Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid
Abstract
:Featured Application
Abstract
1. Introduction
2. EA Chemical Structure and Solubility
3. EA Dietary Assumption
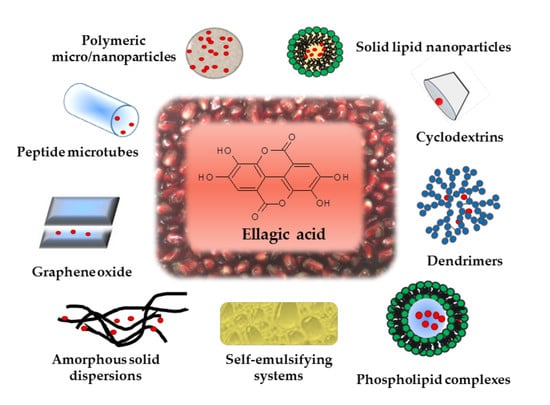
4. Formulation Strategies for Improving EA Oral Bioavailability
4.1. Micronized EA (m-EA)
4.2. EA in Spray Dried and Lyophilized Powders
4.3. Inclusion Complexes
4.3.1. EA Inclusion in Cyclodextrins (CDs)
4.3.2. EA Inclusion in Metalla-Cages
4.4. EA Encapsulated in Polymeric Carriers
4.4.1. Eudragit® Microspheres
4.4.2. Poly (Lactic-Co-Glycolic Acid) (PLGA) and Poly (ε-Caprolactone) (PCL) Nanospheres
4.4.3. Chitosan Micro/Nanospheres
4.4.4. Zein Nanocapsules
4.5. Dendrimers
4.6. Peptide Microtubes
4.7. Functionalized Graphene Oxide (GO) Carriers
4.8. Lipid-Based Carriers
4.8.1. Solid Lipid Nanoparticles (SLNs)
4.8.2. Liposomes (LPs)
4.8.3. Self-Emulsifying Delivery Systems (SNEDDS)
4.9. EA Formulations in Fixed Combination with Other Bioactive Molecules
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miguel, M.G.; Neves, M.A.; Antunes, M.D. Pomegranate (Punica granatum L.): A medicinal plant with myriad biological properties—A short review. J. Med. Plants Res. 2010, 4, 2836–2847. [Google Scholar]
- Ríos, J.L.; Giner, R.M.; Marín, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Zhang, J.; Chen, N.G.; Shi, Z.; Qiu, J.; He, C.; Chen, M. Recent Advances in Anticancer Activities and Drug Delivery Systems of Tannins. Med. Res. Rev. 2017, 37, 665–701. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Sayahi, M.; Sayahi, M. The antidiabetic and antioxidant properties of some phenolic phytochemicals: A review study. Diabetes Metab. Syndr. Clin. Res. 2019, 13, 854–857. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Ghadiri, M.; Ramezani, M.; Askari, V.R. Anti-inflammatory and anti-cancer activities of pomegranate and its constituent, ellagic acid: Evidence from cellular, animal, and clinical studies. Phytother. Res. 2020, in press. [Google Scholar] [CrossRef]
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Grilli, M.; Pittaluga, A.M.; Boggia, R. Ellagic acid a multi-target bioactive compound for drug discovery in CNS? A narrative review. Eur. J. Med. Chem. 2019, 183, 111724. [Google Scholar] [CrossRef]
- Kang, I.; Buckner, T.; Shay, N.F.; Gu, L.; Chung, S. Improvements in Metabolic Health with Consumption of Ellagic Acid and Subsequent Conversion into Urolithins: Evidence and Mechanisms. Adv. Nutr. 2016, 7, 961–972. [Google Scholar] [CrossRef] [Green Version]
- Sarkaki, A.; Farbood, Y.; Dolatshahi, M.; Mansouri, S.M.; Khodadadi, A. Neuroprotective Effects of Ellagic Acid in a Rat Model of Parkinson’s Disease. Acta Med. Iran. 2016, 54, 494–502. [Google Scholar]
- Das, U.; Biswas, S.; Chattopadhyay, S.; Chakraborty, A.; Dey Sharma, R.; Banerji, A.; Dey, S. Radiosensitizing effect of ellagic acid on growth of Hepatocellular carcinoma cells: An in vitro study. Sci. Rep. 2017, 7, 14043. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.; Phan, A.N.H.; Choi, J.W. Anti-cancer Effects of Polyphenolic Compounds in Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor-resistant Non-small Cell Lung Cancer. Pharmacogn. Mag. 2017, 13, 595–599. [Google Scholar] [CrossRef]
- Vanella, L.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Li Volti, G.; Cardile, V.; Abraham, N.; Sorrenti, V. Effects of Ellagic Acid on Angiogenic Factors in Prostate Cancer Cells. Cancers 2013, 5, 726–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Z.; Nair, V.; Khan, M.; Ciolino, H.P. Pomegranate extract inhibits the proliferation and viability of MMTV-Wnt-1 mouse mammary cancer stem cells in vitro. Oncol. Rep. 2010, 24, 1087–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowshik, J.; Giri, H.; Kishore, T.; Kesavan, R.; Vankudavath, R.; Reddy, G.; Dixit, M.; Nagini, S. Ellagic Acid Inhibits VEGF/VEGFR2, PI3K/Akt and MAPK Signaling Cascades in the Hamster Cheek Pouch Carcinogenesis Model. Anticancer Agents Med. Chem. 2014, 14, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Ceci, C.; Tentori, L.; Atzori, M.; Lacal, P.; Bonanno, E.; Scimeca, M.; Cicconi, R.; Mattei, M.; de Martino, M.; Vespasiani, G.; et al. Ellagic Acid Inhibits Bladder Cancer Invasiveness and In Vivo Tumor Growth. Nutrients 2016, 8, 744. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, Z.; Wang, S.; Li, T.; Mastriani, E.; Li, Q.H.; Bao, H.X.; Zhou, Y.J.; Wang, X.; Liu, Y.; et al. Main components of pomegranate, ellagic acid and luteolin, inhibit metastasis of ovarian cancer by down-regulating MMP2 and MMP9. Cancer Biol. Ther. 2017, 18, 990–999. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Chen, Q.; Tan, Y.; Liu, B.; Liu, C. Ellagic acid inhibits human glioblastoma growth in vitro and in vivo. Oncol. Rep. 2017, 37, 1084–1092. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Xu, J.; Wang, T.; Liu, W.; Wei, H.; Yang, X.; Yan, W.; Zhou, W.; Xiao, J. Ellagic acid and Sennoside B inhibit osteosarcoma cell migration, invasion and growth by repressing the expression of c-Jun. Oncol. Lett. 2018, 16, 898–904. [Google Scholar] [CrossRef]
