Improved Permeate Flux of PVDF Ultrafiltration Membrane Containing PVDF-g-PHEA Synthesized via ATRP
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials and Reagents
2.2. Synthesis of PVDF-g-PHEA Copolymer

2.3. Preparation of Membranes
| Membranes | PVDF(g) | DMSO(g) | PVDF-g-PHEA (g) |
|---|---|---|---|
| A-1 | 9.5 g | 90.50 g | 0.0 g |
| B-1 | 10.0 g | 90.00 g | 0.0 g |
| A-2 | 9.0 g | 90.40 g | 0.6 g |
| B-2 | 9.0 g | 90.10 g | 0.9 g |
2.4. FT-IR and NMR Analysis
2.5. TGA (Thermogravimetric Analysis) Performance
3. Membrane Characterization
3.1. Contact Angle Analysis
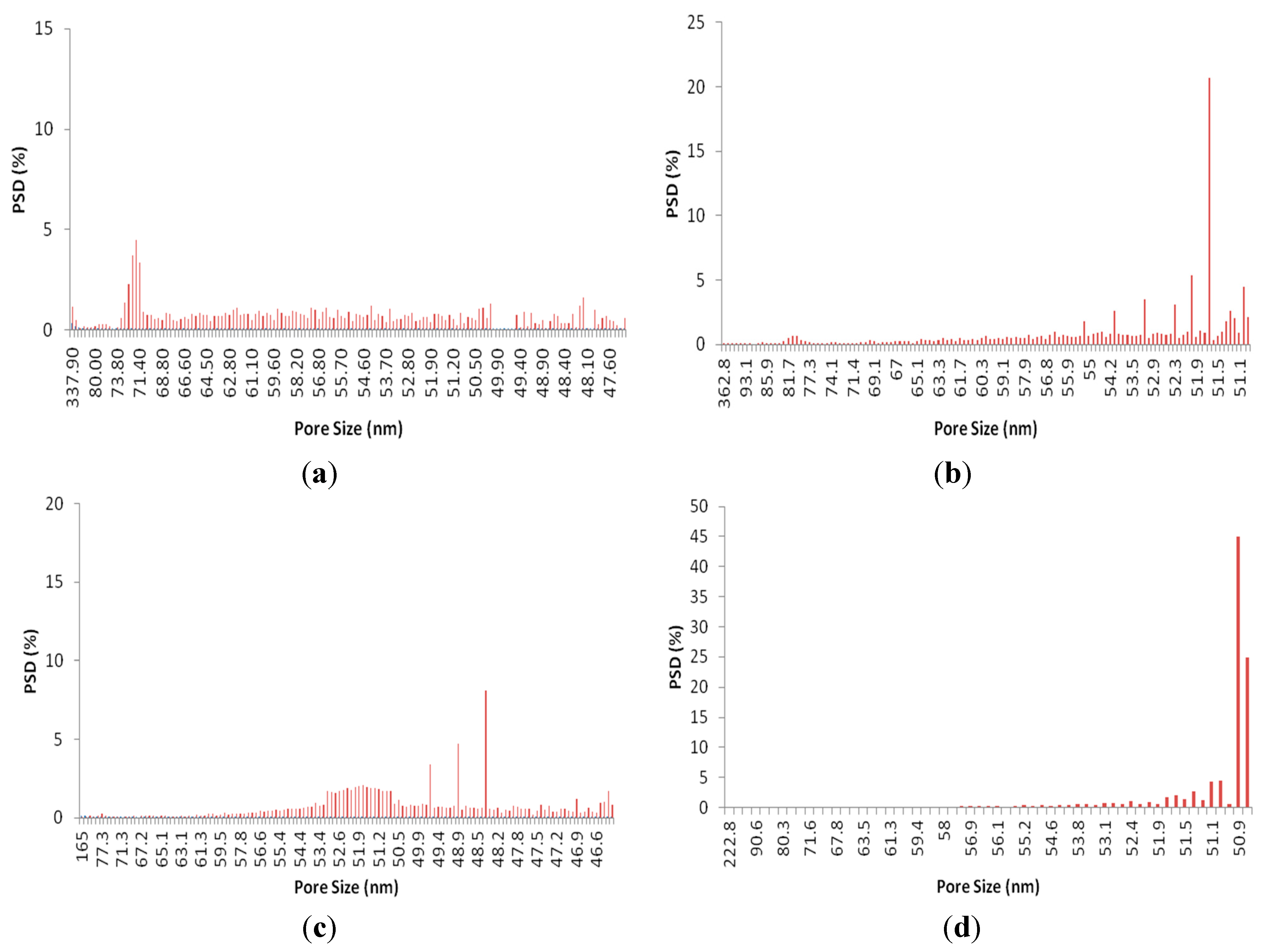
3.2. Pore Size Measurement
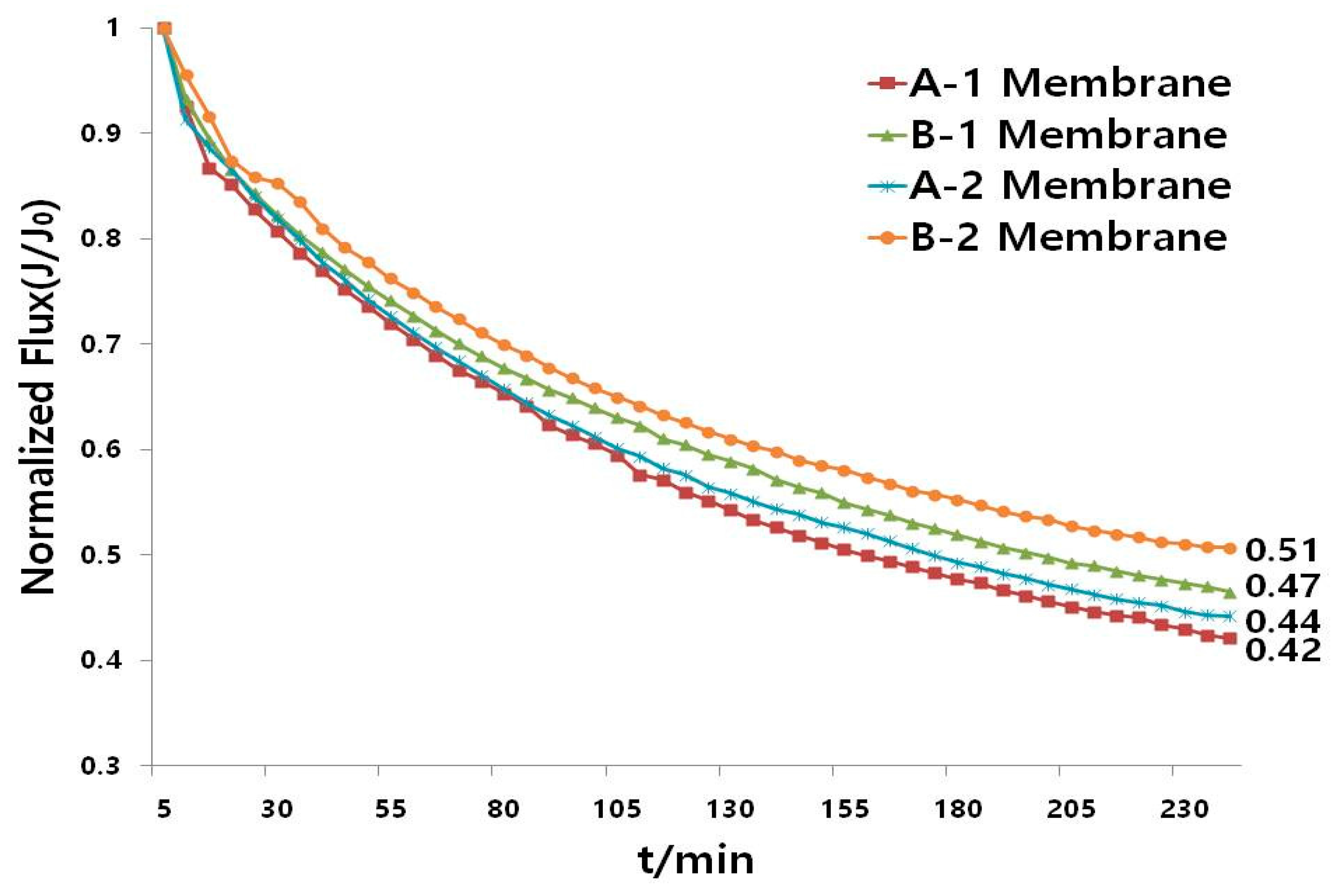
3.3. Flux Characterization
3.4. Humic Acid Rejection Test
3.5. SEM (Scanning Electron Microscopy) Analysis
4. Results and Discussion



5. Characterization of Membranes
5.1. Contact Angle Analysis
5.2. Pore size and distribution measurement
| Membrane | A-1 | A-2 | B-1 | B-2 |
|---|---|---|---|---|
| Contact Angle | 68.6° | 64.3 | 68.0° | 61.8° |
| Mean pore size (nm) | 60.6 | 60.5 | 53.4 | 53.2 |
5.3. Morphology Analysis






6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mehrparvar, A.; Rahimpour, A.; Jahanshahi, M. Modified ultrafiltration membranes for humic acid removal. J. Taiwan Inst. Chem. Eng. 2014, 45, 275–282. [Google Scholar] [CrossRef]
- Liu, B.; Chen, C.; Zhang, W.; Crittenden, J.; Chen, Y. Low-cost antifouling PVC ultrafiltration membrane fabrication with Pluronic F 127: Effect of additives on properties and performance. Desalination 2012, 307, 26–33. [Google Scholar] [CrossRef]
- Woo, S.H.; Park, J.; Min, B. Relationship between permeate flux and surface roughness of membranes with similar water conract angle values. Sep. Purif. Technol. 2015, 146, 187–191. [Google Scholar] [CrossRef]
