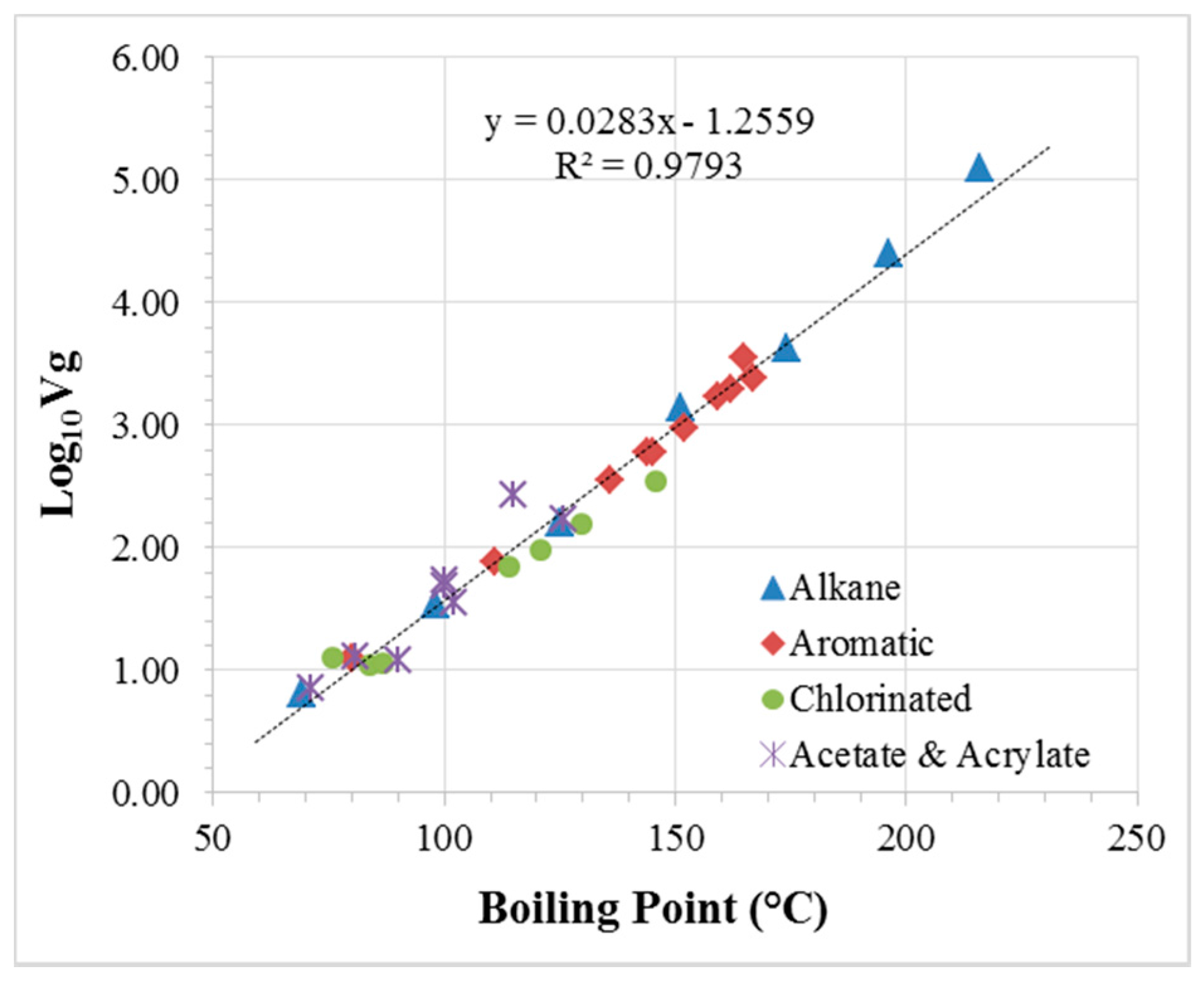
3.3. Compilation of Ideal and Effective URs
3.4. Analytical Performance and MDLs
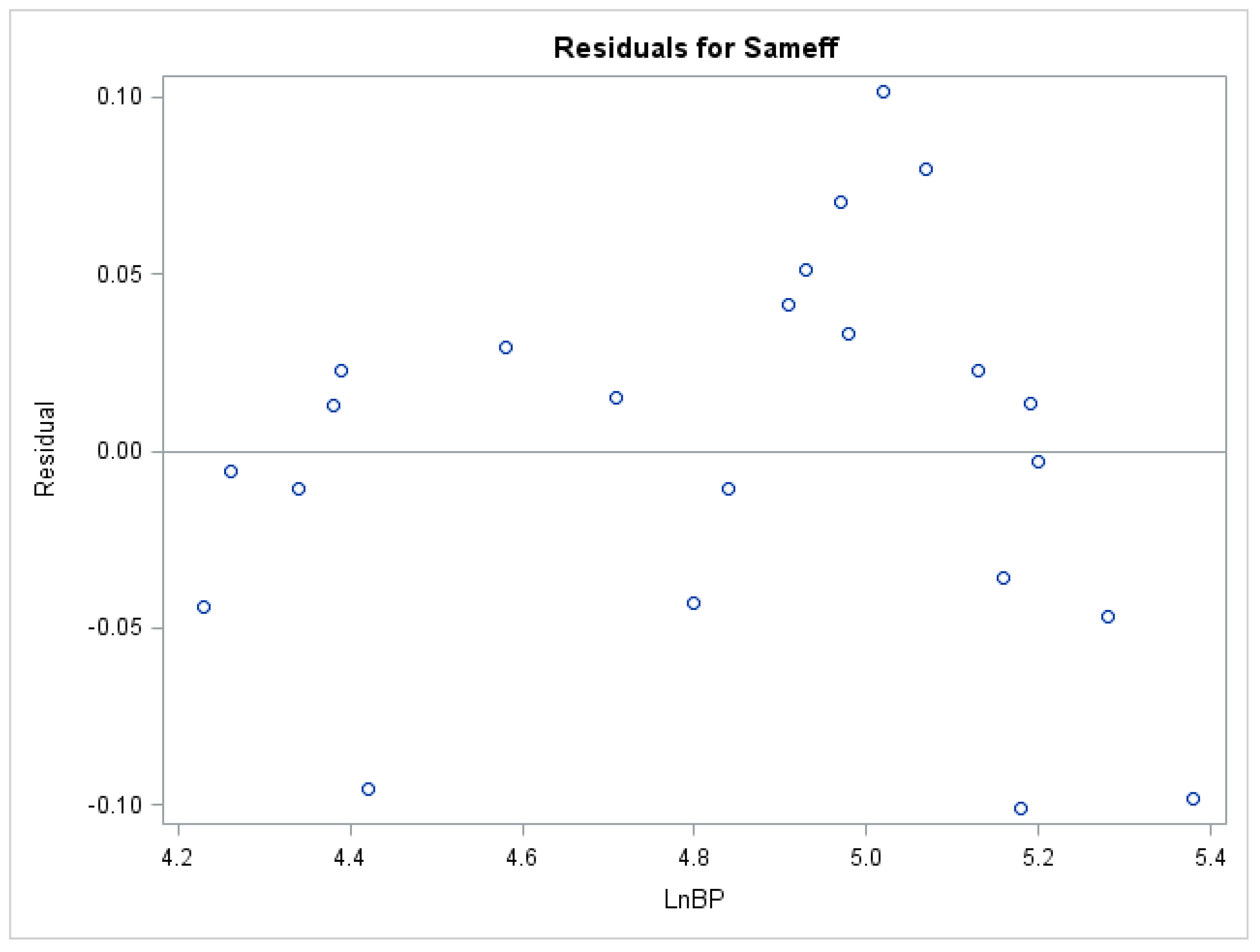
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| VOCs | CAS# | MW | BP 1 | D298 2 | URideal 3 | 8h-UR 4 | 8h-UR | Vg 5 | Log10Vg | α 6 | Pred. UR 7 | Bias 8 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g/mol) | (°C) | (cm2/sec) | (mL/min) | (ng/ppm·min) | (mL/min) | (L) | (mL/min) | (%) | ||||
| Alkanes | ||||||||||||
| n-Heptane | 142-82-5 | 100.2 | 98 | 0.0723 | 0.568 | 1.57 | 0.383 | 34 | 1.53 | 0.636 | 0.361 | −5.8 |
| n-Octane | 111-65-9 | 114.2 | 126 | 0.0671 | 0.527 | 1.67 | 0.357 | 160 | 2.20 | 0.739 | 0.390 | 9.0 |
| n-Nonane | 111-84-2 | 128.3 | 151 | 0.0628 | 0.493 | 1.75 | 0.334 | 1400 | 3.15 | 0.885 | 0.436 | 30.8 |
| n-Decane | 124-18-5 | 142.3 | 174 | 0.0592 | 0.465 | 1.96 | 0.337 | 4200 | 3.62 | 0.958 | 0.445 | 32.2 |
| n-Undecane | 1120-21-4 | 156.3 | 196 | 0.0562 | 0.441 | 1.97 | 0.308 | 25,000 | 4.40 | 1.000 | 0.441 | 43.2 |
| n-Dodecane | 112-40-3 | 170.3 | 216 | 0.0535 | 0.420 | 2.08 | 0.299 | 126,000 | 5.10 | 1.000 | 0.420 | 40.7 |
| n-Tridecane | 629-50-5 | 184.4 | 235 | 0.0512 | 0.402 | 2.33 | 0.309 | 5.40 | 1.000 | 0.402 | 30.1 | |
| n-Tetradecane | 629-59-4 | 198.4 | 253 | 0.0492 | 0.386 | 2.41 | 0.297 | 5.91 | 1.000 | 0.386 | 30.1 | |
| n-Pentadecane | 629-62-9 | 212.4 | 270 | 0.0473 | 0.371 | 2.19 | 0.252 | 6.39 | 1.000 | 0.371 | 47.4 | |
| n-Hexadecane | 544-76-3 | 226.5 | 287 | 0.0457 | 0.359 | 2.36 | 0.255 | 6.88 | 1.000 | 0.359 | 40.9 | |
| Cyclohexane | 110-82-7 | 86.2 | 81 | 0.0832 | 0.653 | 1.32 | 0.375 | 1.03 | 0.558 | 0.365 | −2.6 | |
| Methylcyclohexane | 108-87-2 | 98.2 | 101.1 | 0.0759 | 0.596 | 1.55 | 0.386 | 1.60 | 0.646 | 0.385 | −0.2 | |
| 2-Methylhexane | 591-76-4 | 100.2 | 90 | 0.0724 | 0.569 | 1.48 | 0.361 | 1.28 | 0.597 | 0.340 | −5.9 | |
| 3-Methylhexane | 589-34-4 | 100.2 | 91 | 0.0724 | 0.569 | 1.48 | 0.361 | 1.31 | 0.602 | 0.342 | −5.2 | |
| 2-Methylheptane | 592-28-8 | 114.2 | 116 | 0.0672 | 0.528 | 1.95 | 0.417 | 2.02 | 0.711 | 0.375 | −10.1 | |
| Aromatic compounds | ||||||||||||
| Benzene | 71-43-2 | 78.1 | 80.1 | 0.0928 | 0.729 | 1.3 | 0.407 | 12.5 | 1.10 | 0.569 | 0.415 | 1.9 |
| Toluene | 108-88-3 | 92.1 | 111 | 0.0829 | 0.651 | 1.67 | 0.443 | 76 | 1.88 | 0.690 | 0.449 | 1.3 |
| Ethylbenzene | 100-41-4 | 106.2 | 136 | 0.0756 | 0.594 | 2.00 | 0.461 | 360 | 2.56 | 0.794 | 0.471 | 2.3 |
| m-Xylene | 108-38-3 | 106.2 | 139 | 0.0756 | 0.594 | 1.82 | 0.419 | 600 | 2.78 | 0.828 | 0.492 | 17.3 |
| p-Xylene | 106-42-3 | 106.2 | 139 | 0.0756 | 0.594 | 1.82 | 0.419 | 600 | 2.78 | 0.828 | 0.492 | 17.3 |
| o-Xylene | 95-47-6 | 106.2 | 144 | 0.0756 | 0.594 | 1.82 | 0.419 | 600 | 2.78 | 0.828 | 0.492 | 17.3 |
| Styrene | 100-42-5 | 104.1 | 145 | 0.0757 | 0.595 | 2.40 | 0.563 | 600 | 2.78 | 0.828 | 0.492 | −12.6 |
| Isopropylbenzene | 98-82-8 | 120.2 | 153 | 0.0687 | 0.540 | 2.50 | 0.509 | 960 | 2.98 | 0.859 | 0.464 | −8.8 |
| Propylbenzene | 103-65-1 | 120.2 | 158 | 0.0669 | 0.525 | 2.37 | 0.482 | 1700 | 3.23 | 0.897 | 0.472 | −2.2 |
| 1,3,5-Trimethylbenzene | 108-67-8 | 120.2 | 165 | 0.0698 | 0.548 | 2.37 | 0.482 | 3600 | 3.56 | 0.948 | 0.520 | 7.8 |
| 1,2,4-Trimethylbenzene | 95-63-6 | 120.2 | 168 | 0.0686 | 0.539 | 2.37 | 0.482 | 3600 | 3.56 | 0.948 | 0.511 | 5.9 |
| o-Ethyltoluene | 611-14-3 | 120.2 | 165 | 0.0686 | 0.539 | 2.44 | 0.496 | 3.41 | 0.925 | 0.499 | 0.5 | |
| m-Ethyltoluene | 620-14-4 | 120.2 | 159 | 0.0687 | 0.540 | 2.25 | 0.458 | 3.24 | 0.899 | 0.485 | 6.0 | |
| p-Ethyltoluene | 622-96-8 | 120.2 | 161.7 | 0.0686 | 0.539 | 2.21 | 0.450 | 3.32 | 0.911 | 0.491 | 9.2 | |
| 1,3-Dimethyl-4-ethylbenzene | 874-41-9 | 134.2 | 185 | 0.0641 | 0.503 | 2.45 | 0.446 | 3.98 | 1.000 | 0.503 | 12.8 | |
| 1,4-Diethylbenzene | 105-05-5 | 134.2 | 184 | 0.0642 | 0.504 | 2.56 | 0.466 | 3.95 | 1.000 | 0.504 | 8.1 | |
| Naphthalene | 91-20-3 | 128.2 | 218 | 0.0691 | 0.543 | 2.14 | 0.408 | 4.92 | 1.000 | 0.543 | 32.9 | |
