Stain Resistance of Cotton Fabrics before and after Finishing with Admicellar Polymerization
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Samples, Admicellar Polymerization
2.3. Characterization of Treated Fabric
2.3.1. Water Repellency Tests
2.3.2. Contact Angle Analysis
2.3.3. SEM Study
2.3.4. Stain Recovery and Stain Resistance Tests


2.3.5. Tensile Strength Measurement
3. Results and Discussion
3.1. appearance of Thin Film



3.2. Mechanical Characterization

3.3. Water Repellency Tests

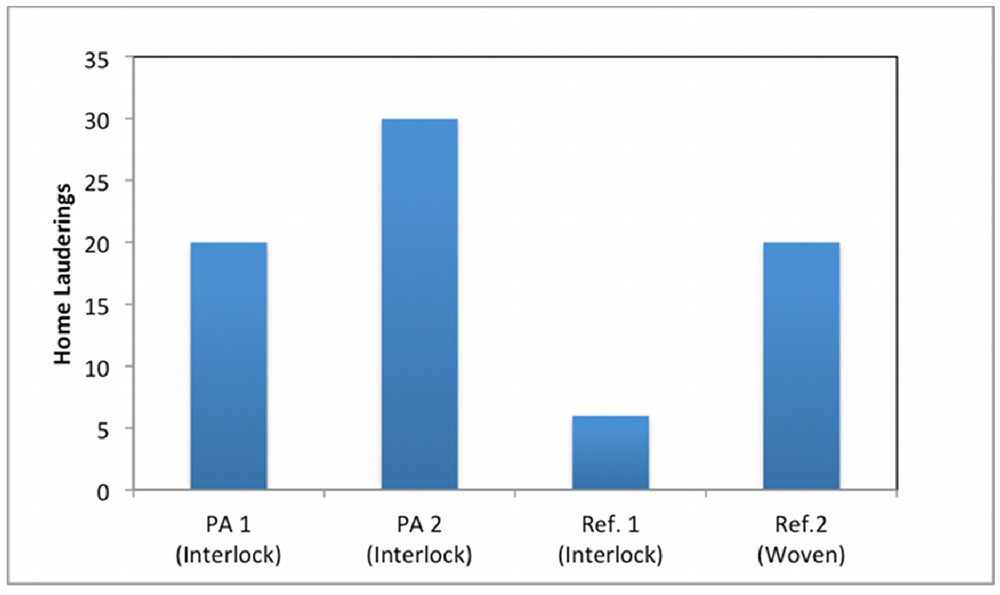

3.4. Oil Repellency

3.5. Stain Recovery and Stain Resistance
| Types of fabric | Stain release grade |
|---|---|
| Interlock (PA1) | 1 |
| Interlock (PA2) | 4.5 |
| Interlock (Ref. 1) | 3 |
| Woven (Ref. 2) | 2 |