- Ceci, C.; Lacal, P.; Tentori, L.; De Martino, M.; Miano, R.; Graziani, G. Experimental Evidence of the Antitumor, Antimetastatic and Antiangiogenic Activity of Ellagic Acid. Nutrients 2018, 10, 1756. [Google Scholar] [CrossRef] [Green Version]
- Umesalma, S.; Nagendraprabhu, P.; Sudhandiran, G. Ellagic acid inhibits proliferation and induced apoptosis via the Akt signaling pathway in HCT-15 colon adenocarcinoma cells. Mol. Cell. Biochem. 2015, 399, 303–313. [Google Scholar] [CrossRef]
- Goyal, Y.; Koul, A.; Ranawat, P. Ellagic acid ameliorates cisplatin induced hepatotoxicity in colon carcinogenesis. Environ. Toxicol. 2019, 34, 804–813. [Google Scholar] [CrossRef]
- Lin, M.; Yin, M. Preventive Effects of Ellagic Acid Against Doxorubicin-Induced Cardio-Toxicity in Mice. Cardiovasc. Toxicol. 2013, 13, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Sonaje, K.; Italia, J.L.; Sharma, G.; Bhardwaj, V.; Tikoo, K.; Kumar, M.N.V.R. Development of Biodegradable Nanoparticles for Oral Delivery of Ellagic Acid and Evaluation of Their Antioxidant Efficacy Against Cyclosporine A-Induced Nephrotoxicity in Rats. Pharm. Res. 2007, 24, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, L.; Ascacio, A.; Rodríguez-Herrera, R.; Aguilera-Carbó, A.; Aguilar, C.N. Ellagic acid biological properties and biotechnological development. Afr. J. Biotechnol. 2011, 10, 4518–4523. [Google Scholar] [CrossRef]
- Notka, F.; Meier, G.; Wagner, R. Concerted inhibitory activities of Phyllanthus amarus on HIV replication in vitro and ex vivo. Antivir. Res. 2004, 64, 93–102. [Google Scholar] [CrossRef]
- Govindarajan, R.; Vijayakumar, M.; Rao, C.V.; Shirwaikar, A.; Mehrotra, S.; Pushpangadan, P. Healing potential of Anogeissus latifolia for dermal wounds in rats. Acta Pharm. 2004, 54, 331–338. [Google Scholar]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Larrosa, M.; Tomás-Barberán, F.; Espín, J.C.; García-Conesa, M.T. Intestinal ellagitannin metabolites ameliorate cytokine-induced inflammation and associated molecular markers in human colon fibroblasts. J. Agric. Food Chem. 2012, 60, 8866–8876. [Google Scholar] [CrossRef]
- Prabha, B.; Sini, S.; Priyadarshini, T.S.; Sasikumar, P.; Gopalan, G.; Jayesh, P.J.; Jithin, M.M.; Sivan, V.V.; Jayamurthy, P.; Radhakrishnan, K.V. Anti-inflammatory effect and mechanism of action of ellagic acid-3,3’,4-trimethoxy-4’-O-α-L-rhamnopyranoside isolated from Hopea parviflora in lipopolysaccharide-stimulated RAW 264.7 macrophages. Nat. Prod. Res. 2019, 12, 1–5. [Google Scholar] [CrossRef]
- Mele, L.; Mena, P.; Piemontese, A.; Marino, V.; López-Gutiérrez, N.; Bernini, F.; Brighenti, F.; Zanotti, I.; Del Rio, D. Antiatherogenic effects of ellagic acid and urolithins in vitro. Arch. Biochem. Biophys. 2016, 599, 42–50. [Google Scholar] [CrossRef]
- Jordão, J.B.R.; Porto, H.K.P.; Lopes, F.M.; Batista, A.C.; Rocha, M.L. Protective Effects of Ellagic Acid on Cardiovascular Injuries Caused by Hypertension in Rats. Planta Med. 2017, 83, 830–836. [Google Scholar] [CrossRef]
- Turrini, F.; Boggia, R.; Donno, D.; Parodi, B.; Beccaro, G.; Baldassari, S.; Signorello, M.G.; Catena, S.; Alfei, S.; Zunin, P. From pomegranate marcs to a potential bioactive ingredient: A recycling proposal for pomegranate squeezed-marcs. Eur. Food Res. Technol. 2019, 246, 273–285. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, K.H.; Han, C.S.; Yang, H.C.; Park, S.H.; Jang, H.-I.; Kim, J.-W.; Choi, Y.-S.; Lee, N.H. Anti-wrinkle activity of Platycarya strobilacea extract and its application as a cosmeceutical ingredient. J. Cosmet. Sci. 2018, 14, 211–223. [Google Scholar]
- Liu, R.; Li, J.; Cheng, Y.; Huo, T.; Xue, J.; Liu, Y.; Liu, J.; Chen, X. Effects of ellagic acid-rich extract of pomegranates peel on regulation of cholesterol metabolism and its molecular mechanism in hamsters. Food Funct. 2015, 6, 780–787. [Google Scholar] [CrossRef]