- Yan, L.; Wang, J. Development of a new polymer membrane—PVB/PVDF blended membrane. Desalination 2011, 281, 455–461. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Liu, Z.; Deng, B.; Yu, M.; Li, L.; Jiang, H.; Li, J. Preparation of the antifouling microfiltration membranes from poly(N,N-dimethylacrylamide) grafted poly(vinylidene fluoride) (PVDF) powder. J. Mater. Chem. 2011, 21, 11908–11915. [Google Scholar] [CrossRef]
- Meng, J.; Chen, C.; Huang, L.; Du, Q.; Zhang, Y. Surface modification of PVDF membrane via AGET ATRP directly from the membrane surface. Appl. Surf. Sci. 2011, 257, 6282–6290. [Google Scholar] [CrossRef]
- Hashim, N.A.; Liu, F.; Li, K. A simplified method for preraration of hydrophilic PVDF membranes from an amphiphilic graft copolymer. J. Membr. Sci. 2009, 345, 134–141. [Google Scholar] [CrossRef]
- Abed, M.R.M.; Kumbharkar, S.C.; Groth, A.M.; Li, K. Economical production of PVDF-g-POEM for use as a blend in preparation of PVDF based hydrophilic hollow fibre membranes. Sep. Purif. Technol. 2013, 106, 47–55. [Google Scholar] [CrossRef]
- Woo, S.H.; Kim, K.; Park, J.; Min, B. Preparation and Characterization of Poly(vinylidene fluoride) (PVDF) Membrane. Chem. Lett. 2015, 44, 85–87. [Google Scholar] [CrossRef]
- Sui, Y.; Wang, Z.; Gao, X.; Gao, C. Antifouling PVDF ultrafiltration membranes incorporating PVDF-g-PHEMA additive via atom transfer radical graft polymerizations. J. Membr. Sci. 2012, 413–414, 38–47. [Google Scholar] [CrossRef]
- Hashim, N.A.; Liu, F.; Abed, M.R.M.; Li, K. Chemistry in spinning solutions: Surface modification of PVDF membranes during phase inversion. J. Membr. Sci. 2012, 415–416, 399–411. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, D.; Deng, Q.; He, X.; Wang, X. Atom Transfer Radical Polymerization Directly from Poly(vinylidene fluoride): Surface and Antifouling Properties. J. Polym. Sci. A Polym. Chem. 2006, 44, 3434–3443. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L. “Smart” Membrane Materials: Preparation and Characterization of PVDF-g-PNIPAAm Graft Copolymer. J. Appl. Polym. Sci. 2007, 104, 1482–1486. [Google Scholar] [CrossRef]
- Liu, B.; Chen, C.; Li, T.; Crittenden, C.; Chen, Y. High performance ultrafiltration membrane composed of PVDF blended with its derivative copolymer PVDF-g-PEGMA. J. Membr. Sci. 2013, 445, 66–75. [Google Scholar] [CrossRef]
- Akthakul, A.; Salinaro, R.F.; Mayes, A.M. Antifouling Polymer Membranes with Subnanometer Size Selectivity. Macromolucules 2004, 37, 7663–7668. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M. Precipitation and characterization of membranes formed by nonsolvent induced phase separation: A review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Woo, S.H.; Lee, J.S.; Lee, H.H.; Park, J.; Min, B. Preparation method of crack-free PVDF microfiltration membrane with enhanced antifouling characteristics. Appl. Mater. Interfaces 2015, 7, 16466–16477. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Maruyama, T.; Sotani, T.; Matsuyama, H. Effect of surface morphology on membrane fouling by humic acid with the use of cellulose acetate butyrate hollow fiber membranes. J. Membr. Sci. 2008, 320, 483–491. [Google Scholar] [CrossRef]
- Kim, Y.W.; Park, J.T.; Koh, J.H.; Roh, D.K.; Kim, J.H. Anhydrous proton conducting membranes based on crosslinked graft copolymer electrolytes. J. Membr. Sci. 2008, 325, 319–325. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, D.K.; Lee, K.J.; Kim, J.H. Single-step synthesis of proton conducting poly(vinylidene fluoride) (PVDF) graft copolymer electrolytes. Eur. Polym. J. 2008, 44, 932–939. [Google Scholar] [CrossRef]
