Sustainable Utility of Magnetically Recyclable Nano-Catalysts in Water: Applications in Organic Synthesis
Abstract
:1. Introduction

2. Highlights of the Review
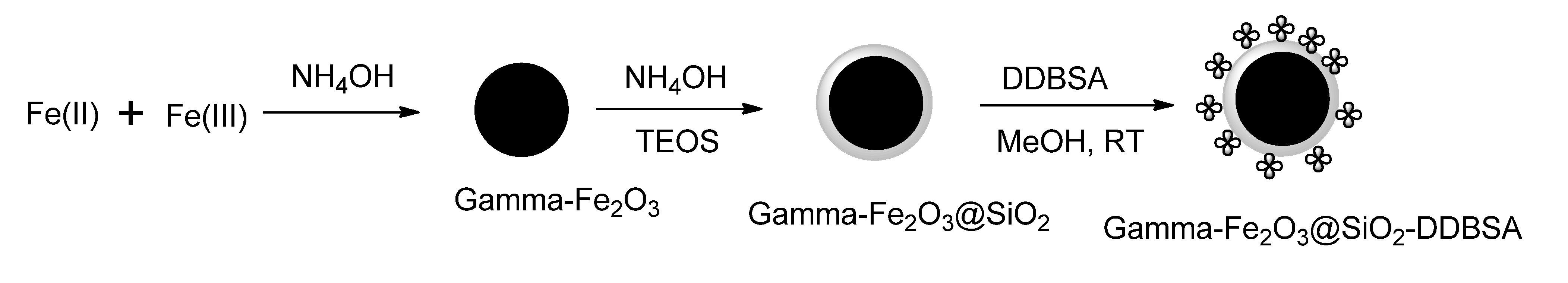
3. Applications of Magnetically Recyclable Nanocatalysis (MRNCs) in Water


















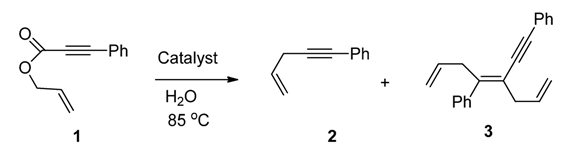
| Entry | Catalyst | T/°C | t/h | Isolated Yield (%) |
|---|---|---|---|---|
| 1 | 5 mol% Fe3O4–Pd | 85 | 2 | 25(3)(70) a |
| 2 | 5 mol% Fe3O4–Pd | 85 | 12 | 95(3) |
| 3 | Recovered from entry 2 | 85 | 12 | 88(3) |
| 4 | 5 mol% Pd/C | 85 | 12 | 20(4(2):1(3)) b |
| 5 | 5 mol% Pd(PPh3)4 | 85 | 6 | 92(4(2):1(3)) b |

4. Reusability of Magnetic Nanocatalysts
5. Conclusions and Future Perspectives
Conflicts of Interest
References
- Gawande, M.B.; Branco, P.S.; Varma, R.S. Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev. 2013, 42, 3371–3393. [Google Scholar] [CrossRef]
- Gawande, M.B.; Bonifacio, V.D.B.; Varma, R.S.; Nogueira, I.D.; Bundaleski, N.; Ghumman, C.A.A.; Teodoro, O.M.N.D.; Branco, P.S. Magnetically recyclable magnetite-ceria (Nanocat-Fe-Ce) nanocatalyst—Applications in multicomponent reactions under benign conditions. Green Chem. 2013, 15, 1226–1231. [Google Scholar]
- Gawande, M.B.; Guo, H.; Rathi, A.K.; Branco, P.S.; Chen, Y.; Varma, R.S.; Peng, D.-L. First application of core-shell Ag@Ni magnetic nanocatalyst for transfer hydrogenation reactions of aromatic nitro and carbonyl compounds. RSC Adv. 2013, 3, 1050–1054. [Google Scholar]
- Vaddula, B.R.; Saha, A.; Leazer, J.; Varma, R.S. A simple and facile Heck-type arylation of alkenes with diaryliodonium salts using magnetically recoverable Pd-catalyst. Green Chem. 2012, 14, 2133–2136. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. A highly active and magnetically retrievable nanoferrite-DOPA-copper catalyst for the coupling of thiophenols with aryl halides. Chem. Commun. 2012, 48, 2582–2584. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Baruwati, B.; Varma, R.S. Nanoparticle-supported and magnetically recoverable nickel catalyst: A robust and economic hydrogenation and transfer hydrogenation protocol. Green Chem. 2009, 11, 127–131. [Google Scholar] [CrossRef]
- Baruwati, B.; Polshettiwar, V.; Varma, R.S. Magnetically recoverable supported ruthenium catalyst for hydrogenation of alkynes and transfer hydrogenation of carbonyl compounds. Tetrahedron Lett. 2009, 50, 1215–1218. [Google Scholar] [CrossRef]
- Gawande, M.B.; Rathi, A.; Nogueira, I.D.; Varma, R.S.; Branco, P.S. Magnetite-supported sulfonic acid: A retrievable nanocatalyst for Ritter reaction and multicomponent reactions. Green Chem. 2013, 15, 1895–1899. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Gawande, M.B.; Rathi, A.; Nogueira, I.D.; Ghumman, C.A.A.; Bundaleski, N.; Teodoro, O.M.N.D.; Branco, P.S. A recyclable ferrite-Co magnetic nanocatalyst for the oxidation of alcohols to carbonyl compounds. ChemPlusChem 2012, 77, 865–871. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S.; Nogueira, I.D.; Ghumman, C.A.A.; Bundaleski, N.; Santos, A.; Teodoro, O.M.N.D.; Luque, R. Catalytic applications of a versatile magnetically separable Fe-Mo (Nanocat-Fe-Mo) nanocatalyst. Green Chem. 2013, 15, 682–689. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, C.; Sun, Z.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Magnetically recyclable nanocatalysts (MRNCs): A versatile integration of high catalytic activity and facile recovery. Nanoscale 2012, 4, 6244–6255. [Google Scholar] [CrossRef]
- Cano, R.; Yus, M.; Ramon, D.J. First practical cross-alkylation of primary alcohols with a new and recyclable impregnated iridium on magnetite catalyst. Chem. Commun. 2012, 48, 7628–7630. [Google Scholar] [CrossRef]
- Zeng, T.Q.; Yang, L.; Hudson, R.; Song, G.H.; Moores, A.R.; Li, C.J. Fe3O4 nanoparticle-supported copper(I) pybox catalyst: Magnetically recoverable catalyst for enantioselective direct-addition of terminal alkynes to imines. Org. Lett. 2011, 13, 442–445. [Google Scholar]
- Schatz, A.; Hager, M.; Reiser, O. Cu(II)-Azabis(oxazoline)-complexes immobilized on superparamagnetic magnetite@silica-nanoparticles: A highly selective and recyclable catalyst for the kinetic resolution of 1,2-diols. Adv. Funct. Mater. 2009, 19, 2109–2115. [Google Scholar] [CrossRef]
- Gawande, M.B.; Rathi, A.K.; Branco, P.S.; Nogueira, I.D.; Velhinho, A.; Shrikhande, J.J.; Indulkar, U.U.; Jayaram, R.V.; Ghumman, C.A.A.; Bundaleski, N.; et al. Regio- and chemoselective reduction of nitroarenes and carbonyl compounds over recyclable magnetic ferrite-nickel nanoparticles (Fe3O4-Ni) by using glycerol as a hydrogen source. Chem. Eur. J. 2012, 18, 12628–12632. [Google Scholar]
- Gawande, M.B.; Velhinho, A.; Nogueira, I.D.; Ghumman, C.A.A.; Teodoro, O.M.N.D.; Branco, P.S. A facile synthesis of cysteine-ferrite magnetic nanoparticles for application in multicomponent reactions-a sustainable protocol. RSC Adv. 2012, 2, 6144–6149. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, H.; Luo, J.; Wei, Y. “Click” magnetic nanoparticle-supported palladium catalyst: A phosphine-free, highly efficient and magnetically recoverable catalyst for Suzuki-Miyaura coupling reactions. Catal. Sci. Technol. 2013, 3, 235–243. [Google Scholar] [CrossRef]
- Hudson, R.; Riviere, A.; Cirtiu, C.M.; Luska, K.L.; Moores, A. Iron-iron oxide core-shell nanoparticles are active and magnetically recyclable olefin and alkyne hydrogenation catalysts in protic and aqueous media. Chem. Commun. 2012, 48, 3360–3362. [Google Scholar] [CrossRef]
- Gawande, M.B.; Bonifacio, V.D.B.; Luque, R.; Branco, P.S.; Varma, R.S. Benign by design: Catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem. Soc. Rev. 2013, 42, 5522–5551. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S. An efficient and expeditious Fmoc protection of amines and amino acids in aqueous media. Green Chem. 2011, 13, 3355–3359. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Aqueous microwave chemistry: A clean and green synthetic tool for rapid drug discovery. Chem. Soc. Rev. 2008, 37, 1546–1557. [Google Scholar] [CrossRef]
