Effects of Pretreatments on Yields, Selectivity and Properties of Products from Pyrolysis of Phragmites australis (Common Reeds)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Biomass Samples
2.2. Biomass Pretreatment Steps
2.3. Analytical Fast Pyrolysis
3. Results and Discussion
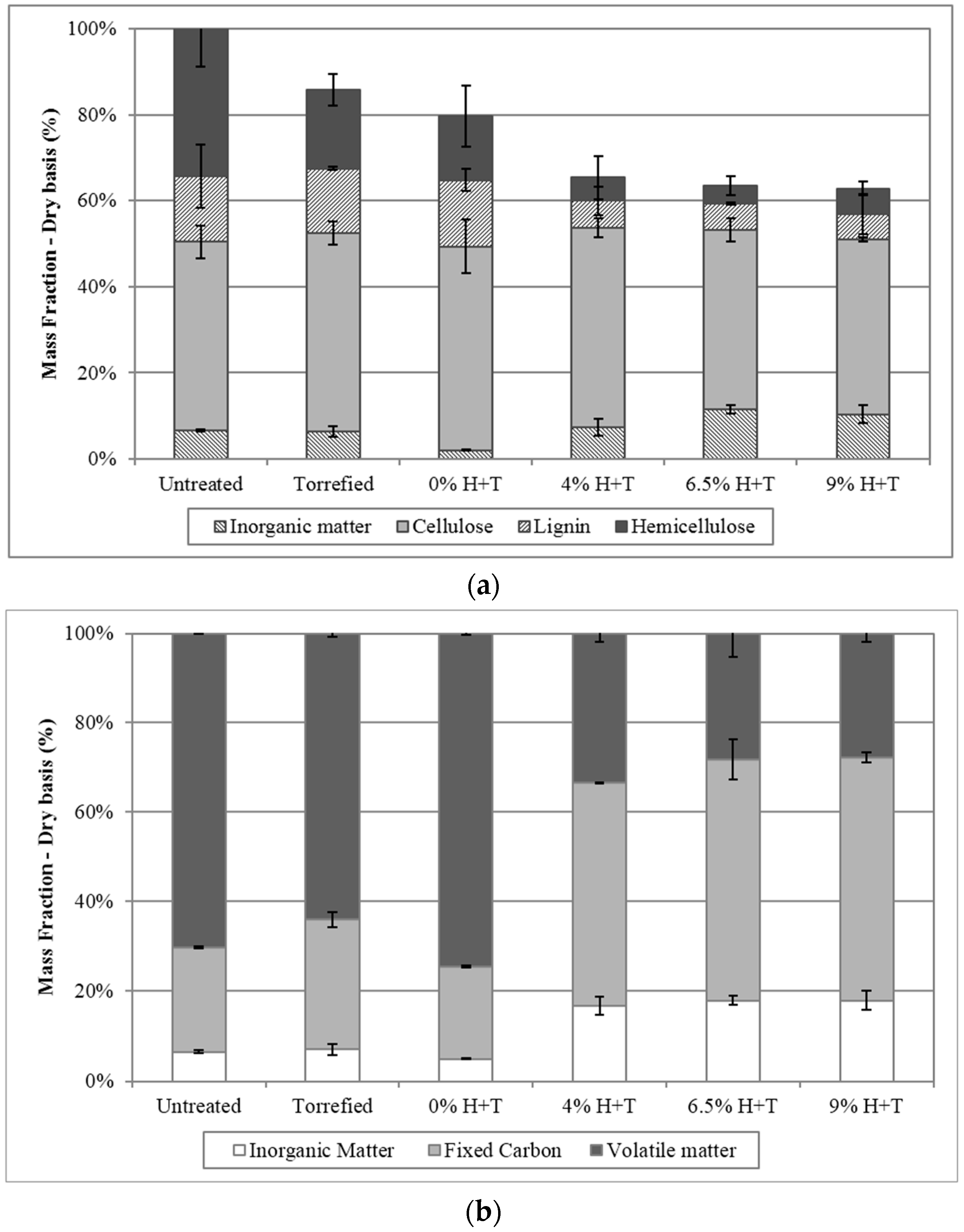
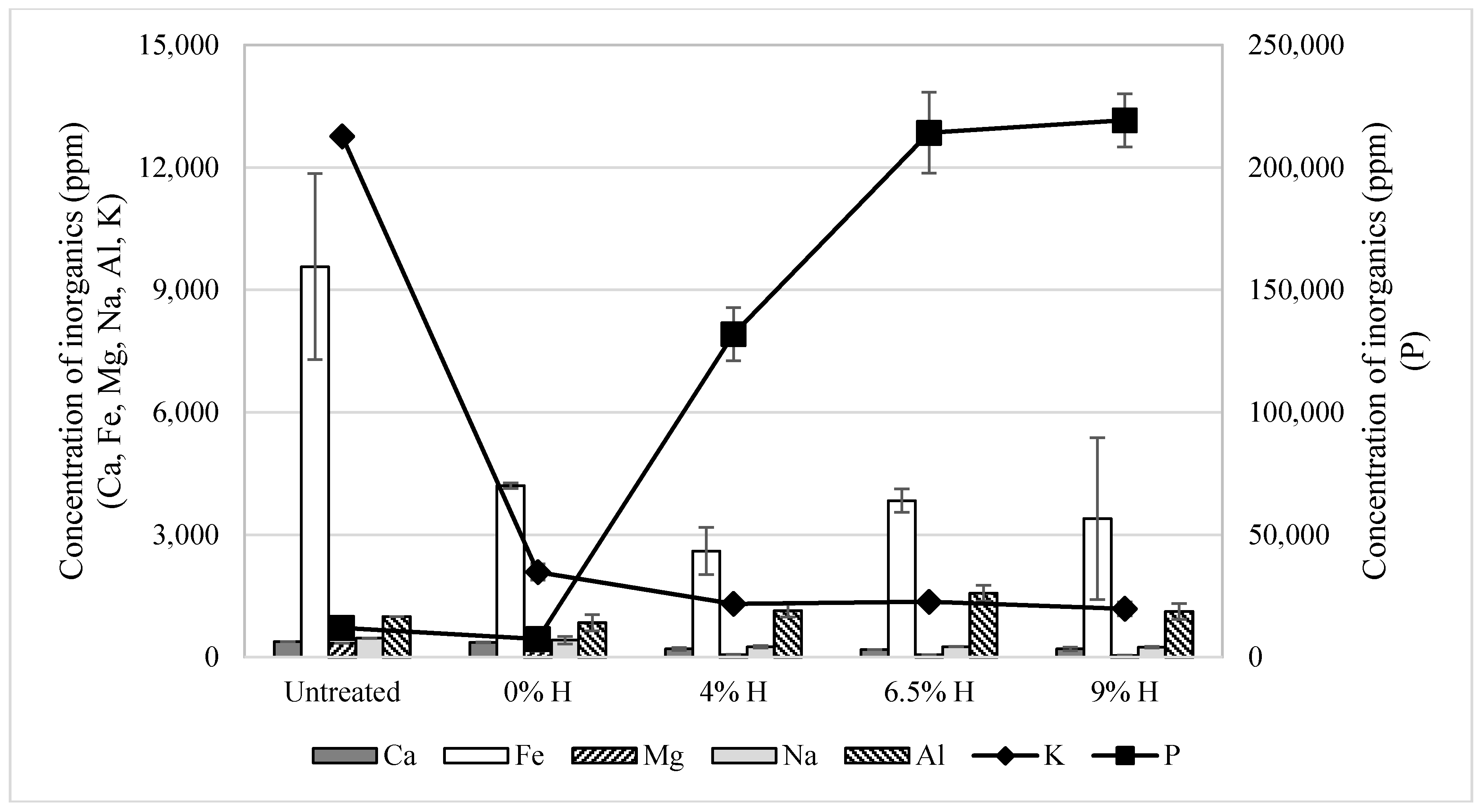
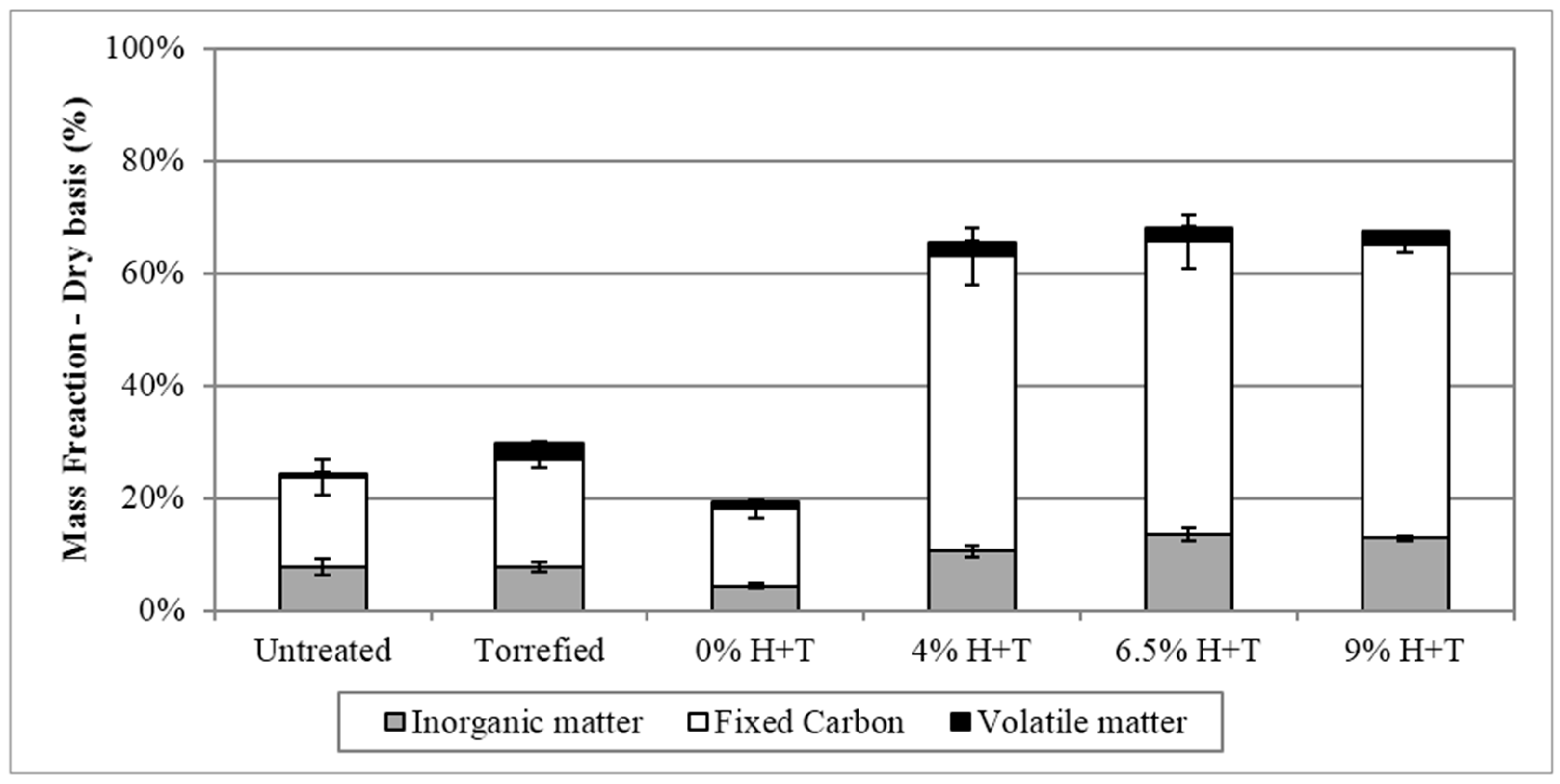
3.1. Pretreatment Effects on Physicochemical Properties of Phragmites
