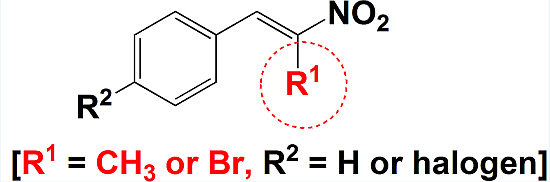
Comparisons of Halogenated β-Nitrostyrenes as Antimicrobial Agents
Abstract
:1. Introduction

2. Experimental Section
2.1. Methods and Materials

2.2. General β-Nitrostyrene Synthesis Method
3. Results and Discussion
3.1. Analysis of Assays
| Strain | A-1 | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 29213 | 16 | 16 | 32 | 16 | 128 | 32 | 32 | 32 |
| Bacillus subtilis ATCC 6633 | 16 | 16 | 32 | 8 | 128 | 32 | 64 | 64 |
| Enterococcus faecalis ATCC 29212 | 32 | 512 | 64 | 64 | 128 | 256 | 128 | 128 |
| Escherichia coli ATCC 25922 | 362 | 512 | 197 | 181 | 362 | 128 | 128 | 128 |
| Candida albicans ATCC 10231 | 23 | 44 | 23 | 23 | 362 | 23 | 44 | 23 |
| Strain | A-1 | I | D | F |
|---|---|---|---|---|
| Staphylococcus aureus ATCC 29213 | 32 | 32 | 32 | 32 |
| Bacillus subtilise ATCC 6633 | 16 | 16 | 16 | 32 |
| Enterococcus faecalis ATCC 29212 | 64 | 128 | 128 | 256 |
| Escherichia coli ATCC 25922 | 512 | 256 | 256 | 128 |
| Candida albicans ATCC 10231 | 32 | 32 | 32 | 32 |
3.2. Mechanism of Antibacterial Activity