- Simon, M.-O.; Li, C.-J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev 2012, 41, 1415–1427. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Microwave-assisted organic synthesis and transformations using benign reaction media. Acc. Chem. Res. 2008, 41, 629–639. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Baruwati, B.; Varma, R.S. Magnetic nanoparticle-supported glutathione: A conceptually sustainable organocatalyst. Chem. Commun. 2009, 1837–1839. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Nanoparticle-supported and magnetically recoverable ruthenium hydroxide catalyst: Efficient hydration of nitriles to amides in aqueous medium. Chem. Eur. J. 2009, 15, 1582–1586. [Google Scholar] [CrossRef]
- Garcia-Garrido, S.E.; Francos, J.; Cadierno, V.; Basset, J.M.; Polshettiwar, V. Chemistry by nanocatalysis: First example of a solid-supported rapta complex for organic reactions in aqueous medium. ChemSusChem 2011, 4, 104–111. [Google Scholar] [CrossRef]
- Hildebrand, H.; Mackenzie, K.; Kopinke, F.-D. Highly active pd-on-magnetite nanocatalysts for aqueous phase hydrodechlorination reactions. Environ. Sci. Technol. 2009, 43, 3254–3259. [Google Scholar] [CrossRef]
- Rezaeifard, A.; Jafarpour, M.; Farshid, P.; Naeimi, A. Nanomagnet-supported partially brominated manganese-porphyrin as a promising catalyst for the selective heterogeneous oxidation of hydrocarbons and sulfides in water. Eur. J. Inorg. Chem. 2012, 2012, 5515–5524. [Google Scholar]
- Baig, R.B.N.; Varma, R.S. Magnetically retrievable catalysts for organic synthesis. Chem. Commun. 2013, 49, 752–770. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Nano-organocatalyst: Magnetically retrievable ferrite-anchored glutathione for microwave-assisted Paal-Knorr reaction, aza-Michael addition, and pyrazole synthesis. Tetrahedron 2010, 66, 1091–1097. [Google Scholar] [CrossRef]
- Dydio, P.; Lichosyt, D.; Jurczak, J. Amide- and urea-functionalized pyrroles and benzopyrroles as synthetic, neutral anion receptors. Chem. Soc. Rev. 2011, 40, 2971–2985. [Google Scholar] [CrossRef]
- Ma, F.-P.; Li, P.-H.; Li, B.-L.; Mo, L.-P.; Liu, N.; Kang, H.-J.; Liu, Y.-N.; Zhang, Z.-H. A recyclable magnetic nanoparticles supported antimony catalyst for the synthesis of N-substituted pyrroles in water. Appl. Catal. A 2013, 457, 34–41. [Google Scholar] [CrossRef]
- Wang, B.G.; Ma, B.C.; Wang, Q.; Wang, W. Superparamagnetic nanoparticle-supported (s)-diphenylprolinol trimethylsilyl ether as a recyclable catalyst for asymmetric michael addition in water. Adv. Synth. Catal. 2010, 352, 2923–2928. [Google Scholar] [CrossRef]