- Boggia, R.; Turrini, F.; Villa, C.; Lacapra, C.; Zunin, P.; Parodi, B. Green extraction from pomegranate marcs for the production of functional foods and cosmetics. Pharmaceuticals 2016, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Sánchez, M.A.; García-Villalba, R.; Monedero-Saiz, T.; GarcíaTalavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; Rodríguez-Gil, F.J.; Ruiz-Marín, M.; Pastor-Quirante, F.A.; et al. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol. Nutr. Food Res. 2014, 58, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Bellone, J.A.; Murray, J.R.; Jorge, P.; Fogel, T.G.; Kim, M.; Wallace, D.R.; Hartman, R.E. Pomegranate supplementation improves cognitive and functional recovery following ischemic stroke: A randomized trial. Nutr. Neurosci. 2019, 22, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Kumar, M.N. Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. Anal. 2006, 40, 206–210. [Google Scholar] [CrossRef]
- Muzolf, M.; Szymusiak, H.; Gliszczynska-Swiglo, A.; Rietjens, I.M.C.M.; Tyrakowska, B. pH-Dependent radical scavenging capacity of green tea catechins. J. Agric. Food Chem. 2008, 56, 816–823. [Google Scholar] [CrossRef]
- Panichayupakaranant, P.; Itsuriya, A.; Sirikatitham, A. Preparation method and stability of ellagic acid-rich pomegranate fruit peel extract. Pharm. Biol. 2010, 48, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Wu, L.-F.; Zhao, H.; Liang, W.-Y.; Chen, W.-J.; Han, S.-X.; Qi, Q.; Cui, Y.-P.; Li, S.; Yang, G.-H.; et al. Transport of Corilagin, Gallic Acid, and Ellagic Acid from Fructus Phyllanthi Tannin Fraction in Caco-2 Cell Monolayers. Evid. Based Complementary Altern. Med. 2016, 2016, 9205379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertens-talcot, S.U.; Jilma-Stohlawetz, P.; Rios, J.; Hingorani, L.; Derendorf, H. Absorption metabolism and antioxidant effects of pomegranate. J. Agric. Food Chem. 2006, 54, 8956–8961. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Zhang, Y.; Suchard, M.; Li, Z.; Heber, D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J. Nutr. 2006, 136, 2481–2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; González-Sarrias, A.; Garcıa-Villalba, R.; Nunez-Sanchez, M.A.; Selma, M.V.; Garcıa-Conesa, M.T.; Espın, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, A.; García-Villalba, R.; González-Sarrías, A.; Romo-Vaquero, M.; Loria-Kohen, V.; Ramírez-de-Molina, A.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. The gut microbiota urolithin metabotypes revisited: The human metabolism of ellagicacid is mainly determined by aging. Food Funct. 2018, 9, 4100–4106. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.Á.; Tomé-Carneiro, J.; Zafrilla, P.; Mulero, J.; Tomás-Barberán, F.A.; Espín, J.C. Identifying the limits for ellagic acid bioavailability: A crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts. J. Funct. Foods 2015, 19, 225–235. [Google Scholar] [CrossRef]
- Tennant, D.R.; Davidson, J.; Day, A.J. Phytonutrient intakes in relation to European fruit and vegetable consumption patterns observed in different food surveys. Br. J. Nutr. 2014, 112, 1214–1225. [Google Scholar] [CrossRef] [Green Version]
- Whitley, A.C.; Stoner, G.D.; Darby, M.V.; Walle, T. Intestinal epithelial cell accumulation of the cancer preventive polyphenol ellagic acid—Extensive binding to protein and DNA. Biochem. Pharmacol. 2003, 66, 907–915. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Bhatnagar, P.; Singh, M.; Mishra, S.; Kumar, P.; Shukla, Y.; Gupta, K.C. Synthesis of PLGA nanoparticles of tea polyphenols and their strong in vivo protective effect against chemically induced DNA damage. Int. J. Nanomed. 2019, 14, 7001–7002. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA Nanoparticles Improve the Oral Bioavailability of Curcumin in Rats: Characterizations and Mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef]
- Sanna, V.; Siddiqui, I.A.; Sechi, M.; Mukhtar, H. Resveratrol-Loaded Nanoparticles Based on Poly (epsilon-caprolactone) and Poly (d l-lactic-co-glycolic acid)–Poly (ethylene glycol) Blend for Prostate Cancer Treatment. Mol. Pharmaceut. 2013, 10, 3871–3881. [Google Scholar] [CrossRef] [Green Version]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1987, 19, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, X.; Zu, Y.; Zhang, Y.; Ge, Y.; Zhong, C.; Wu, W. Preparation and characterization of micronized ellagic acid using antisolvent precipitation for oral delivery. Int. J. Pharmaceut. 2015, 486, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Committee for Human Medicinal Products ICH guideline Q3C (R6) on impurities: Guideline for residual solvents. EMA/CHMP/ICH/82260/ 2006, 2006, 1–39.