| Haloginated Hydrocarbons | ||||||||||||
| Tetrachloroethylene | 127-18-4 | 165.8 | 121 | 0.0767 | 0.602 | 2.80 | 0.413 | 96 | 1.98 | 0.705 | 0.425 | 2.9 |
| Benzyl chloride | 100-44-7 | 126.6 | 178.9 | 0.0728 | 0.572 | 2.72 | 0.525 | 3.81 | 0.986 | 0.564 | 7.3 | |
| Tetrachloroethene | 127-18-4 | 165.8 | 121.1 | 0.0767 | 0.602 | 2.80 | 0.413 | 2.17 | 0.734 | 0.442 | 7.0 | |
| Bromobenzene | 108-86-1 | 157.0 | 156 | 0.0789 | 0.620 | 3.31 | 0.515 | 3.16 | 0.886 | 0.549 | 6.5 | |
| Esters and Glycol Ethers | ||||||||||||
| Ethyl acetate | 141-78-6 | 88.1 | 77.1 | 0.0892 | 0.701 | 1.60 | 0.444 | 7 | 0.86 | 0.532 | 0.373 | −16.1 |
| n-Butyl acetate | 123-86-4 | 116.2 | 125.6 | 0.0738 | 0.580 | 2.26 | 0.476 | 2.29 | 0.753 | 0.436 | −8.3 | |
| Isobutyl acetate | 110-19-0 | 116.2 | 117.2 | 0.0739 | 0.580 | 1.91 | 0.402 | 265 | 2.42 | 0.773 | 0.449 | 11.6 |
| sec-Butyl acetate | 105-46-4 | 116.2 | 112.2 | 0.074 | 0.581 | 1.90 | 0.400 | 1.91 | 0.695 | 0.404 | 1.0 | |
| tert-Butyl acetate | 540-88-5 | 116.2 | 97.8 | 0.0742 | 0.583 | 1.79 | 0.377 | 1.50 | 0.632 | 0.368 | −2.3 | |
| Methyl acrylate | 96-33-3 | 86.1 | 80 | 0.0918 | 0.721 | 1.500 | 0.426 | 13 | 1.11 | 0.572 | 0.412 | −3.3 |
| Methyl methacrylate | 80-62-6 | 100.1 | 100 | 0.0825 | 0.648 | 1.77 | 0.432 | 55 | 1.74 | 0.668 | 0.433 | 0.1 |
| Butyl acrylate | 141-32-2 | 128.2 | 145 | 0.0697 | 0.547 | 2.60 | 0.496 | 2.84 | 0.838 | 0.459 | −7.5 | |
| Ethylhexyl acrylate | 1322-13-0 | 184.3 | 227.7 | 0.0552 | 0.434 | 2.99 | 0.397 | 5.19 | 1.000 | 0.434 | 9.3 | |
| Halothane | 151-67-7 | 197.4 | 50 | 0.0824 | 0.647 | 2.59 | 0.321 | 0.15 | 0.423 | 0.273 | −14.8 | |
| Enflurane | 13838-16-9 | 184.5 | 56 | 0.078 | 0.613 | 2.29 | 0.303 | 0.32 | 0.449 | 0.275 | −9.4 | |
| Isoflurane | 26675-46-7 | 184.5 | 49 | 0.0782 | 0.614 | 2.20 | 0.292 | 0.12 | 0.418 | 0.257 | −11.9 | |
| 2-Methoxyethyl acetane | 110-49-6 | 118.1 | 145 | 0.077 | 0.605 | 1.64 | 0.339 | 2.84 | 0.838 | 0.507 | 49.3 | |
| 2-Ethoxyethyl acetate | 111-15-9 | 132.2 | 156.1 | 0.0711 | 0.558 | 2.10 | 0.389 | 3.16 | 0.887 | 0.495 | 27.4 | |
| 2-Ethoxyethanol | 110-80-5 | 90.1 | 135 | 0.0859 | 0.675 | 1.80 | 0.488 | 2.56 | 0.794 | 0.536 | 9.7 | |
| 2-Propoxyethanol | 2807-30-9 | 104.1 | 153 | 0.0781 | 0.613 | 1.65 | 0.387 | 3.07 | 0.873 | 0.536 | 38.2 | |
| 2-Butoxyethanol | 117-76-2 | 118.2 | 171 | 0.0718 | 0.564 | 1.90 | 0.393 | 3.58 | 0.952 | 0.537 | 36.5 | |
| 1-Methoxyprppan-2-ol | 107-98-2 | 90.1 | 121 | 0.0862 | 0.677 | 1.56 | 0.423 | 2.16 | 0.733 | 0.496 | 17.3 | |
| 2-Methoxypropan-2-ol | 72360-66-8 | 90.1 | 94.8 | 0.0865 | 0.679 | 1.52 | 0.412 | 1.42 | 0.618 | 0.420 | 1.9 | |
| Ketones and Aldehydes | ||||||||||||
| Butan-2-one | 78-93-3 | 72.1 | 75.6 | 0.0943 | 0.741 | 1.34 | 0.454 | 0.87 | 0.534 | 0.396 | −12.9 | |
| Methyl isobutyl ketone | 108-10-1 | 100.2 | 118 | 0.0766 | 0.602 | 1.71 | 0.417 | 2.08 | 0.720 | 0.433 | 3.8 | |
| Cyclohexanone | 108-94-1 | 98.2 | 155.6 | 0.0786 | 0.617 | 2.30 | 0.573 | 340 | 2.53 | 0.790 | 0.488 | −14.9 |
| 2-Methylcyclohexanone | 583-60-8 | 112.2 | 162.8 | 0.0739 | 0.580 | 2.31 | 0.504 | 3.35 | 0.916 | 0.532 | 5.6 | |
| 3-Methylcyclohexanone | 591-24-2 | 112.2 | 170 | 0.0738 | 0.580 | 2.22 | 0.484 | 3.55 | 0.947 | 0.549 | 13.5 | |
| 4-Methylcyclohexanone | 589-92-4 | 112.2 | 171 | 0.0738 | 0.580 | 2.14 | 0.466 | 3.58 | 0.952 | 0.552 | 18.3 | |
| Furfural | 98-01-1 | 96.1 | 162 | 0.0889 | 0.698 | 2.5 | 0.636 | 600 | 2.78 | 0.828 | 0.578 | −9.1 |
| Hexanal | 66-25-1 | 100.2 | 131 | 0.0765 | 0.601 | 1.64 | 0.400 | 2.45 | 0.777 | 0.467 | 16.6 | |
| Decanal | 112-31-2 | 156.2 | 209 | 0.0584 | 0.459 | 2.32 | 0.363 | 4.66 | 1.000 | 0.459 | 26.3 | |
| Alcohols and Others | ||||||||||||
| Isobutanol | 78-83-1 | 74.1 | 108 | 0.0910 | 0.715 | 1.260 | 0.416 | 6 | 0.75 | 0.515 | 0.368 | −11.4 |
| Furfuryl alcohol | 98-00-0 | 98.1 | 170 | 0.0862 | 0.677 | 2.5 | 0.623 | 3.55 | 0.947 | 0.641 | 2.9 | |