- Saha, A.; Leazer, J.; Varma, R.S. O-Allylation of phenols with allylic acetates in aqueous media using a magnetically separable catalytic system. Green Chem. 2012, 14, 67–71. [Google Scholar] [CrossRef]
- Tanaka, S.; Saburi, H.; Ishibashi, Y.; Kitamura, M. (CpRuPF6)-P-II/quinaldic acid-catalyzed chemoselective allyl ether cleavage. A simple and practical method for hydroxyl deprotection. Org. Lett. 2004, 6, 1873–1875. [Google Scholar] [CrossRef]
- Williamson, A.W. XXII.—On etherification. J. Chem. Soc. 1852, 4, 229–239. [Google Scholar]
- Rajabi, F.; Karimi, N.; Saidi, M.R.; Primo, A.; Varma, R.S.; Luque, R. Unprecedented selective oxidation of styrene derivatives using a supported iron oxide nanocatalyst in aqueous medium. Adv. Synth. Catal. 2012, 354, 1707–1711. [Google Scholar] [CrossRef]
- Shipley, H.J.; Engates, K.E.; Guettner, A.M. Study of iron oxide nanoparticles in soil for remediation of arsenic. J. Nanopart. Res. 2011, 13, 2387–2397. [Google Scholar] [CrossRef]
- Beygzadeh, M.; Alizadeh, A.; Khodaei, M.M.; Kordestani, D. Biguanide/Pd(OAc)2 immobilized on magnetic nanoparticle as a recyclable catalyst for the heterogeneous Suzuki reaction in aqueous media. Catal. Commun. 2013, 32, 86–91. [Google Scholar]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Yin, J.J.; Rainka, M.P.; Zhang, X.X.; Buchwald, S.L. A highly active Suzuki catalyst for the synthesis of sterically hindered biaryls: Novel ligand coordination. J. Am. Chem. Soc. 2002, 124, 1162–1163. [Google Scholar]
- Deng, J.; Mo, L.-P.; Zhao, F.-Y.; Zhang, Z.-H.; Liu, S.-X. One-pot, three-component synthesis of a library of spirooxindole-pyrimidines catalyzed by magnetic nanoparticle supported dodecyl benzenesulfonic acid in aqueous media. ACS Comb. Sci. 2012, 14, 335–341. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Kochi, J.K. Metal-Catalysed Oxidation of Organic Compounds; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Hudlicky, M. Oxidation in Organic Chemsitry (ACS Monograph Series); ACS: Washington, DC, USA, 1990. [Google Scholar]
- Aurich, H.G.; Hahn, K.; Stork, K.; Weiss, W. Aminyl oxides (nitroxides). 24. Empirical determination of spin-density distribution in aminyl oxides. Tetrahedron 1977, 33, 969–975. [Google Scholar] [CrossRef]
- Karimi, B.; Farhangi, E. A highly recyclable magnetic core-shell nanoparticle-supported tempo catalyst for efficient metal- and halogen-free aerobic oxidation of alcohols in water. Chem. Eur. J. 2011, 17, 6056–6060. [Google Scholar] [CrossRef]
- Kiasat, A.R.; Nazari, S. Magnetic nanoparticles grafted with beta-cyclodextrin-polyurethane polymer as a novel nanomagnetic polymer brush catalyst for nucleophilic substitution reactions of benzyl halides in water. J. Mol. Catal. A 2012, 365, 80–86. [Google Scholar] [CrossRef]
- Can, K.; Ozmen, M.; Ersoz, M. Immobilization of albumin on aminosilane modified superparamagnetic magnetite nanoparticles and its characterization. Colloids Surf. B 2009, 71, 154–159. [Google Scholar]