- Beshbishy, A.M.; Batiha, G.E.-S.; Yokoyama, N.; Igarashi, I. Ellagic acid microspheres restrict the growth of Babesia and Theileria in vitro and Babesia microti in vivo. Parasites Vectors 2019, 12, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montes, A.; Wehner, L.; Pereyra, C.; Martínez de la Ossa, E.J. Generation of microparticles of ellagic acid by supercritical antisolvent process. J. Supercrit. Fluids 2016, 116, 101–110. [Google Scholar] [CrossRef]
- Laine, P.; Kylli, P.; Heinonen, M.; Jouppila, K. Storage Stability of Microencapsulated Cloudberry (Rubus chamaemorus) Phenolics. J. Agric. Food Chem. 2008, 56, 11251–11261. [Google Scholar] [CrossRef]
- Li, B.; Harich, K.; Wegiel, L.; Taylor, L.S.; Edgar, K.J. Stability and solubility enhancement of ellagic acid in cellulose ester solid dispersions. Carbohyd. Polym. 2013, 92, 1443–1450. [Google Scholar] [CrossRef]
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Parodi, B.; Zuccari, G.; Pittaluga, A.M.; Boggia, R. Preparation of ellagic acid micro and nano formulations with amazingly increased water solubility by its entrapment in pectin or non-PAMAM dendrimers suitable for clinical applications. New J. Chem. 2019, 43, 2438–2448. [Google Scholar] [CrossRef]
- Food Additive Database. Available online: https://webgate.ec.europa.eu/foods_system/main/?sector=FAD&auth=SANCAS (accessed on 2 February 2017).
- El-Missiry, M.A.; Othman, A.I.; Amer, M.A.; Sedki, M.; Ali, S.M.; El-Sherbiny, I.M. Nanoformulated ellagic acid ameliorates pentylenetetrazol-induced experimental epileptic seizures by modulating oxidative stress, inflammatory cytokines and apoptosis in the brains of male mice. Metab. Brain Dis. 2020, 35, 385–399. [Google Scholar] [CrossRef]
- Bulani, V.D.; Kothavade, P.S.; Nagmoti, D.M.; Kundaikar, H.S.; Degani, M.S.; Juvekar, A.R. Characterisation and anti-inflammatory evaluation of the inclusion complex of ellagic acid with hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2015, 82, 361–372. [Google Scholar] [CrossRef]
- Bulani, V.D.; Kothavade, P.S.; Kundaikar, H.S.; Gawali, N.B.; Chowdhury, A.A.; Degani, M.S.; Juvekar, A.R. Inclusion complex of ellagic acid with β-cyclodextrin: Characterization and in vitro anti-inflammatory evaluation. J. Mol. Struct. 2016, 1105, 308–315. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Tian, Z.; Ma, J.; Kang, M.; Ding, C.; Ming, D. Preparation of β-CD-Ellagic Acid Microspheres and Their Effects on HepG2 Cell Proliferation. Molecules 2017, 22, 2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mady, F.M.; Ibrahim, S.R.M. Cyclodextrin-based nanosponge for improvement of solubility and oral bioavailability of Ellagic acid. Pak. J. Pharm. Sci. 2018, 31, 2069–2076. [Google Scholar] [PubMed]
- Joseph, E.; Singhvi, G. Nanomaterials for Drug Delivery and Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–111. [Google Scholar]
- Lu, G.W.; Gao, P. Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 59–94. [Google Scholar]
- Fan, G.; Cai, Y.; Fu, E.; Yuan, X.; Tang, J.; Sheng, H.; Gong, J. Preparation and process optimization of pomegranate ellagic acid-hydroxypropyl-β-cyclodextrin inclusion complex and its antibacterial activity in vitro. Acta Medica. Mediterr. 2019, 35, 383–389. [Google Scholar] [CrossRef]
- Gontijo, A.V.G.; Sampaio, A.; Da, G.; Koga-Ito, C.Y.; Salvador, M.J. Biopharmaceutical and antifungal properties of ellagic acid-cyclodextrin using an in vitro model of invasive candidiasis. Future Microbiol. 2019, 14, 957–967. [Google Scholar] [CrossRef]
- An, S.S.; Chi, K.-W.; Kang, S.C.; Dubey, A.; Park, D.W.; Kwon, J.E.; Jeong, Y.J.; Kim, T.; Kim, I. Investigation of the biological and anti-cancer properties of ellagic acid-encapsulated nano-sized metalla-cages. Int. J. Nanomed. 2015, 10, 227–240. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.-I.; Yv, R.P.; Ohno, T.; Yoshikawa, Y.; Shibata, N.; Kato, S.; Takeuchi, K.; Takada, K. Application of Eudragit P-4135F for the delivery of ellagic acid to the rat lower small intestine. J. Phar. Pharmacol. 2001, 53, 1079–1085. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Kharade, S.V.; Roy, N.; Ravi Kumar, M.N.V. Sustained release nanoparticulate formulation containing antioxidant-ellagic acid as potential prophylaxis system for oral administration. J. Drug Target. 2006, 14, 27–34. [Google Scholar] [CrossRef]