| Tetrahydrofurfuryl alcohol | 97-99-4 | 102.1 | 178 | 0.0817 | 0.642 | 1.9 | 0.455 | 3.78 | 0.982 | 0.630 | 38.6 | |
| Allyl glycidyl ether | 106-92-3 | 114.2 | 154 | 0.0759 | 0.596 | 1.83 | 0.392 | 3.10 | 0.877 | 0.523 | 33.5 | |
| Butyl glycidyl ether | 2426-08-6 | 130.2 | 164 | 0.069 | 0.542 | 2.36 | 0.443 | 3.38 | 0.921 | 0.499 | 12.6 | |
| n-Methylpyrrolidone | 872-50-4 | 99.1 | 204 | 0.0808 | 0.635 | 1.83 | 0.451 | 4.52 | 1.000 | 0.635 | 40.6 | |
| n-Vinylpyrrolidone | 88-12-0 | 111.1 | 95 | 0.0771 | 0.606 | 2.51 | 0.552 | 1.42 | 0.619 | 0.375 | −32.1 | |
| α-Pinene | 80-56-8 | 136.2 | 156 | 0.0634 | 0.498 | 2.35 | 0.422 | 3.16 | 0.886 | 0.441 | 4.6 |
| VOCs | CAS# | BP 1 | D298 2 | URideal 3 | 7d-UReff 4 (Obs.) | 7d-α 5 (Obs.) | 7d-UReff 6 (Mod.) | Bias 7 |
|---|---|---|---|---|---|---|---|---|
| (°C) | (cm2/s) | (mL/min) | (mL/min) | (mL/min) | (%) | |||
| n-Hexane | 110-54-3 | 69 | 0.0788 | 0.619 | 0.16 | 0.259 | 0.190 | 18.7 |
| n-Heptane | 142-82-5 | 98 | 0.0723 | 0.568 | 0.26 | 0.458 | 0.246 | −5.5 |
| n-Octane | 111-65-9 | 126 | 0.0671 | 0.527 | 0.27 | 0.512 | 0.275 | 2.0 |
| n-Nonane | 111-84-2 | 150.8 | 0.0628 | 0.493 | 0.34 | 0.689 | 0.289 | −14.9 |
| n-Decane | 124-18-5 | 174.1 | 0.0592 | 0.465 | 0.28 | 0.602 | 0.297 | 6.0 |
| n-Undecane | 1120-21-4 | 195.5 | 0.0562 | 0.441 | 0.28 | 0.634 | 0.300 | 7.2 |
| n-Dodecane | 112-40-3 | 216.3 | 0.0535 | 0.420 | 0.26 | 0.619 | 0.301 | 15.7 |
| Methylcyclopentane | 96-37-7 | 71 | 0.0827 | 0.650 | 0.20 | 0.308 | 0.206 | 3.0 |
| Cyclohexane | 110-82-7 | 81 | 0.0832 | 0.653 | 0.25 | 0.383 | 0.238 | −4.8 |
| Benzene | 71-43-2 | 80.1 | 0.0928 | 0.729 | 0.27 | 0.370 | 0.263 | −2.8 |
| Toluene | 108-88-3 | 111 | 0.0829 | 0.651 | 0.32 | 0.491 | 0.311 | −2.9 |
| Ethylbenzene | 100-41-4 | 136 | 0.0756 | 0.594 | 0.35 | 0.589 | 0.327 | −6.7 |
| m & p-Xylene | 108-38-3 | 139 | 0.0756 | 0.594 | 0.36 | 0.606 | 0.331 | −8.0 |
| o-Xylene | 95-47-6 | 144 | 0.0756 | 0.594 | 0.38 | 0.640 | 0.339 | −10.9 |
| Propylbenzene | 103-65-1 | 159.2 | 0.0669 | 0.525 | 0.36 | 0.685 | 0.319 | −11.5 |
| 1,2,4-Trimethylbenzene | 95-63-6 | 169.5 | 0.0686 | 0.539 | 0.35 | 0.650 | 0.339 | −3.2 |
| Styrene | 100-42-5 | 145 | 0.0757 | 0.595 | 0.36 | 0.606 | 0.341 | −5.4 |
| Phenol | 108-95-2 | 182 | 0.0844 | 0.663 | 0.43 | 0.649 | 0.434 | 0.9 |
| Benzaldehyde | 100-52-7 | 179 | 0.0788 | 0.619 | 0.41 | 0.662 | 0.401 | −2.1 |
| Ethyl acetate | 141-78-6 | 77 | 0.0881 | 0.692 | 0.23 | 0.332 | 0.239 | 4.1 |
| Hexanal | 66-25-1 | 130.5 | 0.0765 | 0.601 | 0.17 | 0.283 | n.a. | n.a. |
| 1,2-Dichloroethane | 107-06-2 | 83.5 | 0.0922 | 0.724 | 0.20 | 0.276 | 0.272 | 35.8 |
| Tetrachloroethylene | 127-18-4 | 121 | 0.0767 | 0.602 | 0.28 | 0.465 | 0.306 | 9.3 |
| α-Pinene | 80-56-8 | 155 | 0.0634 | 0.498 | 0.20 | 0.402 | n.a. | n.a. |
| d-Limonene | 5989-27-5 | 177 | 0.0632 | 0.496 | 0.27 | 0.544 | 0.320 | 18.5 |
References
- Bolden, A.L.; Kwiatkowski, C.F.; Colborn, T. New look at BTEX: Are ambient levels a problem? Environ. Sci. Technol. 2015, 49, 5261–5276. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.S.; Zhang, J.F.J.; Sigsgaard, T.; Jantunen, M.; Lioy, P.J.; Samson, R.; Karol, M.H. Current state of the science: Health effects and indoor environmental quality. Environ. Health Perspect. 2007, 115, 958–964. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Sampling Strategies for Volatile Organic Compounds (VOCs) in Indoor Air (Report EUR 16051 EN); European Commission: Brussels, Belgium; Luxembourg, 1995. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA). Technical Assistance Document for the National Air Toxics Trends Stations Program, Revision 3; USEPA: Research Triangle Park, NC, USA, 2016.