4. Conclusion
Acknowledgements
Conflict of Interest
References
- Holme, I. Innovative technologies for high performance textiles. Color Technol. 2007, 123, 59–73. [Google Scholar] [CrossRef]
- Sawhney, A.P.S.; Condon, B.; Singh, K.V.; Pang, S.S.; Li, G.; Hui, D. Modern applications of nanotechnology in textiles. Text. Res. J. 2008, 78, 731–739. [Google Scholar] [CrossRef]
- Vazquez, F. Silicone softeners for stain repellent and stain resistance fabric finishing. Dyes and Chemicals. 2004. Available online: http://www.fibre2fashion.com/industry-article/7/619/silicone-softeners-for-stain-repellent-and-stain-release-fabric-finishing6.asp (accessed on 27 January 2012).
- Kathirvelu, S.; D’Souza, L.; Dhurai, B. Study of stain-eliminating textiles using ZnO nanoparticles. J. Tex. I 2010, 101, 520–526. [Google Scholar] [CrossRef]
- Badyal, J.P. Beyond the surface-cold plasmas are streamlining the surface coating. Chem. Br. 2001, 37, 45–46. [Google Scholar]
- Bubert, H. Surface and thin film analysis. Ullmann’s Encycl. Ind. Chem. 2011. pub2.. [Google Scholar] [CrossRef]
- Ma, M.L.; Gupta, M.; Zhai, L.; Gleason, K. K.; Cohen, R. E.; Rubner, M. F.; Rutledge, G. C. Decorated electrospun fibers exhibiting superhydrophobicity. Adv. Mater. 2007, 19, 255–259. [Google Scholar]
- Maity, J.; Jacob, C.; Das, C.K.; Singh, R.P. Direct fluorination of Twaron fiber and investigation of mechanical thermal and morphological properties of high density polyethylene and Twaron fiber composites. J. Appl. Polym. Sci. 2008, 107, 3739–3749. [Google Scholar] [CrossRef]
- Tarimala, S.; Kothari, N.; Abidi, N.; Hequet, E.; Fralick, J.; Dai, L.L. New approach to antibacterial treatment of cotton fabric with silver nanoparticle–doped silica using sol-gel process. J. Appl. Polym. Sci. 2006, 101, 2938–2943. [Google Scholar]
- Yeh, J.-T.; Chen, C.-L.; Huang, K.-S. Preparation and application of fluorocarbon polymer/SiO2 hybrid materials, part 2: Water and oil repellent processing for cotton fabrics by sol-gel method. J. Appl. Polym. Sci. 2007, 103, 3019–3024. [Google Scholar] [CrossRef]
- Wang, T.; Hu, X.; Dong, S. A general route to transform normal hydrophilic cloths into superhydrophobic surfaces. Chem. Commun. 2007, 1849–1851. [Google Scholar]
- Hoefnagels, H.F.; Wu, D.; de With, G.; Ming, W. Biomimetic superhydrophobic and highly oleophobic cotton textiles. Langmuir 2007, 23, 13158–13163. [Google Scholar] [CrossRef]
- Xue, C.H.; Jia, S.T.; Chen, H.Z.; Wang, M. Superhydrophobic cotton fabrics prepared by sol-gel coating of TiO2 and surface hydrophobization. Sci. Technol. Adv. Mater. 2008, 9. [Google Scholar] [CrossRef]
- Wang, H.; Fang, J.; Cheng, T.; Ding, J.; Qu, L.; Dai, L.; Wang, X.; Lin, T. One-step coating of fluoro-containing silica nanoparticles for universal generation of surface superhydrophobicity. Chem. Commun. 2008, 877–879. [Google Scholar]
- Leng, B.; Shao, Z.Z.; de With, G.; Ming, W. Superoleophobic cotton textiles. Langmuir 2009, 25, 2456–2460. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, Y.W.; Wang, X.G.; Lin, T. Superhydrophobic cotton fabric fabricated by electrostatic assembly of silica nanoparticles and its remarkable buoyancy. Appl. Surf. Sci. 2010, 256, 6736–6742. [Google Scholar]
- Luna-Xavier, J.-L.; Guyot, A.; Bourgeat-Lami, E. Synthesis and characterization of silica/poly (methyl methacrylate) nanocomposite latex particles through emulsion polymerization using a cationic azo initiator. J. Colloid Interface Sci. 2002, 250, 82–92. [Google Scholar] [CrossRef]
- O’Haver, J.H.; Harwell, J.H.; O’Rear, E.A.; Snodgrass, L.J.; Waddell, W.H. In situ formation of polystyrene in adsorbed surfactant bilayers on precipitated silica. Langmuir 1994, 10, 2588–2593. [Google Scholar]
- Wang, S.; Russo, T.; Qiao, G.G.; Solomon, D.H.; Shanks, R.A. Admicellar polymerization of styrene with divinyl benzene on alumina particles: The synthesis of white reinforcing fillers. J. Mater. Sci. 2006, 41, 7474–7482. [Google Scholar]
- Karlsson, P.M.; Esbjornsson, N.B.; Holmberg, K. Admicellar polymerization of methyl methacrylate on aluminum pigments. J. Colloid Interface Sci. 2009, 337, 364–368. [Google Scholar]
- Maity, J.; Kothary, P.; O’Rear, E.A.; Jacob, C. Preparation and comparison of hydrophobic cotton fabric obtained by direct fluorination and admicellar polymerization of fluoromonomers. Ind. Eng. Chem. Res. 2010, 49, 6075–6079. [Google Scholar]
- Siriviriyanun, A.; O’Rear, E.A.; Yanumet, N. Improvement in the flame retardancy of cotton fabric by admicellar polymerization of 2-acryloyloxyethyl diethyl phosphate using an anionic surfactant. J. Appl. Polym. Sci. 2008, 109, 3859–3866. [Google Scholar] [CrossRef]
- Tragoonwichian, S.; O’Rear, E.A.; Yanumet, N. Broad ultraviolet protection by copolymerization of 2-[3-(2H-benzotriazol-2-yl)-4-hydroxyphenyl]ethyl methacrylate and 2-hydroxy-4-acryloyloxybenzophenone on cotton via admicellar polymerization. J. Appl. Polym. Sci. 2008, 108, 4004–4013. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hanumansetty, S.; Maity, J.; Foster, R.; O’Rear, E.A. Stain Resistance of Cotton Fabrics before and after Finishing with Admicellar Polymerization. Appl. Sci. 2012, 2, 192-205. https://doi.org/10.3390/app2010192
Hanumansetty S, Maity J, Foster R, O’Rear EA. Stain Resistance of Cotton Fabrics before and after Finishing with Admicellar Polymerization. Applied Sciences. 2012; 2(1):192-205. https://doi.org/10.3390/app2010192
Chicago/Turabian StyleHanumansetty, Srinivas, Jayanta Maity, Rod Foster, and Edgar A. O’Rear. 2012. "Stain Resistance of Cotton Fabrics before and after Finishing with Admicellar Polymerization" Applied Sciences 2, no. 1: 192-205. https://doi.org/10.3390/app2010192