- Reliene, R.; Shirode, A.; Coon, J.; Nallanthighal, S.; Bharali, D.; Mousa, S. Nanoencapsulation of pomegranate bioactive compounds for breast cancer chemoprevention. Int. J. Nanomed. 2015, 10, 475–484. [Google Scholar] [CrossRef]
- Abd-Rabou, A.A.; Ahmed, H.H. CS-PEG decorated PLGA nano-prototype for delivery of bioactive compounds: A novel approach for induction of apoptosis in HepG2 cell line. Adv. Med. Sci. 2017, 62, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Mady, F.; Shaker, M. Enhanced anticancer activity and oral bioavailability of ellagic acid through encapsulation in biodegradable polymeric nanoparticles. Int. J. Nanomed. 2017, 12, 7405–7417. [Google Scholar] [CrossRef] [Green Version]
- Arulmozhi, V.; Pandian, K.; Mirunalini, S. Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids Surf. B 2013, 110, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, L.; Ramana, L.N.; Sethuraman, S.; Krishnan, U.M. Ellagic acid encapsulated chitosan nanoparticles as anti-hemorrhagic agent. Carbohydr. Polym. 2014, 111, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Bi, H.; Wang, D.; Kang, M.; Tian, Z.; Zhang, Y.; Wang, H.; Zhu, T.; Ma, J. Preparation of Chitosan/Alginate-ellagic acid sustained-release microspheres and their Inhibition of preadipocyte adipogenic differentiation. Curr. Pharm. Biotechnol. 2019, 20, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Yang, Y.; Yang, F.; Wan, K.; Fan, D.; Wang, D. Novel oral administrated ellagic acid nanoparticles for enhancing oral bioavailability and anti-inflammatory efficacy. J. Drug Deliv. Sci. Technol. 2018, 46, 215–222. [Google Scholar] [CrossRef]
- Padilla De Jesus, O.L.; Ihre, H.R.; Gagne, L.; Frechet, J.M.J.; Szoka, F.C. Polyester dendritic systems for drug delivery applications: In vitro and in vivo evaluation. Bioconjug. Chem. 2002, 13, 453–461. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Domenicotti, C. Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic andmPro-Oxidant Effect on Human Neuroblastoma Cells. Antioxidants 2020, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Tesauro, D.; Accardo, A.; Diaferia, C.; Milano, V.; Guillon, J.; Ronga, L.; Rossi, F. Peptide based drug delivery systems in biotechnological applications. Molecules 2019, 24, 351. [Google Scholar] [CrossRef] [Green Version]
- Barnaby, S.N.; Fath, K.R.; Tsiola, A.; Banerjee, I.A. Fabrication of ellagic acid incorporated self-assembled peptide microtubes and their applications. Colloid Surf. B 2012, 95, 154–161. [Google Scholar] [CrossRef]
- Rahmanian, N.; Hamishehkar, H.; Dolatabadi, J.E.; Arsalani, N. Nano graphene oxide: A novel carrier for oral delivery of flavonoids. Colloids Surf. B 2014, 123, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Kakran, M.G.; Sahoo, N.; Bao, H.; Pan, Y.; Li, L. Functionalized graphene oxide as nanocarrier for loading and delivery of ellagic acid. Curr. Med. Chem. 2011, 18, 4503–4512. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [PubMed]
- Yoon, G.; Park, J.W.; Yoon, I.S. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): Recent advances in drug delivery. J. Pharm. Investig. 2013, 43, 353–362. [Google Scholar] [CrossRef]
- Makwana, V.; Jain, R.; Patel, K.; Nivsarkar, M.; Joshi, A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: Elucidation of mechanism of uptake using chylomicron flow blocking approach. Int. J. Pharm. 2015, 495, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, H.; Hamishehkar, H.; Rahmati-yamchi, M.; Shanehbandi, D.; Nazari Soltan Ahmad, S.; Hasani, A. Enhanced anti-cancer capability of ellagic acid using solid lipid nanoparticles (SLNs). Int. J. Cancer Manag. 2018, 11, e9402. [Google Scholar] [CrossRef]
- Murugan, V.; Mukherjee, K.; Maiti, K.; Mukherjee, P.K. Enhanced oral bioavailability and antioxidant profile of ellagic acid by phospholipids. J. Agric. Food Chem. 2009, 57, 4559–4565. [Google Scholar] [CrossRef]