- Weisel, C.P.; Zhang, J.F.; Turpin, B.J.; Morandi, M.T.; Colome, S.; Stock, T.H.; Spektor, D.M.; Korn, L.; Winer, A.; Alimokhtari, S.; et al. Relationship of Indoor, Outdoor and Personal Air (RIOPA) Study: Study design, methods and quality assurance/control results. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; D’Souza, J.; Batterman, S. Distributions of personal VOC exposures: A population-based analysis. Environ. Int. 2008, 34, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Batterman, S.; Godwin, C. VOCs in industrial, urban and suburban neighborhoods, Part 1: Indoor and outdoor concentrations, variation, and risk drivers. Atmos. Environ. 2008, 42, 2083–2100. [Google Scholar] [CrossRef]
- Gorecki, T.; Namiesnik, J. Passive sampling. TrAC-Trends Anal. Chem. 2002, 21, 276–291. [Google Scholar] [CrossRef]
- Krol, S.; Zabiegala, B.; Namiesnik, J. Monitoring VOCs in atmospheric air II. Sample collection and preparation. TrAC-Trends Anal. Chem. 2010, 29, 1101–1112. [Google Scholar] [CrossRef]
- Jia, C.; Batterman, S.; Godwin, C. Continuous, intermittent and passive sampling of airborne VOCs. J. Environ. Monit. 2007, 9, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (USEPA). Passive Samplers for Investigations of Air Quality: Method Description, Implementation, and Comparison to Alternative Sampling Methods (EPA/600/R-14/434); USEPA: Cincinnati, OH, USA, 2014.
- Batterman, S.; Metts, T.; Kalliokoski, P. Diffusive uptake in passive and active adsorbent sampling using thermal desorption tubes. J. Environ. Monit. 2002, 4, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.A.; Marlow, D.J.; Henderson, M.H.; Goody, B.A.; Quincey, P.G. Studies using the sorbent Carbopack X for measuring environmental benzene with Perkin-Elmer-type pumped and diffusive samplers. Atmos. Environ. 2003, 37, 871–879. [Google Scholar] [CrossRef]
- Simpson, A.T.; Wright, M.D. Diffusive sampling of C(7)-C(16) hydrocarbons in workplace air: Uptake rates, wall effects and use in oil mist measurements. Ann. Occup. Hyg. 2008, 52, 249–257. [Google Scholar] [PubMed]
- Brown, V.M.; Crump, D.R.; Gardiner, D.; Yu, C.W.F. Long-term diffusive sampling of volatile organic-compounds in indoor air. Environ. Technol. 1993, 14, 771–777. [Google Scholar] [CrossRef]
- McClenny, W.A.; Oliver, K.D.; Jacumin, H.H.; Daughtrey, E.H.; Whitaker, D.A. 24 h diffusive sampling of toxic VOCs in air onto Carbopack X solid adsorbent followed by thermal desorption/GC/MS analysis—Laboratory studies. J. Environ. Monit. 2005, 7, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Hellen, H.; Hakola, H.; Laurila, T.; Hiltunen, V.; Koskentalo, T. Aromatic hydrocarbon and methyl tert-butyl, ether measurements in ambient air of Helsinki (Finland) using diffusive samplers. Sci. Total Environ. 2002, 298, 55–64. [Google Scholar] [CrossRef]
- Walgraeve, C.; Demeestere, K.; Dewulf, J.; Van Huffel, K.; Van Langenhove, H. Diffusive sampling of 25 volatile organic compounds in indoor air: Uptake rate determination and application in Flemish homes for the elderly. Atmos. Environ. 2011, 45, 5828–5836. [Google Scholar] [CrossRef]
- Brown, R.H. Monitoring the ambient environment with diffusive samplers: Theory and practical considerations. J. Environ. Monit. 2000, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Markes International. Application Note 3: National and International Standard Methods Relating to Speciated Monitoring of Vapour-Phase Organic Compounds in Air; Markes International Ltd.: Llantrisant, UK, 2012. [Google Scholar]
- Seethapathy, S.; Górecki, T.; Li, X. Passive sampling in environmental analysis. J. Chromatogr. A 2008, 1184, 234–253. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO). ISO16017-2: Indoor, Ambient and Workplace Air -Sampling and Analysis of Volatile Organic Compounds by Sorbent Tube/Thermal Desorption/Capillary Gas Chromatography—Part 2: Diffusive Sampling; ISO: Geneva, Switzerland, 2003. [Google Scholar]
- ASTM International. Standard Practice for Choosing Sorbents, Sampling Parameters and Thermal Desorption Analytical Conditions for Monitoring Volatile Organic Chemicals in Air; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA). EPA Method 325B, Volatile Organic Compounds from Fugitive and Area Sources: Sampler Preparation and Analysis; USEPA: Washington, DC, USA, 2015.