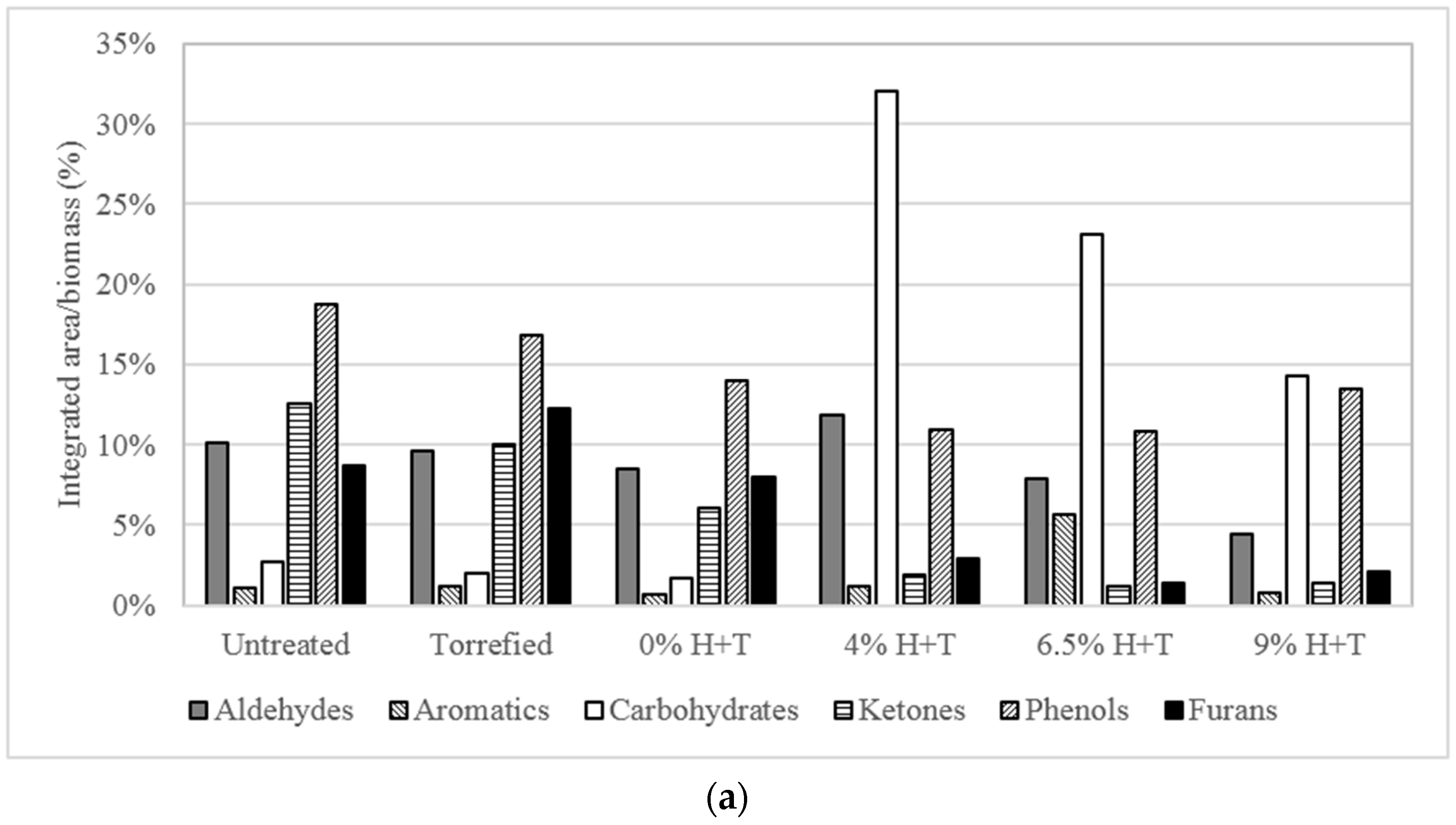
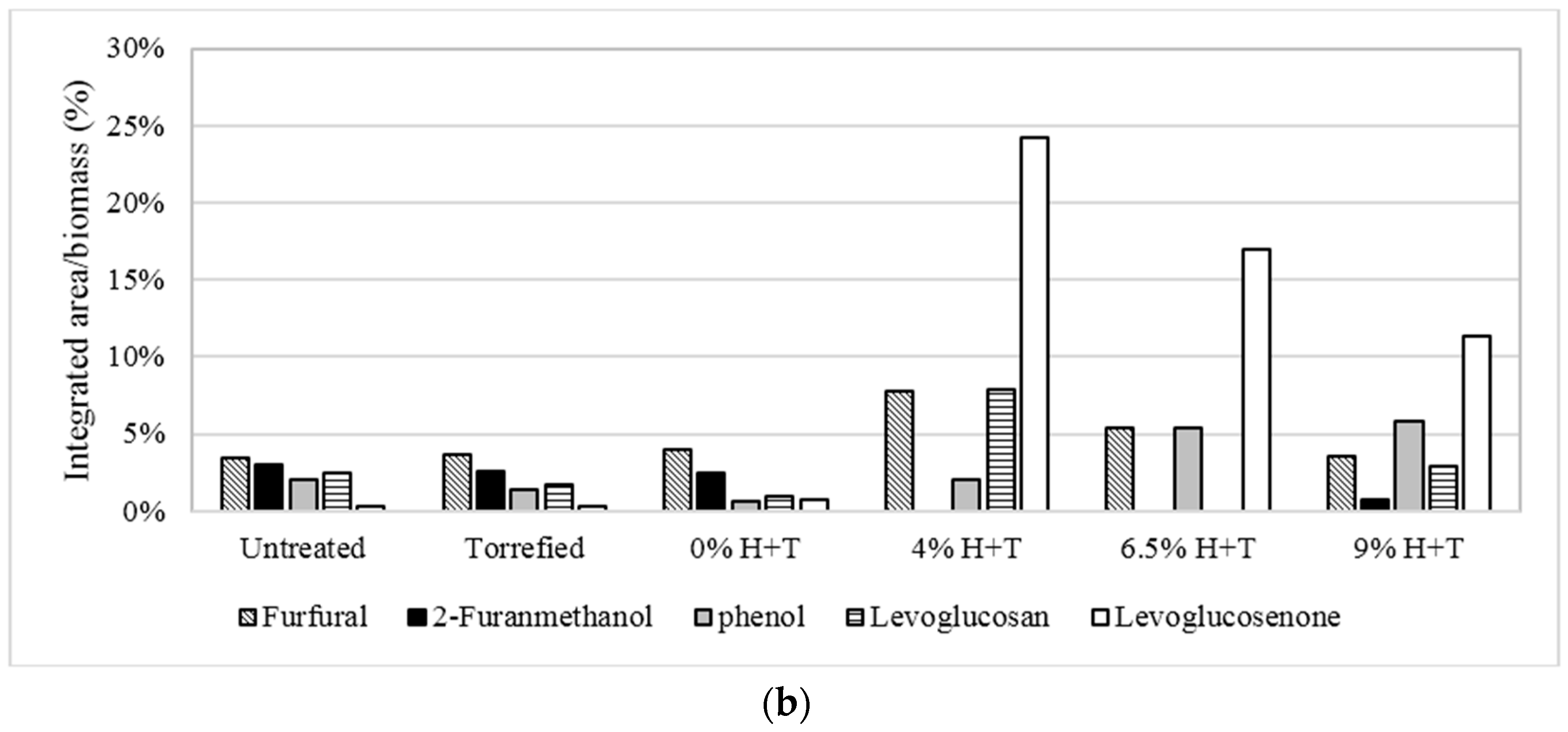
3.2. Fast Pyrolysis of Treated and Untreated Phragmites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Category | Compound | Retention Time(RT) [min] |
|---|---|---|
| aldehyde | Butanedial | 4.156 |
| aldehyde | Furfural | 5.573 |
| furans | 2-furanmethanol | 6.414 |
| aromatic | Styrene | 7.379 |
| ketone | 2(5H)-Furanone | 8.528 |
| ketone | 2-hydroxy-2-cyclopenten-1-one | 9.011 |