- Stojiljković, N.; Ilić, S.; Stojanović, N.; Janković-Veličković, L.; Stojnev, S.; Kocić, G.; Radenković, G.; Arsić, I.; Stojanović, M.; Petković, M. Nanoliposome-encapsulated ellagic acid prevents cyclophosphamide-induced rat liver damage. Mol. Cell. Biochem. 2019, 458, 185–195. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Wang, Y.-W.; Huang, M.-T.; Ho, C.-T.; Huang, Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008, 108, 419–424. [Google Scholar] [CrossRef]
- Avachat, A.M.; Patel, V.G. Self nanoemulsifying drug delivery system of stabilized ellagic acid–phospholipid complex with improved dissolution and permeability. Saudi Pharm. J. 2015, 23, 276–289. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-T.; Chou, C.-T.; Su, N.-W. A food-grade self-nanoemulsifying delivery system for enhancing oral bioavailability of ellagic acid. J. Funct. Foods 2017, 34, 207–215. [Google Scholar] [CrossRef]
- Dokania, S.; Joshi, A.K. Self-microemulsifying drug delivery system (SMEDDS)—Challenges and road ahead. Drug Deliv. 2015, 22, 675–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, D.; Lv, C.; Sun, X.; Wang, J.; Zhao, Z. Preparation of a supersaturatable self-microemulsion as drug delivery system for ellagic acid and evaluation of its antioxidant activities. J. Drug Deliv. Sci. Technol. 2019, 53, 101209. [Google Scholar] [CrossRef]
- Tavano, L.; Muzzalupo, R.; Picci, N.; de Cindio, B. Co-encapsulation of antioxidants into niosomal carriers: Gastrointestinal release studies for nutraceutical applications. Colloid Surf. B 2014, 114, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, D.V.; Chandraiah, G.; Meena, A.K.; Ramarao, P.; Kumar, M.N. The co-encapsulated antioxidant nanoparticles of ellagic acid and coenzyme Q10 ameliorates hyperlipidemia in high fat diet fed rats. Nanosci. Nanotechnol. 2009, 9, 6741–6746. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Mirza Mohd, A.; Anwer, M.K.; Alshetaili, A.S.; Alshahrani, S.M.; Ahmed, F.J.; Iqbal, Z. Development of NIPAAm-PEG acrylate polymeric nanoparticles for co-delivery of paclitaxel with ellagic acid for the treatment of breast cancer. J. Polym. Eng. 2019, 39, 271–278. [Google Scholar] [CrossRef]
- Fahmy, U.A. Augmentation of fluvastatin cytotoxicity against prostate carcinoma PC3 cell line utilizing alpha lipoic–ellagic acid nanostructured lipid carrier formula. AAPS PharmSciTech 2018, 19, 3454–3461. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 23–43. [Google Scholar] [CrossRef]
- Abd Elwakil, M.M.; Mabrouk, M.T.; Helmy, M.W.; Abdelfattah, E.-Z.A.; Khiste, S.K.; Elkhodairy, K.A.; Elzoghby, A.O. Inhalable lactoferrin–chondroitin nanocomposites for combined delivery of doxorubicin and ellagic acid to lung carcinoma. Nanomedicine 2018, 13, 2015–2035. [Google Scholar] [CrossRef]


| Vehicles | Solubility (mg/mL) | Temperature | Ref. | |
|---|---|---|---|---|
| Solvents | N-methyl-2-pyrrolidone | 25 | 37 °C | [36] |
| DMSO | 2.5 | 37 °C | [36] | |
| Pyridine | 2.0 | 37 °C | [36] | |
| Methanol | (671 ± 17) × 10−3 | 37 °C | [36] | |
| Ethanol | 1.02 ± 0.04 | 25 °C | [96] | |
| Cosolvents | PEG 200 | 4.178 | 25 °C | [94] |
| PEG 400 | 11.0 ± 0.5 | 25 °C | [96] | |
| Propylene glycol | 2.1 ± 0.1 | 25 °C | [96] | |
| Oils | Palmester 3575 1 | 0.030 | 25 °C | [94] |
| Cottonseed oil | 0.005 | 25 °C | [94] | |
| Soybean oil | 0.29 ± 0.01 | 25 °C | [95] | |
| Castor oil | 1.63 ± 0.07 | 25 °C | [96] | |
| Oleic acid | 0.29 ± 0.01 | 25 °C | [96] | |
| Ethyl oleate | 2.34 ± 0.06 | 25 °C | [96] | |
| Surfactants | Tween 20 | 1.605 | 25 °C | [94] |
| Sucrose esters | 0.115 | 25 °C | [94] | |
| Isopropyl myristate | 1.94 ± 0.07 | 25 °C | [96] | |
| Cremophor RH40 2 | 2.5 ± 0.1 | 25 °C | [96] | |
| Tween 80 | 3.5 ± 0.1 | 25 °C | [96] | |
| Lecithin | 0.085 ± 0.004 | 25 °C | [96] | |
| Poloxamer F68 | 0.036 ± 0.002 | 25 °C | [96] | |
| Aqueous Solutions | Phosphate buffer pH 7.4 | (33 ± 16) × 10−3 | 37 °C | [36] |
| Phosphate buffer pH 6.8 | (11.1 ± 0.4) × 10−3 | 25 °C | [96] | |
| Acetate buffer pH 4.5 | (6.9 ± 0.3) × 10−3 | 25 °C | [96] | |
| Distilled water | (8.2 ± 0.4) × 10−3 | 25 °C | [96] | |
| HCl 0.1 M in water | (1.03 ± 0.06) × 10−3 | 25 °C | [96] |
| Formulation Type | Fabrication Method | Excipients | Mean Size | Remarkable Features | Highlights | Ref. |
|---|---|---|---|---|---|---|
| Micro-sized EA | Anti-solvent precipitation | - | n.a. | Use of a syringe pump Good dispersion | In vitro and in vivo inhibition of blood parasites | [54] |