- U.S. Environmental Protection Agency (USEPA). Compendium Method TO-17, Determination of Volatile Organic Compounds in Ambient Air Using Active Sampling onto Sorbent Tubes; USEPA: Cincinnati, OH, USA, 1999.
- Brown, R.H. The use of diffusive samplers for monitoring of ambient air. Pure Appl. Chem. 1993, 65, 1859–1874. [Google Scholar] [CrossRef]
- Oury, B.; Lhuillier, F.; Protois, J.C.; Morele, Y. Behavior of the GABIE, 3M 3500, PerkinElmer Tenax TA, and RADIELLO 145 diffusive samplers exposed over a long time to a low concentration of VOCs. J. Occup. Environ. Hyg. 2006, 3, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Hewitt, C.N. Evaluation of Tenax-GR adsorbent for the passive sampling of volatile organic-compounds at low concentrations. Atmos. Environ. Part A 1993, 27, 1865–1872. [Google Scholar] [CrossRef]
- Camel, V.; Caude, M. Trace enrichment methods for the determination of organic pollutants in ambient air. J. Chromatogr. A 1995, 710, 3–19. [Google Scholar] [CrossRef]
- Dettmer, K.; Engewald, W. Adsorbent materials commonly used in air analysis for adsorptive enrichment and thermal desorption of volatile organic compounds. Anal. Bioanal. Chem. 2002, 373, 490–500. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (USEPA). EPA On-Line Tools for Site Assessment Calculation; USEPA: Washington, DC, USA, 2016.
- Lyman, W.J. Handbook of Chemical Property Estimation Methods: Environmental Behavior of Organic Compounds, Chapter 17; Reehl, W.F., Rosenblatt, D.H., Eds.; McGraw-Hill: New York, NY, USA, 1982. [Google Scholar]
- Wright, M.D. Diffusive Uptake Rates for the Perkin Elmer Tube—BCR Air Sampling Intercomparison At Vito. Mol, Belgium Feb 1991—April 1992; HSE Internal Report 1993 IR/L/IA/93/3; Health and Safety Laboratory: Buxton, UK, 1993.
- Markes International. Application Note 002: Prediction of Uptake Rates for Diffusive Tubes; Markes International Ltd.: Llantrisant, UK, 2012. [Google Scholar]
- Brown, R.H.; Purnell, C.J. Collection and analysis of trace organic vapor pollutants in ambient atmospheres—Performance of a Tenax-GC adsorbent tube. J. Chromatogr. 1979, 178, 79–90. [Google Scholar] [CrossRef]
- Markes International. Application Note 001: Uptake Rates for Tube-Type Axial Diffusive Samplers; Markes International Ltd.: Llantrisant, UK, 2015. [Google Scholar]
- McAlary, T.; Groenevelt, H.; Disher, S.; Arnold, J.; Seethapathy, S.; Sacco, P.; Crump, D.; Schumacher, B.; Hayes, H.; Johnson, P.; et al. Passive sampling for volatile organic compounds in indoor air-controlled laboratory comparison of four sampler types. Environ. Sci. Process. Impacts 2015, 17, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Xian, Q.M.; Feng, Y.L.; Chan, C.C.; Zhu, J.P. Use of reference chemicals to determine passive uptake rates of common indoor air VOCs by collocation deployment of active and passive samplers. J. Environ. Monit. 2011, 13, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Feigley, C.E.; Lee, B.M. Determination of sampling rates of passive samplers for organic vapors based on estimated diffusion-coefficients. Am. Ind. Hyg. Assoc. J. 1988, 49, 266–269. [Google Scholar] [CrossRef]
- Walgraeve, C.; Demeestere, K.; Dewulf, J.; Van Huffel, K.; Van Langenhove, H. Uptake rate behavior of tube-type passive samplers for volatile organic compounds under controlled atmospheric conditions. Atmos. Environ. 2011, 45, 5872–5879. [Google Scholar] [CrossRef]
- Kilic, N.; Ballantine, J.A. Comparison of various adsorbents for long-term diffusive sampling of volatile organic compounds. Analyst 1998, 123, 1795–1797. [Google Scholar] [CrossRef]
- Calogirou, A.; Larsen, B.R.; Brussol, C.; Duane, M.; Kotzias, D. Decomposition of terpenes by ozone during sampling on Tenax. Anal. Chem. 1996, 68, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Schrader, W.; Geiger, J.; Klockow, D.; Korte, E.H. Degradation of alpha-pinene on Tenax during sample storage: Effects of daylight radiation and temperature. Environ. Sci. Technol. 2001, 35, 2717–2720. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Batterman, S.; Chernyak, S. Development and comparison of methods using MS scan and selective ion monitoring modes for a wide range of airborne VOCs. J. Environ. Monit. 2006, 8, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.M.; Crump, D.R.; Gardiner, D. Measurement of volatile organic-compounds in indoor air by a passive technique. Environ. Technol. 1992, 13, 367–375. [Google Scholar] [CrossRef]
- Chemexper.com. Available online: http://www.chemexper.com/ (accessed on 28 October 2017).