| aldehyde | 5-methyl-2-furancarboxaldehyde | 10.694 |
| phenols | phenol | 11.731 |
| ketone | 3-methyl-1,2-cyclopentendione | 13.609 |
| furans | 5,6-dihydro-4-methoxy-2H-Furan | 14.368 |
| phenols | 4-methylphenol | 16.082 |
| phenols | 2-methoxyphenol | 16.514 |
| ketone | 3-ethyl-2-hydroxy-2-cyclopenten-1-one | 17.94 |
| carbohydrate | Levoglucosenone | 18.689 |
| phenols | 4-ethylphenol | 20.362 |
| phenols | 2-methoxy-4-methylphenol | 21.378 |
| phenols | 1,2-Benzenediol | 22.015 |
| furans | 2,3-dihydrobenzofuran | 22.785 |
| phenols | 3-methoxy-1,2-benzenediol | 24.437 |
| phenols | 4-ethyl-2-methoxyphenol | 25.309 |
| aromatic | Indole | 25.864 |
| phenols | 2-methoxy-4-vinylphenol | 26.839 |
| phenols | 2,6-dimethoxyphenol | 28.512 |
| phenols | 2-methoxy-4-(1-propenyl)phenol | 32.535 |
| carbohydrate | Levoglucosan | 33.941 |
| aldehyde | 4-methyl-2,5-dimethoxy-benzoaldehyde | 36.374 |
| phenols | 2,6-methoxy-4-(2-propenyl)phenol | 39.381 |
References
- Szijártó, N.; Kádár, Z.; Varga, E.; Thomsen, A.B.; Costa-Ferreira, M.; Réczey, K. Pretreatment of reed by wet oxidation and subsequent utilization of the pretreated fibers for ethanol production. Appl. Biochem. Biotechnol. 2009, 155, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Swearingen, J.; Saltonstall, K. Distinguishing Native and Exotic Forms of Common Reed (Phragmites australis) in the United States; Natural Resources Conservation Service: Boise, ID, USA, 2010.