- Fujii, S.; Hamasaki, H.; Abe, H.; Yamanaka, S.; Ohtaka, A.; Nakamura, E.; Nakamura, Y. One-step synthesis of magnetic iron-conducting polymer-palladium ternary nanocomposite microspheres with applications as a recyclable catalyst. J. Mater. Chem. A 2013, 1, 4427–4430. [Google Scholar]
- Kale, S.R.; Kahandal, S.S.; Gawande, M.B.; Jayaram, R.V. Magnetically recyclable γ-Fe2O3-HAP nanoparticles for the cycloaddition reaction of alkynes, halides and azides in aqueous media. RSC Adv. 2013, 3, 8184–8192. [Google Scholar]
- Meldal, M.; Tornoe, C.W. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. A highly active magnetically recoverable nano ferrite-glutathione-copper (nano-FGT-Cu) catalyst for Huisgen 1,3-dipolar cycloadditions. Green Chem. 2012, 14, 625–632. [Google Scholar] [CrossRef]
- Hudson, R.; Li, C.J.; Moores, A. Magnetic copper-iron nanoparticles as simple heterogeneous catalysts for the azide-alkyne click reaction in water. Green Chem. 2012, 14, 622–624. [Google Scholar] [CrossRef]
- Yeo, K.M.; Lee, S.I.; Lee, Y.T.; Chung, Y.K.; Lee, I.S. Core-satellite heterostruture of Fe3O4-Pd nanocomposite: Selective and magnetically recyclable catalyst for decarboxylative coupling reaction in aqueous media. Chem. Lett. 2008, 37, 116–117. [Google Scholar] [CrossRef]
- Mori, K.; Yoshioka, N.; Kondo, Y.; Takeuchi, T.; Yamashita, H. Catalytically active, magnetically separable, and water-soluble FePt nanoparticles modified with cyclodextrin for aqueous hydrogenation reactions. Green Chem. 2009, 11, 1337–1342. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, G.; Gu, H.; Huang, T.; Zhang, Y.; Li, H. Magnetically recoverable SiO2-coated Fe3O4 nanoparticles: A new platform for asymmetric transfer hydrogenation of aromatic ketones in aqueous medium. Chem. Commun. 2011, 47, 2583–2585. [Google Scholar] [CrossRef]
- Rezaeifard, A.; Jafarpour, M.; Naeimi, A.; Haddad, R. Aqueous heterogeneous oxygenation of hydrocarbons and sulfides catalyzed by recoverable magnetite nanoparticles coated with copper(II) phthalocyanine. Green Chem. 2012, 14, 3386–3394. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gawande, M.B.; Rathi, A.K.; Branco, P.S.; Varma, R.S. Sustainable Utility of Magnetically Recyclable Nano-Catalysts in Water: Applications in Organic Synthesis. Appl. Sci. 2013, 3, 656-674. https://doi.org/10.3390/app3040656
Gawande MB, Rathi AK, Branco PS, Varma RS. Sustainable Utility of Magnetically Recyclable Nano-Catalysts in Water: Applications in Organic Synthesis. Applied Sciences. 2013; 3(4):656-674. https://doi.org/10.3390/app3040656
Chicago/Turabian StyleGawande, Manoj B., Anuj K. Rathi, Paula S. Branco, and Rajender S. Varma. 2013. "Sustainable Utility of Magnetically Recyclable Nano-Catalysts in Water: Applications in Organic Synthesis" Applied Sciences 3, no. 4: 656-674. https://doi.org/10.3390/app3040656