- Adley, D.P.; Underhill, D.W. Fundamental factors in the performance of diffusive samplers. Anal. Chem. 1989, 61, 843–847. [Google Scholar] [CrossRef]
- Underhill, D.W. Efficiency of Passive Sampling by Adsorbents. Am. Ind. Hyg. Assoc. J. 1984, 45, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Batterman, S.; Metts, T.; Kalliokoski, P.; Barnett, E. Low-flow active and passive sampling of VOCs using thermal desorption tubes: Theory and application at an offset printing facility. J. Environ. Monit. 2002, 4, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Nordstrand, E.; Kristensson, J. A computer-program for simulating the performance of thick bed diffusive samplers. Am. Ind. Hyg. Assoc. J. 1994, 55, 935–941. [Google Scholar] [CrossRef]
- Vandenhoed, N.; Vanasselen, O.L.J. A computer-model for calculating effective uptake rates of tube-type diffusive air samplers. Ann. Occup. Hyg. 1991, 35, 273–285. [Google Scholar]
- Hafkenscheid, T.L.; Mowrer, J. Intercomparison of tube-type diffusive sampling for the determination of volatile hydrocarbons in ambient air. Analyst 1996, 121, 1249–1252. [Google Scholar] [CrossRef]
- Civan, M.Y.; Tuncel, G. Evaluation of adsorbents with passive sampling and their analytical methods to determine volatile organic compounds emitted from vehicular exhaust. Energy Educ. Sci. Technol. Part A 2012, 29, 563–576. [Google Scholar]
- Paciência, I.; Madureira, J.; Rufo, J.; Fernandes, E.; Moreira, A.; Teixeira, J.P. Trends of Volatile Organic Compounds in different indoor microenvironments: A review. In Occupational Safety and Hygiene IV; CRC Press: Boca Raton, FL, USA, 2016; pp. 7–10. [Google Scholar]
- Strum, M.; Scheffe, R. National review of ambient air toxics observations. J. Air Waste Manag. Assoc. 2016, 66, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Tolnai, B.; Gelencser, A.; Hlavay, J. Theoretical approach to non-constant uptake rates for tube-type diffusive samplers. Talanta 2001, 54, 703–713. [Google Scholar] [CrossRef]

| Instrument | Parameter | Settings |
|---|---|---|
| Thermal Desorption (TD) | Dry purge flow rate | 20 mL/min |
| Dry purge time | 1 min | |
| Desorption temperature | 260 °C | |
| Desorption flow rate | 50 mL/min | |
| Desorption time | 5 min | |
| Split ratio | 6.3:1 | |
| Trap low | 40 °C | |
| Heating rate | 40 °C/s | |
| Trap high | 280 °C | |
| Trap hold | 3 min | |
| Gas Chromatography (GC) | Injector | Splitless |
| Column | HP-5MSUI, 30 m × 250 µm × 0.25 µm | |
| Flow rate | 1.2 mL/min | |
| Temperature program | 35 °C hold for 3 min; 15 °C/min to 95 °C, hold for 2 min; | |
| 15 °C/min to 140 °C, no hold; 35 °C/min to 220 °C, no hold; | ||
| 40 °C/min to 300 °C, hold for 4 min | ||
| Total run time | 20.29 min | |
| Mass Spectrometry (MS) | Mass mode and range | Scan mode, solvent delay 1.5 min |
| 1.5–20.29 min: 35–350 amu, 4.5 scans/s, 0.1 m/z step size | ||
| MS quad temperature | 150 °C | |
| MS source temperature | 230 °C |
| VOCs | CAS # | MW 1 | BP 2 | Vg 3 | D 4 | URideal 5 | 8h-UReff 6 | 7d-UReff 7 |
|---|---|---|---|---|---|---|---|---|
| (g/mol) | (°C) | (L) | (cm2/s) | (mL/min) | (mL/min) | (mL/min) | ||
| Acrylonitrile | 107-13-1 | 53.1 | 77 | 0.1120 | 0.88 | 0.48 | 0.30 | |
| trans-1,2-Dichloroethylene | 156-60-5 | 96.9 | 48 | 0.0958 | 0.75 | 0.31 | 0.13 | |
| Methyl tert-butyl ether | 1634-04-4 | 88.1 | 55 | 0.0803 | 0.63 | 0.28 | 0.14 | |
| 1,1-Dichloroethane | 75-34-3 | 99.0 | 57 | 0.0927 | 0.73 | 0.33 | 0.17 | |
| Propionitrile | 107-12-0 | 55.1 | 97 | 0.1080 | 0.85 | 0.54 | 0.36 | |
| Methacrylonitrile | 126-98-7 | 67.1 | 91 | 0.0967 | 0.76 | 0.46 | 0.31 | |
| cis-1,2-Dichloroethylene | 156-59-2 | 96.9 | 60 | 0.0956 | 0.75 | 0.35 | 0.19 | |
| Methyl acrylate | 96-33-3 | 86.1 | 80 | 13 | 0.0918 | 0.72 | 0.43 | 0.26 |
| 2,2-Dichloropropane | 594-20-7 | 113.0 | 96 | 0.0805 | 0.63 | 0.40 | 0.27 | |
| Chloroform | 67-66-3 | 119.4 | 61 | 0.0917 | 0.72 | 0.34 | 0.19 | |
| Tetrahydrofuran | 109-99-9 | 72.1 | 66 | 0.0978 | 0.77 | 0.38 | 0.22 | |
| 1,1,1-Trichloroethane | 71-55-6 | 133.4 | 75 | 0.0826 | 0.65 | 0.35 | 0.22 | |
| 1,2-Dichloroethane | 107-06-2 | 99.0 | 83 | 11 | 0.0923 | 0.73 | 0.41 | 0.20 |
| 1,1-Dichloropropene | 563-58-6 | 111.0 | 78 | 0.0854 | 0.67 | 0.37 | 0.23 | |
| Benzene | 71-43-2 | 78.1 | 80 | 13 | 0.0928 | 0.73 | 0.41 | 0.27 |
| Carbon tetrachloride | 56-23-5 | 153.8 | 76 | 12 | 0.0809 | 0.64 | 0.59 | 0.22 |
| 1,2-Dichloropropane | 78-87-5 | 113.0 | 95 | 0.0830 | 0.65 | 0.41 | 0.27 | |
| Trichloroethylene | 79-01-6 | 131.4 | 87 | 11 | 0.0846 | 0.67 | 0.37 | 0.28 |
| Dibromomethane | 74-95-3 | 173.8 | 97 | 0.0953 | 0.75 | 0.47 | 0.32 | |
| Bromodichloromethane | 75-27-4 | 163.8 | 87 | 0.0880 | 0.69 | 0.41 | 0.27 | |
| 2,5-Dimethylfuran | 625-86-5 | 96.0 | 93 | 0.0781 | 0.62 | 0.38 | 0.25 | |
| Methyl methacrylate | 80-62-6 | 100.1 | 100 | 55 | 0.0825 | 0.65 | 0.43 | 0.28 |
| trans-1,3-Dichloropropene (E) | 10061-02-6 | 111.0 | 108 | 0.0850 | 0.67 | 0.45 | 0.31 | |
| cis-1,3-Dichloropropene(z) | 10061-01-5 | 111.0 | 108 | 0.0850 | 0.67 | 0.45 | 0.31 | |
| Toluene | 108-88-3 | 92.1 | 111 | 76 | 0.0829 | 0.65 | 0.44 | 0.32 |