- Sathitsuksanoh, N.; Zhu, Z.; Templeton, N.; Rollin, J.A.; Harvey, S.P.; Zhang, Y.-H.P. Saccharification of a potential bioenergy crop, Phragmites australis (Common Reed), by lignocellulose fractionation followed by enzymatic hydrolysis at decreased cellulase loadings. Ind. Eng. Chem. Res. 2009, 48, 6441–6447. [Google Scholar] [CrossRef]
- Vasquez, E.A.; Glenn, E.P.; Brown, J.J.; Guntenspergen, G.R.; Nelson, S.G. Salt tolerance underlies the cryptic invasion of North American salt marshes by an introduced haplotype of the common reed Phragmites australis (Poaceae). Mar. Ecol. Prog. Ser. 2005, 298, 1–8. [Google Scholar] [CrossRef]
- Del Río, J.; Gutiérrez, A.; Rodríguez, I.; Ibarra, D.; Martínez, Á.T. Composition of non-woody plant lignins and cinnamic acids by Py-GC/MS, Py/TMAH and FT-IR. J. Anal. Appl. Pyrolysis 2007, 79, 39–46. [Google Scholar] [CrossRef]
- Chambers, R.M.; Osgood, D.T.; Bart, D.J.; Montalto, F. Phragmites australis invasion and expansion in tidal wetlands: Interactions among salinity, sulfide, and hydrology. Estuaries 2003, 26, 398–406. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, H.; Zhang, C.; Sun, B.; Zhang, Y.; Dong, S.; Xue, Y.; Qin, S. Thermogravimetry study of pyrolytic characteristics and kinetics of the giant wetland plant Phragmites australis. J. Therm. Anal. Calorim. 2012, 110, 611–617. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, H.; Zhang, C.; Liu, X.; Xue, Y.; Qiao, Y.; Tian, Y.; Qin, S. Pyrolytic characteristics and kinetics of Phragmites australis. Evid. Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Wichtmann, W.; Couwenberg, J. Reed as a Renewable Resource and Other Aspects of Paludiculture. In Proceedings of the International Conference on the Utilization of Emergent Wetland Plants, Greifswald Germany, 14–16 February 2013; Volume 13, pp. 1–2. [Google Scholar]
- Wartha, C.; Rixrath, D.; Krail, J. Life cycle assessment of energy conversion from reed. In Proceedings of the International Conference on the Utilization of Emergent Wetland Plants, Greifswald Germany, 14–16 February 2013. [Google Scholar]
- Patuzzi, F.; Mimmo, T.; Cesco, S.; Gasparella, A.; Baratieri, M. Common reeds (Phragmites australis) as sustainable energy source: Experimental and modelling analysis of torrefaction and pyrolysis processes. GCB Bioenergy 2013, 5, 367–374. [Google Scholar] [CrossRef]
- Allirand, J.-M.; Gosse, G. An above-ground biomass production model for a common reed (Phragmites communis Trin.) stand. Biomass Bioenergy 1995, 9, 441–448. [Google Scholar] [CrossRef]
- Komulainen, M.; Simi, P.; Hagelberg, E.; Ikonen, I.; Lyytinen, S. Reed Energy: Possibilities of Using the Common Reed for Energy Generation in Southern Finland; Turku University of Applied Sciences: Turku, Finland, 2008. [Google Scholar]
- Kresovich, S.; Wagner, C.K.; Scantland, D.A.; Groet, S.S.; Lawhon, W.T. The Utilization of Emergent Aquatic Plants for Biomass Energy Systems Development; Solar Energy Research Institute: Golden, CO, USA, 1982. [Google Scholar]
- Kresovich, S.; Wagner, C.K.; Scantland, D.A.; Lawhon, W.T. Emergent aquatic plants: Biological and economic perspectives. Biomass 1981, 1, 127–144. [Google Scholar] [CrossRef]
- Brix, H.; Ye, S.; Laws, E.A.; Sun, D.; Li, G.; Ding, X.; Yuan, H.; Zhao, G.; Wang, J.; Pei, S. Large-scale management of common reed, Phragmites australis, for paper production: A case study from the Liaohe Delta. China Ecol. Eng. 2014, 73, 760–769. [Google Scholar] [CrossRef]
- Granéli, W. Reed Phragmites australis (Cav.) Trin. ex Steudel as an energy source in Sweden. Biomass 1984, 4, 183–208. [Google Scholar] [CrossRef]
- Bjork, S.; Graneli, W. Energy reeds and the environment. Ambio 1978, 7, 150–156. [Google Scholar]