| Strain |  |  |
|---|---|---|
| Staphylococcus aureus | 128 | 2 |
| Bacillus subtilis | 256 | 2 |
| Enterococcus faecalis | 64 | 5.5 |
| Escherichia coli | 256 | 27 |
| Candida albicans | 32 | 2 |
3.3. Summary of SARs of the Antimicrobial Activity of Nitrostyrenes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klutmans, J. Enterobacteria: Ban resistant strains from food chain. Nature 2013, 501, 316. [Google Scholar] [CrossRef]
- McKenna, M. Antibiotic resistance: The last resort. Nature 2013, 499, 394–396. [Google Scholar] [CrossRef]
- Fischer, J.; Rodrïguez, I.; Schmoger, S.; Friese, A.; Roesler, U.; Helmuth, R.; Guerra, B. Salmonella enterica subsp. Enterica producing VIM-1 carbapenemase isolated from livestock farms. J. Antimicrob. Chemother. 2013, 68, 478–480. [Google Scholar] [CrossRef]
- Magnes, A.R.; Johnson, J.R. Food-borne origins of Escherichia coli causing extra intestinal infections. Clin. Infect. Dis. 2012, 55, 712–719. [Google Scholar] [CrossRef]
- Smith, R.; Coast, J. The true cost of antimicrobial resistance. Br. Med. J. 2013, 346, f1493. [Google Scholar] [CrossRef]
- Pettit, R.K.; Pettit, G.R.; Hamel, E.; Hogan, F.; Moser, B.R.; Wolf, S.; Pon, S.; Chapuis, J.C.; Schmid, J.M. E-Combretastatin and E-resveratrol structural modifications: Antimicrobial and cancer cell growth inhibitory β-E-nitrostyrenes. Bioorg. Med. Chem. 2009, 17, 6606–6612. [Google Scholar] [CrossRef]
- Galano, J.J.; Alias, M.; Pérez, R.; Velåzquez-Campoy, A.; Hoffman, P.S.; Sanch, J. Improved flavodoxin inhibitors with potential therapeutic effects against Heliobacter pylori infection. J. Med. Chem. 2013, 56, 6248–6258. [Google Scholar] [CrossRef]
- Nicoletti, A.; White, K.S. β-Nitrostyrene Derivative Protein Tyrosine Phosphatase Modulators, and Their Therapeutic Use. Patent WO/2008/061308, 29 May 2008. [Google Scholar]
- Valdez, C.A.; Tripp, J.C.; Miyamoto, Y.; Kalisiak, J.; Hruz, P.; Anderson, Y.S.; Brown, S.E.; Kangas, K.; Arzu, L.V.; Davids, B.J.; et al. Synthesis and electrochemistry of 2-Ethenyl and 2-Ethanyl derivatives of 5-Nitroimidazole and antimicrobial activity against Giardia lamblia. J. Med. Chem. 2009, 52, 4038–4053. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Kalisiak, J.; Korthals, K.; Lauwaet, T.; Cheung, D.Y.; Lozano, R.; Cobo, E.R.; Upcroft, P.; Upcroft, J.A.; Berg, D.E.; et al. Expanded therapeutic potential in activity space of next-generation 5-nitroimidazole antimicrobials with broad structural diversity. Proc. Natl. Acad. Sci. USA 2013, 110, 17564–17569. [Google Scholar] [CrossRef]
- Scholz, T.; Heyl, C.L.; Bernardi, D.; Zimmermann, S.; Kattner, L.; Klein, C.D. Chemical, biochemical and microbiological properties of a brominated nitrovinylfuran with broad-spectrum antibacterial activity. Bioorg. Med. Chem. 2013, 21, 795–804. [Google Scholar] [CrossRef]
- Lo, K.; Cornell, H.; Nicoletti, G.; Jackson, N.; Hügel, H. A study of fluorinated β-nitrostyrenes as antimicrobial agents. Appl. Sci. 2012, 2, 114–128. [Google Scholar] [CrossRef]
- Nicoletti, G.; Cornell, H.; Hügel, H.M.; White, K.S.; Nguyen, T.; Zalizniak, L.; Nugegoda, D. Synthesis and antimicrobial activity of nitroalkenyl arenes. Anti-Infective Agents 2013, 11, 179–191. [Google Scholar] [CrossRef]
- Fleming, P.J.; Wallace, J.J. How not to lie with statistics: The correct way to summarize benchmark results. Commun. ACM 1986, 29, 218–221. [Google Scholar] [CrossRef]
- White, K.S. The Antimicrobial Mechanism of Action of 3,4-Methylenedioxy-β-Nitropropene. Ph.D. Thesis, RMIT University, Melbourne, Australia, March 2008. [Google Scholar]
- Wang, W.Y.; Hsieh, P.W.; Wu, Y.C.; Wu, C.C. Synthesis and pharmacological evaluation of novel β-nitrostyrene derivatives as tyrosine kinase inhibitors with potent antiplatelet activity. Biochem. Pharmacol. 2007, 74, 601–611. [Google Scholar] [CrossRef]
- Hsieh, P.W.; Chang, Y.T.; Chuang, W.Y.; Shih, H.C.; Chiang, S.Z.; Wu, C.C. The synthesis and biologic evaluation of anti-platelet and cytotoxic β-nitrostyrenes. Bioorg. Med. Chem. 2010, 18, 7621–7627. [Google Scholar] [CrossRef]
- He, Y.; Varadarajan, S.; Muñoz-Planillo, R.; Burberry, A.; Nakamura, Y.; Nüñez, G. 3,4-Methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J. Biol. Chem. 2014, 289, 1142–1150. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Miao, Y.; Geertsema, E.M.; Tepper, P.G.; Zandvoort, E.; Poelarend, G.J. Promiscuous catalysis of asymmetric michael-type additions of linear aldehydes to β-nitrostyrene by the proline-based Enzyme 4-Oxalocrotonate tautomerase. ChemBioChem 2013, 14, 191–194. [Google Scholar] [CrossRef]
- Naranic, T.; Radiojevic, J.; Jovanovic, P.; Francuski, D.; Bigovic, M.; Maslak, V.; Savic, V.; Vasiljevic, B.; O’Connor, K.E.; Nikodinovic-Runic, J. Highly efficient Michael-type addition of acetaldehyde to β-nitrostyrenes by whole resting cells of Escherichia coli expressing 4-oxalocrotonate tautomerase. Bioresour. Technol. 2013, 142, 462–468. [Google Scholar] [CrossRef]
- Park, J.; Pei, D. Trans-β-nitrostyrene derivatives as slow-binding inhibitors of protein tyrosine phosphatases. Biochemistry 2004, 43, 15014–15021. [Google Scholar] [CrossRef]
- Lu, L.Q.; Cao, Y.J.; Liu, X.P.; An, J.; Yao, C.J.; Ming, Z.H.; Xiao, W.J. A new entry to cascade organocatalysis: Reactions of stable sulfur ylides and nitroolefins sequentially catalyzed by thiourea and dmap. J. Am. Chem. Soc. 2008, 130, 6946–6948. [Google Scholar]
- Baker, L.M.S.; Baker, P.R.S.; Golin-Bisello, F.; Schopfer, F.J.; Fink, M.; Woodcock, S.R.; Branchaud, B.P.; Radi, R.; Freeman, B.A. Nitro-fatty acid reaction with glutathione and cysteine: Kinetic analysis of thiol alkylation by a Michael addition reaction. J. Biol. Chem. 2007, 282, 31085–31093. [Google Scholar] [CrossRef]
- Bernasconi, C.F.; Schuck, D.F. Kinetics of reversible thiolate ion addition to substituted beta-nitrostyrenes in water. Radicaloid transition state or principle of nonperfect synchronization? J. Org. Chem. 1992, 57, 2365–2373. [Google Scholar] [CrossRef]
- Berner, O.M.; Tedeschi, L.; Enders, D. Asymmetric michael additions to nitroalkenes. Eur. J. Org. Chem. 2002, 2002, 1877–1894. [Google Scholar] [CrossRef]
- Milhazes, N.; Calheiros, R.; Marques, M.P.M.; Garrido, J.; Cordeiro, M.N.D.S.; Rodrigues, C.; Quinteira, S.; Novais, C.; Peixe, L.; Borges, F. β-Nitrostyrene derivatives as potential antibacterial agents: A structure-property-activity relationship study. Bioorg. Med. Chem. 2006, 14, 4078–4088. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cornell, H.; Nguyen, T.; Nicoletti, G.; Jackson, N.; Hügel, H. Comparisons of Halogenated β-Nitrostyrenes as Antimicrobial Agents. Appl. Sci. 2014, 4, 380-389. https://doi.org/10.3390/app4030380
Cornell H, Nguyen T, Nicoletti G, Jackson N, Hügel H. Comparisons of Halogenated β-Nitrostyrenes as Antimicrobial Agents. Applied Sciences. 2014; 4(3):380-389. https://doi.org/10.3390/app4030380
Chicago/Turabian StyleCornell, Hugh, Thu Nguyen, Gina Nicoletti, Neale Jackson, and Helmut Hügel. 2014. "Comparisons of Halogenated β-Nitrostyrenes as Antimicrobial Agents" Applied Sciences 4, no. 3: 380-389. https://doi.org/10.3390/app4030380