| Micro-sized EA | Supercritical anti-solvent process | Eudragit® L 100 | 3.73 µm | Co-precipitate product EA content 49% Residual NMP 148 ppm | Increased EA dissolution rate | [55] |
| Amorphous solid dispersion | Freeze drying | Maltodextrin | n.a. | Use of cloudberry extract | Higher storage stability up to 32 days Food supplement formulation | [56] |
| Amorphous solid dispersion | Spray drying | Hydroxypropyl-methyl cellulose acetate succinate | n.a. | EA solubility 280 µg/mL EA content 25%Stable supersaturated EA solution at pH 6.8 | pH-sensitive polymer Minimal release in the stomach, quite fast at pH 6.8 (35% after 0.5 h) | [57] |
| Amorphous solid dispersion | Spray drying | Pectin | 10 µm | EA solubility 63 µg/mL EA content 21% No organic solvent used | High biocompatibility Suitable to formulate antioxidant-rich functional food | [58] |
| Amorphous solid dispersion | Spray drying | Alginic acid | 670 nm | EA solubilized in basic solution Crosslinking with CaCl2 Complete release after 8 h | Highly biocompatible formulation Improved in vivo neuroprotective and anticonvulsant effect in orally treated mice | [60] |
| Polymeric microspheres | Emulsion-evaporation technique in oil phase (acetone/light liquid paraffin) | Eudragit® P-4135F | 113 µm | EA content 35% EE 81% Total release after 6 h | pH responsive release Ileocaecal targeting | [70] |
| Polymeric microspheres | Ionotropic gelation by sodium alginate | Chitosan | 4.36 µm | EE 29% Sustained release | Obesity prevention and treatment | [78] |
| Peptide microtubes | Self-assembling | bis(N-α-amido threonine)-1, 5-pentane dicarboxylate | 0.5–1 µm | EE 80% Rate release dependent on the EA deprotonation process at different pH | High biocompatibility Enhanced antibacterial activity | [83] |
| Phospholipidvesicles | Anti-solvent precipitation | Hydrogenated soy phosphatidyl-choline | 1–3 µm | EE 29% | High biocompatibility Liver protection Relative AUC increase by2.8-fold | [83] |
| Formulation Type | Fabrication Method | Excipients | Mean Size | Remarkable Features | Highlights | Ref. |
|---|---|---|---|---|---|---|
| Nano-sized EA | Anti-solvent precipitation | - | 428 nm | EA water solubility 11.67 µg/mL Lyophilized product with maltodextrin as diluent Residual NMP 405 ppm | Higher radical scavenging activity Enhanced relative AUC by 2 times | [52] |
| Polymer nanospheres | Emulsion-diffusion-evaporation | PLGA or PCL | 125 nm | EA content 62% Slow EA release (about 24% after 6 days) | Sustained release for 20 days Good stability Potential prophylaxis system Higher in situ uptake and greater in vivo nephron-protection in CyA-treated rats | [22,72] |
| Dendrimer | Self-assembling | Aminoacid-modifed hetero dendrimer | 70 nm | EA solubility 9 mg/mL EA content 53% | EA solubility increase | [58] |
| Inclusion complex | Freeze drying | HP-β-CD | n.a. | Formation of 1:2 EA:HP-β-CD complex Increased total EA solubility up to 54.40 µg/mL 60% EA released after 0.5 h | Enhanced in vivo anti-inflammatory effect | [61] |
| Inclusion complex | Freeze drying | β-CD | n.a. | Formation of 1:2 EA:β-CD complex Increased EA solubility up to 39.14 µg/mL Less than 30% EA released after 0.5 h | Enhanced in vivo anti-inflammatory effect | [62,63] |
| Inclusion complex | Overnight shaking | β-CD nanosponge | 423 nm | EA solubility up to 49.79 µg/mL EA content 69% Prolonged release | High biocompatibility Increased relative AUC by 2.2-fold | [64] |
| Inclusion complex | Stirring-ultrasonic and final freeze drying | HP-β-CD | n.a. | Optimized production process EE 84% | Antibacterial activity | [67] |
| Inclusion complex | Precipitation | Arene-Ru metalla-prisms | n.a. | Yield 92% High complex stability | Enhanced antitumor activity against A549 cells | [69] |
| Polymeric nanospheres | Double emulsion-evaporation (w/o/w) | PLGA-PEG | 175 nm | Opsonization avoided Prolonged circulation time in blood Suitable for i.v. administration | Inhibition of breast cancer cell growth More than 2-fold IC50 reduction in MCF-7 cells | [73] |
| Polymeric nanospheres | Emulsion-evaporation in aqueous phase | PLGA coated with chitosan and PEG | 255 nm | Opsonization avoided Sustained release Suitable for i.v. administration | 3-fold IC50 reduction in HepG2 and HCT 116 cells | [74] |