- Monti, A.; Di Virgilio, N.; Venturi, G. Mineral composition and ash content of six major energy crops. Biomass Bioenergy 2008, 32, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Klass, D.L. Biomass as a Non-Fossil Fuel Source; Klass, D.L., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1988; Volume 33, ISBN 0-8412-0599-X. [Google Scholar]
- Maschio, G.; Koufopanos, C.; Lucchesi, A. Pyrolysis, a promising route for biomass utilization. Bioresour. Technol. 1992, 42, 219–231. [Google Scholar] [CrossRef]
- Meier, D.; Faix, O. State of the art of applied fast pyrolysis of lignocellulosic materials—A review. Bioresour. Technol. 1999, 68, 71–77. [Google Scholar] [CrossRef]
- Brown, R.C.; Brown, T.R. Biorenewable Resources Engineering New Products from Agriculture; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; ISBN 9781118524954. [Google Scholar]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzyme Res. 2011, 2011, 787532. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Garrido, R.A.; Ruiz-Felix, M.N.; Satrio, J.A. Effects of hydrolysis and torrefaction on pyrolysis product distribution of spent mushroom compost (SMC). Int. J. Environ. Pollut. Remediat. 2012, 1, 98–103. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 2, 472–499. [Google Scholar]
- Hamelinck, C.N.; Hooijdonk, G.; Van Faaij, A.P. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Rollin, J.A.; Zhu, Z.; Sathitsuksanoh, N.; Zhang, Y.H.P. Increasing cellulose accessibility is more important than removing lignin: A comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia. Biotechnol. Bioeng. 2011, 108, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Dobele, G.; Rossinskaja, G.; Dizhbite, T.; Telysheva, G.; Meier, D.; Faix, O. Application of catalysts for obtaining 1,6-anhydrosaccharides from cellulose and wood by fast pyrolysis. J. Anal. Appl. Pyrolysis 2005, 74, 401–405. [Google Scholar] [CrossRef]
- Nowakowski, D.J.; Woodbridge, C.R.; Jones, J.M. Phosphorus catalysis in the pyrolysis behaviour of biomass. J. Anal. Appl. Pyrolysis 2008, 83, 197–204. [Google Scholar] [CrossRef]
- Sui, X.-W.; Wang, Z.; Liao, B.; Zhang, Y.; Guo, Q.-X. Preparation of levoglucosenone through sulfuric acid promoted pyrolysis of bagasse at low temperature. Bioresour. Technol. 2012, 103, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.A. An Improved Preparation of Levoglucosenone from Cellulose. Master’s Thesis, Iowa State University, Ames, IN, USA, January 2008. [Google Scholar]
- Pach, M.; Zanzi, R.; Björnbom, E. Torrefied biomass a substitue for wood and charcoal. In Proceedings of the 6th Asian-Pacific International Symposium on Combustion and Energy Utilization, Kuala Lumpur, Malaysia, 20–22 May 2002; p. 6. [Google Scholar]
- Hamaguchi, M.; Cardoso, M.; Vakkilainen, E. Alternative technologies for biofuels production in kraft pulp mills-potential and prospects. Energies 2012, 5, 2288–2309. [Google Scholar] [CrossRef]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubiera, F.; Pis, J.J. Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, V.; Adhikari, S.; Chattanathan, S.A.; Park, S. Catalytic pyrolysis of torrefied biomass for hydrocarbons production. Energy Fuels 2012, 26, 7347–7353. [Google Scholar] [CrossRef]
- Meng, J.; Park, J.; Tilotta, D.; Park, S. The effect of torrefaction on the chemistry of fast-pyrolysis bio-oil. Bioresour. Technol. 2012, 111, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Lei, H.; Wang, L.; Bu, Q.; Chen, S.; Wu, J.; Julson, J.; Ruan, R. The effects of torrefaction on compositions of bio-oil and syngas from biomass pyrolysis by microwave heating. Bioresour. Technol. 2013, 135, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Reckamp, J.M.; Garrido, R.A.; Satrio, J.A. Selective pyrolysis of paper mill sludge by using pretreatment processes to enhance the quality of bio-oil and biochar products. Biomass Bioenergy 2014, 71, 235–244. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses; United States Department of Agriculture (USDA): Washington, DC, USA, 1970.
- Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, A.; Sluiter, J.; Templeton, D. Preparation of Samples for Compositional Analysis; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Wise, L.E.; Maxine, M.; D’Addieco, A.A. Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Pap. Trade J. 1946, 122, 35–43. [Google Scholar]
- Van Soest, P.J. Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar]
- Haykiri-Acma, H.; Yaman, S.; Alkan, M.; Kucukbayrak, S. Mineralogical characterization of chemically isolated ingredients from biomass. Energy Convers. Manag. 2014, 77, 221–226. [Google Scholar] [CrossRef]
- Gani, A.; Naruse, I. Effect of cellulose and lignin content on pyrolysis and combustion characteristics for several types of biomass. Renew. Energy 2007, 32, 649–661. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.V.V.; Donnison, I.; Yates, N.; Jones, J.M.M. The effect of lignin and inorganic species in biomass on pyrolysis oil yields, quality and stability. Fuel 2008, 87, 1230–1240. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Product distribution from fast pyrolysis of glucose-based carbohydrates. J. Anal. Appl. Pyrolysis 2009, 86, 323–330. [Google Scholar] [CrossRef]
- Piskorz, J.; Radlein, D.; Majerski, P.; Scott, D.S. Pyrolysis of Cellulose—From Oligosaccharides to Synthesis Gas. In Fast Pyrolysis of Biomass: A Handbook; CPL Press: Thatcham, UK, 2002. [Google Scholar]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Rondon, M.A.; Lehmann, J.; Ramirez, J.; Hurtado, M.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Ahmed Hared, I.; Dirion, J.L.; Salvador, S.; Lacroix, M.; Rio, S. Pyrolysis of wood impregnated with phosphoric acid for the production of activated carbon: Kinetics and porosity development studies. J. Anal. Appl. Pyrolysis 2007, 79, 101–105. [Google Scholar] [CrossRef]
- Lenihan, P.; Orozco, A.; O’Neill, E.; Ahmad, M.N.M.; Rooney, D.W.; Walker, G.M. Dilute acid hydrolysis of lignocellulosic biomass. Chem. Eng. J. 2010, 156, 395–403. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, R.A.; Reckamp, J.M.; Satrio, J.A. Effects of Pretreatments on Yields, Selectivity and Properties of Products from Pyrolysis of Phragmites australis (Common Reeds). Environments 2017, 4, 96. https://doi.org/10.3390/environments4040096
Garrido RA, Reckamp JM, Satrio JA. Effects of Pretreatments on Yields, Selectivity and Properties of Products from Pyrolysis of Phragmites australis (Common Reeds). Environments. 2017; 4(4):96. https://doi.org/10.3390/environments4040096
Chicago/Turabian StyleGarrido, Rene A., Joseph M. Reckamp, and Justinus A. Satrio. 2017. "Effects of Pretreatments on Yields, Selectivity and Properties of Products from Pyrolysis of Phragmites australis (Common Reeds)" Environments 4, no. 4: 96. https://doi.org/10.3390/environments4040096