| 1,1,2-Trichloroethane | 79-00-5 | 133.4 | 113 | 0.0821 | 0.65 | 0.45 | 0.31 | |
| 1,3-Dichloropropane | 142-28-9 | 113.0 | 121 | 0.0826 | 0.65 | 0.48 | 0.33 | |
| Ethyl methacrylate | 97-63-2 | 114.1 | 118 | 0.0744 | 0.59 | 0.42 | 0.29 | |
| Dibromochloromethane | 124-48-1 | 208.3 | 118 | 0.0850 | 0.67 | 0.48 | 0.33 | |
| n-Octane | 111-65-9 | 114.2 | 126 | 160 | 0.0671 | 0.53 | 0.36 | 0.27 |
| 1,2-Dibromoethane | 106-93-4 | 187.9 | 131 | 0.0480 | 0.38 | 0.29 | 0.20 | |
| Tetrachloroethene | 127-18-4 | 165.8 | 121 | 0.0767 | 0.60 | 0.43 | 0.28 | |
| Chlorobenzene | 108-90-7 | 112.6 | 131 | 52 | 0.0796 | 0.63 | 0.42 | 0.34 |
| 1,1,1,2-Tetrachloroethane | 630-20-6 | 167.8 | 131 | 156 | 0.0749 | 0.59 | 0.44 | 0.32 |
| Ethylbenzene | 100-41-4 | 106.2 | 136 | 360 | 0.0756 | 0.60 | 0.46 | 0.35 |
| m-Xylene | 108-38-3 | 106.2 | 144 | 600 | 0.0756 | 0.60 | 0.42 | 0.36 |
| p-Xylene | 106-42-3 | 106.2 | 144 | 600 | 0.0756 | 0.60 | 0.42 | 0.36 |
| Bromoform | 75-25-2 | 252.7 | 150 | 0.0826 | 0.65 | 0.56 | 0.38 | |
| Styrene | 100-42-5 | 104.1 | 145 | 600 | 0.0757 | 0.60 | 0.56 | 0.36 |
| o-Xylene | 95-47-6 | 106.2 | 144 | 600 | 0.0756 | 0.60 | 0.42 | 0.34 |
| n-Nonane | 111-84-2 | 128.3 | 151 | 1400 | 0.0628 | 0.49 | 0.40 | 0.34 |
| 1,1,2,2-Tetrachloroethane | 79-34-5 | 167.8 | 147 | 340 | 0.0747 | 0.59 | 0.46 | 0.34 |
| 1,2,3-Trichloropropane | 96-18-4 | 147.4 | 154 | 0.0749 | 0.59 | 0.52 | 0.35 | |
| Cumene | 98-82-8 | 120.2 | 153 | 0.0687 | 0.54 | 0.46 | 0.32 | |
| trans-1,4-Dichloro-2-butene | 110-57-6 | 125.0 | 126 | 0.0752 | 0.59 | 0.45 | 0.31 | |
| Bromobenzene | 108-86-1 | 157.0 | 156 | 0.0789 | 0.62 | 0.52 | 0.37 | |
| α-Pinene | 7785-70-8 | 136.2 | 155 | 0.0602 | 0.47 | 0.42 | 0.28 | |
| 2-Chlorotoluene | 95-49-8 | 126.6 | 158 | 0.0730 | 0.58 | 0.51 | 0.35 | |
| n-Propylbenzene | 103-65-1 | 120.2 | 158 | 0.0669 | 0.53 | 0.48 | 0.36 | |
| 4-chlorotoluene | 106-43-4 | 126.6 | 162 | 0.0730 | 0.58 | 0.52 | 0.35 | |
| 1,3,5-Trimethylbenzene | 108-67-8 | 120.2 | 165 | 3600 | 0.0698 | 0.55 | 0.48 | 0.34 |
| Pentachloroethane | 76-01-7 | 202.3 | 162 | 0.0692 | 0.55 | 0.50 | 0.33 | |
| Phenol | 108-95-2 | 94.1 | 182 | 480 | 0.0844 | 0.66 | 0.54 | 0.43 |
| tert-Butylbenzene | 98-06-6 | 134.2 | 169 | 0.0643 | 0.51 | 0.48 | 0.32 | |
| 1,2,4-Trimethylbenzene | 95-63-6 | 120.2 | 168 | 3600 | 0.0686 | 0.54 | 0.44 | 0.35 |
| n-Decane | 124-18-5 | 142.3 | 174 | 4200 | 0.0592 | 0.47 | 0.40 | 0.28 |
| 1,3-Dichlorobenzene | 541-73-1 | 147.0 | 173 | 0.0723 | 0.57 | 0.55 | 0.36 | |
| sec-Butylbenzene | 135-98-8 | 134.2 | 174 | 0.0643 | 0.51 | 0.49 | 0.32 | |
| 1,4-Dichlorobenzene | 106-46-7 | 147.0 | 173 | 0.0723 | 0.57 | 0.54 | 0.36 | |
| p-Isopropyltoluene | 99-87-6 | 134.2 | 177 | 0.0642 | 0.51 | 0.49 | 0.33 | |
| d-Limonene | 5989-27-5 | 136.2 | 176 | 0.0632 | 0.50 | 0.48 | 0.27 | |
| 1,2-Dichlorobenzene | 95-50-1 | 147.0 | 180 | 0.0722 | 0.57 | 0.56 | 0.37 | |
| n-Butylbenzene | 104-51-8 | 134.2 | 183 | 0.0642 | 0.51 | 0.51 | 0.33 | |
| Hexachloroethane | 67-72-1 | 236.7 | 187 | 0.0647 | 0.51 | 0.52 | 0.34 | |
| 1,2-Dibromo-3-chloropropane | 96-12-8 | 236.3 | 196 | 0.0709 | 0.56 | 0.59 | 0.38 | |
| Nitrobenzene | 98-95-3 | 123.1 | 210 | 28,000 | 0.0771 | 0.61 | 0.66 | 0.43 |
| n-Undecane | 1120-21-4 | 156.3 | 196 | 25,000 | 0.0562 | 0.44 | 0.31 | 0.28 |
| 1,2,4-Trichlorobenzene | 120-82-1 | 181.4 | 214 | 0.0668 | 0.53 | 0.60 | 0.38 | |
| Naphthalene | 91-20-3 | 128.2 | 218 | 0.0691 | 0.54 | 0.49 | 0.39 | |
| n-Dodecane | 112-40-3 | 170.3 | 216 | 126,000 | 0.0535 | 0.42 | 0.30 | 0.26 |
| 1,2,3-Trichlorobenzene | 87-61-6 | 181.4 | 218 | 0.0668 | 0.53 | 0.61 | 0.38 | |
| Hexachlorobutadiene | 87-68-3 | 260.8 | 215 | 0.0595 | 0.47 | 0.54 | 0.34 | |
| n-Tridecane | 629-50-5 | 184.0 | 234 | 0.0512 | 0.40 | 0.31 | 0.30 | |
| n-Tetradecane | 629-59-4 | 198.0 | 254 | 0.0506 | 0.40 | 0.30 | 0.31 | |
| n-Pentadecane | 629-62-9 | 212.0 | 271 | 0.0473 | 0.37 | 0.25 | 0.30 |
| VOCs | RT 1 | Precision 2 | R2 | RSD 3 | MDL 4 | MDL_8h 5 | MDL_7d 6 |
|---|---|---|---|---|---|---|---|
| (min) | (%) | (%) | (ng) | (µg/m3) | (µg/m3) | ||
| Acrylonitrile | 1.907 | 8.6 | 0.9992 | 18.9 | 0.13 | 0.56 | 0.042 |
| trans-1,2-Dichloroethylene | 2.074 | 9.3 | 0.9991 | 11.7 | 0.03 | 0.21 | 0.024 |
| Methyl tert-butyl ether | 2.100 | 5.4 | 0.9999 | 11.5 | 0.05 | 0.37 | 0.035 |
| 1,1-Dichloroethane | 2.157 | 5.6 | 0.9990 | 8.0 | 0.03 | 0.22 | 0.020 |
| Propionitrile | 2.168 | 8.7 | 0.9988 | 9.3 | 0.03 | 0.12 | 0.008 |
| Methacrylonitrile | 2.314 | 7.9 | 0.9994 | 7.8 | 0.13 | 0.60 | 0.043 |
| cis-1,2-Dichloroethylene | 2.370 | 8.2 | 0.9993 | 9.0 | 0.03 | 0.18 | 0.016 |
| Methyl acrylate | 2.430 | 7.9 | 0.9994 | 7.9 | 0.03 | 0.14 | 0.011 |
| 2,2-Dichloropropane | 2.430 | 3.7 | 0.9999 | 7.6 | 0.06 | 0.32 | 0.023 |
| Chloroform | 2.456 | 8.1 | 0.9993 | 7.4 | 0.04 | 0.22 | 0.019 |
| Tetrahydrofuran | 2.558 | 10.3 | 0.9988 | 97.0 | 0.02 | 0.12 | 0.010 |
| 1,1,1-Trichloroethane | 2.749 | 2.9 | 0.9998 | 17.3 | 0.04 | 0.26 | 0.020 |
| 1,2-Dichloroethane | 2.786 | 5.6 | 0.9991 | 7.4 | 0.04 | 0.20 | 0.020 |
| 1,1-Dichloropropene | 2.873 | 5.9 | 0.9999 | 6.4 | 0.04 | 0.22 | 0.016 |