| Polymeric nanospheres | Emulsion-diffusion-evaporation | PCL | 193 nm | EA content 58% EE 66% Slow EA release: approx. 48% after 8 days | Long-term release 6.9-fold cytotoxicity increase against HCT 116 cells Improved relative AUC by 3.6 times | [75] |
| Polymeric nanospheres | Ionic gelation by sodium tripolyphosphate | Chitosan | 176 nm | EA content 33% Rapid release up to 8 h, then more controlled up to 48 h | More than 3-fold IC50 reduction in KB cells Faster clotting time | [76,77] |
| Polymeric nanocapsules | Anti-solvent coprecipitation | Zein | 72 nm | Shell thickness of 20 nm 326 mg EA loaded per 1 g of excipient Sustained release up to 6 days | High biocompatibility Enhanced in vivo anti-inflammatory effect Relative AUC increased by 8.7-fold | [79] |
| Adsorption complex | Overnight shaking | Functionalized graphene oxide | Sheets ranged from 20 to 120 nm | Easy EA loading by physisorption EA solubility up to 610 µg/mL 1.22 g EA loaded per 1 g of excipient 23% EA released at pH 4 38% at pH 10 after 72 h | Enhanced in vitro cytotoxicity against MCF7 and HT29 cells | [85] |
| Solid lipid nanoparticle | Hot homogenization method | Precirol® Poloxamer 407 Tween 80 | 100 nm | EE 89% EA content 36% Initial burst effect (>40%) followed by a sustained release | Enhanced antitumor activity against PC3 cells | [89] |
| Liposome | Overnight mixing | Phospholipid nanoparticles solution (10%) in form of nanospheres | n.a. | EE 60% Good EA protection in different pH buffers and metal ion containing solutions | For nutritional supplements as adjuvant therapy in cancer | [91] |
| Self-nanoemulsifying delivery systems | Preliminary EA and soy lecithin complex obtained by anti-solvent precipitation | Soy lecithin Captex® 500 Cremophor® RH40 PEG 400 Tocopherol | 106 nm | EE 95% Fast release of nearly 95% after 1 h | Improved ex vivo intestinal permeability | [93] |
| Self-nanoemulsifying delivery systems | Vortex mixing | Palmester® 3575 Tween 80 PEG 400 | 120 nm | 2.5 mg/mL EA loading into SNEDDS Fast release | Food-grade components Increased relative AUC by 6.6-fold | [94] |
| Self-nanoemulsifying delivery systems | Vortex mixing | Ethyl oleate Tween 80 PEG 400 | 45 nm | 4 mg/g EA loading into SNEDDS Adjunct of PVP 0.5% as stabilizer Less fast release | Enhanced in vitro and in vivo radical scavenging activity | [96] |
| Polymeric nanospheres | Emulsion-diffusion-evaporation | PLGA | 259 nm | Fixed formulation with CoQ10 EE of 70% for EA and of 72% for CoQ10 | Prolonged control on cholesterol levels in rats | [98] |
| Polymeric nanospheres | Self-assembling and dialysis method for drug loading | Poly (N-iso-propylacrylamide-PEG acrylate) | 200 nm | Combined formulation with paclitaxel Controlled release (only 8% after 2 h) EE 98% for EA and 92% for paclitaxel | Enhanced in vitro cytotoxicity against MCF-7 cells | [99] |
| Nanostructured lipid carrier | Hot emulsification-ultrasonication | Almond oil Compritol® ATO Phosphatidylcholine Gelucire® | 85 nm | Co-delivery of EA, fluvastatin, alpha lipoic acid Total drug released within 3 h | Enhanced in vitro cytotoxicity against PC3 cells | [100] |
| Polymeric nanoparticles | EA nanocrystallization by anti-solvent precipitation + polyelectrolyte electrostatic complexation | Lactoferrin Chondroitin sulfate | 192 nm | Inhalable combined formulation with doxorubicin | Low cost of production Easy scalability Excipients usable in food products | [102] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuccari, G.; Baldassari, S.; Ailuno, G.; Turrini, F.; Alfei, S.; Caviglioli, G. Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid. Appl. Sci. 2020, 10, 3353. https://doi.org/10.3390/app10103353
Zuccari G, Baldassari S, Ailuno G, Turrini F, Alfei S, Caviglioli G. Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid. Applied Sciences. 2020; 10(10):3353. https://doi.org/10.3390/app10103353
Chicago/Turabian StyleZuccari, Guendalina, Sara Baldassari, Giorgia Ailuno, Federica Turrini, Silvana Alfei, and Gabriele Caviglioli. 2020. "Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid" Applied Sciences 10, no. 10: 3353. https://doi.org/10.3390/app10103353