| Benzene | 2.940 | 5.8 | 0.9998 | 22.2 | 0.03 | 0.16 | 0.012 |
| Carbon tetrachloride | 2.951 | 1.8 | 1.0000 | 4.3 | 0.04 | 0.14 | 0.018 |
| 1,2-Dichloropropane | 3.480 | 8.4 | 0.9976 | 18.3 | 0.06 | 0.32 | 0.023 |
| Trichloroethylene | 3.491 | 6.2 | 0.9986 | 8.0 | 0.03 | 0.15 | 0.010 |
| Dibromomethane | 3.517 | 5.5 | 0.9991 | 6.8 | 0.03 | 0.13 | 0.009 |
| Bromodichloromethane | 3.600 | 4.7 | 0.9990 | 8.1 | 0.03 | 0.13 | 0.009 |
| 2,5-Dimethylfuran | 3.630 | 5.4 | 0.9995 | 5.8 | 0.06 | 0.33 | 0.024 |
| Methyl methacrylate | 3.716 | 9.4 | 0.9988 | 9.3 | 0.08 | 0.37 | 0.027 |
| trans-1,3-Dichloropropene (E) | 4.170 | 7.5 | 0.9995 | 7.5 | 0.05 | 0.23 | 0.016 |
| cis-1,3-Dichloropropene (z) | 4.601 | 7.0 | 0.9996 | 6.4 | 0.04 | 0.18 | 0.013 |
| Toluene | 4.612 | 5.6 | 0.9996 | 13.1 | 0.06 | 0.26 | 0.017 |
| 1,1,2-Trichloroethane | 4.694 | 6.2 | 0.9999 | 7.2 | 0.04 | 0.18 | 0.012 |
| 1,3-Dichloropropane | 4.927 | 6.5 | 1.0000 | 6.9 | 0.03 | 0.14 | 0.010 |
| Ethyl methacrylate | 4.976 | 5.2 | 0.9999 | 10.2 | 0.06 | 0.28 | 0.020 |
| Dibromochloromethane | 5.077 | 5.3 | 1.0000 | 7.8 | 0.02 | 0.09 | 0.006 |
| n-Octane | 5.167 | 7.1 | 1.0000 | 18.7 | 0.07 | 0.43 | 0.027 |
| 1,2-Dibromoethane | 5.260 | 6.3 | 1.0000 | 7.0 | 0.05 | 0.33 | 0.023 |
| Tetrachloroethene | 5.324 | 4.3 | 0.9998 | 7.2 | 0.02 | 0.10 | 0.007 |
| Chlorobenzene | 5.916 | 2.7 | 0.9999 | 7.2 | 0.02 | 0.11 | 0.006 |
| 1,1,1,2-Tetrachloroethane | 5.980 | 2.1 | 1.0000 | 6.8 | 0.03 | 0.15 | 0.010 |
| Ethylbenzene | 6.160 | 7.7 | 0.9993 | 8.2 | 0.03 | 0.16 | 0.010 |
| m-Xylene | 6.284 | 4.9 | 0.9978 | 10.5 | 0.07 | 0.35 | 0.019 |
| p-Xylene | 6.284 | 4.9 | 0.9978 | 10.5 | 0.07 | 0.35 | 0.019 |
| Bromoform | 6.505 | 5.9 | 0.9999 | 13.0 | 0.05 | 0.19 | 0.013 |
| Styrene | 6.599 | 6.9 | 1.0000 | 10.0 | 0.05 | 0.17 | 0.013 |
| o-Xylene | 6.632 | 7.6 | 0.9996 | 9.4 | 0.05 | 0.23 | 0.013 |
| n-Nonane | 6.722 | 11.6 | 0.9991 | 17.7 | 0.04 | 0.22 | 0.012 |
| 1,1,2,2-Tetrachloroethane | 6.910 | 9.0 | 1.0000 | 10.1 | 0.04 | 0.16 | 0.011 |
| 1,2,3-Trichloropropane | 7.011 | 7.7 | 1.0000 | 8.4 | 0.05 | 0.20 | 0.014 |
| Cumene | 7.086 | 8.7 | 0.9996 | 7.5 | 0.03 | 0.14 | 0.009 |
| trans-1,4-Dichloro-2-butene | 7.131 | 10.6 | 0.9999 | 16.7 | 0.05 | 0.23 | 0.016 |
| Bromobenzene | 7.176 | 9.2 | 0.9999 | 6.4 | 0.06 | 0.25 | 0.016 |
| α-Pinene | 7.232 | 10.6 | 0.9998 | 8.1 | 0.04 | 0.20 | 0.014 |
| 2-Chlorotoluene | 7.483 | 8.5 | 0.9999 | 7.0 | 0.06 | 0.26 | 0.018 |
| n-Propylbenzene | 7.517 | 10.1 | 0.9995 | 9.5 | 0.06 | 0.26 | 0.017 |
| 4-chlorotoluene | 7.570 | 9.0 | 0.9999 | 7.7 | 0.05 | 0.21 | 0.015 |
| 1,3,5-Trimethylbenzene | 7.746 | 9.0 | 1.0000 | 7.4 | 0.04 | 0.19 | 0.013 |
| Pentachloroethane | 7.869 | 6.7 | 0.9998 | 13.7 | 0.05 | 0.20 | 0.014 |
| Phenol | 7.899 | 26.6 | 0.9999 | 30.9 | 0.16 | 0.60 | 0.036 |
| tert-Butylbenzene | 8.158 | 2.5 | 0.9996 | 6.6 | 0.06 | 0.25 | 0.018 |
| 1,2,4-Trimethylbenzene | 8.169 | 9.4 | 0.9995 | 7.2 | 0.06 | 0.31 | 0.018 |
| n-Decane | 8.244 | 17.7 | 0.9998 | 49.6 | 0.07 | 0.34 | 0.023 |
| 1,3-Dichlorobenzene | 8.387 | 5.7 | 0.9998 | 8.7 | 0.04 | 0.15 | 0.011 |
| sec-Butylbenzene | 8.484 | 6.5 | 0.9984 | 10.3 | 0.04 | 0.18 | 0.013 |
| 1,4-Dichlorobenzene | 8.507 | 6.4 | 0.9999 | 6.9 | 0.05 | 0.19 | 0.013 |
| p-Isopropyltoluene | 8.747 | 4.5 | 0.9998 | 9.9 | 0.04 | 0.16 | 0.012 |
| d-Limonene | 8.833 | 9.3 | 0.9995 | 14.5 | 0.12 | 0.53 | 0.046 |
| 1,2-Dichlorobenzene | 8.964 | 4.1 | 0.9999 | 7.7 | 0.04 | 0.13 | 0.010 |
| n-Butylbenzene | 9.384 | 6.6 | 0.9994 | 10.3 | 0.04 | 0.16 | 0.012 |
| Hexachloroethane | 9.733 | 14.5 | 0.9997 | 13.9 | 0.04 | 0.15 | 0.011 |
| 1,2-Dibromo-3-chloropropane | 9.894 | 6.5 | 0.9999 | 19.5 | 0.05 | 0.16 | 0.012 |
| Nitrobenzene | 9.976 | 13.0 | 0.9997 | 29.9 | 0.13 | 0.42 | 0.031 |
| n-Undecane | 10.145 | 9.2 | 0.9993 | 11.1 | 0.05 | 0.34 | 0.018 |
| 1,2,4-Trichlorobenzene | 11.431 | 7.8 | 0.9997 | 8.7 | 0.06 | 0.20 | 0.015 |
| Naphthalene | 11.532 | 9.5 | 0.9975 | 12.9 | 0.08 | 0.35 | 0.021 |
| n-Dodecane | 11.674 | 14.7 | 0.9974 | 13.0 | 0.05 | 0.32 | 0.017 |
| 1,2,3-Trichlorobenzene | 11.933 | 7.8 | 1.0000 | 7.5 | 0.08 | 0.27 | 0.020 |
| Hexachlorobutadiene | 11.978 | 8.0 | 1.0000 | 7.8 | 0.06 | 0.24 | 0.018 |
| n-Tridecane | 12.799 | 12.3 | 0.9978 | 13.6 | 0.06 | 0.41 | 0.020 |
| n-Tetradecane | 13.549 | 15.6 | 0.9960 | 25.5 | 0.10 | 0.67 | 0.031 |
| n-Pentadecane | 14.118 | 19.8 | 0.9944 | 26.2 | 0.10 | 0.85 | 0.034 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, C.; Fu, X. Diffusive Uptake Rates of Volatile Organic Compounds on Standard ATD Tubes for Environmental and Workplace Applications. Environments 2017, 4, 87. https://doi.org/10.3390/environments4040087
Jia C, Fu X. Diffusive Uptake Rates of Volatile Organic Compounds on Standard ATD Tubes for Environmental and Workplace Applications. Environments. 2017; 4(4):87. https://doi.org/10.3390/environments4040087
Chicago/Turabian StyleJia, Chunrong, and Xianqiang Fu. 2017. "Diffusive Uptake Rates of Volatile Organic Compounds on Standard ATD Tubes for Environmental and Workplace Applications" Environments 4, no. 4: 87. https://doi.org/10.3390/environments4040087