- Jena, A.; Gupta, K. Characterization of pore structure of filtration media. Fluid Part. Sep. J. 2002, 14, 227–241. [Google Scholar]
- Li, D.; Frey, M.W.; Joo, Y.L. Characterization of nanofibrous membranes with capillary flow porometry. J. Membr. Sci. 2006, 286, 104–114. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, R.; Tian, M.; Qiu, C.; Fane, A.G. Fabrication of polyvinylidene fluoride (PVDF) nanofiber membranes by electro-spinning for direct contact membrane distillation. J. Membr. Sci. 2013, 425, 30–39. [Google Scholar] [CrossRef]
- Lee, N.; Amy, G.; Croué, J.-P.; Buisson, H. Morphological analyses of natural organic matter (NOM) fouling of low-pressure membranes (MF/UF). J. Membr. Sci. 2005, 261, 7–16. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.K.; Kim, S.C. Surface structure and phase separation mechanism of polysulfone membranes by atomic force microscopy. J. Membr. Sci. 1999, 163, 159–166. [Google Scholar] [CrossRef]
- Tao, M.; Liu, F.; Xue, L. Hydrophilic poly(vinylidene fluoride) (PVDF) membrane by in situ polymerisation of 2-hydroxyethyl methacrylate (HEMA) and micro-phase separation. J. Mater. Chem. 2012, 22, 9131–9137. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-M.; Woo, S.H.; Lee, J.S.; Park, H.S.; Park, J.; Min, B.R. Improved Permeate Flux of PVDF Ultrafiltration Membrane Containing PVDF-g-PHEA Synthesized via ATRP. Appl. Sci. 2015, 5, 1992-2008. https://doi.org/10.3390/app5041992
Kim K-M, Woo SH, Lee JS, Park HS, Park J, Min BR. Improved Permeate Flux of PVDF Ultrafiltration Membrane Containing PVDF-g-PHEA Synthesized via ATRP. Applied Sciences. 2015; 5(4):1992-2008. https://doi.org/10.3390/app5041992
Chicago/Turabian StyleKim, Kwang-Mo, Sahng Hyuck Woo, Ju Sung Lee, Hyun Sic Park, Jinwon Park, and Byoung Ryul Min. 2015. "Improved Permeate Flux of PVDF Ultrafiltration Membrane Containing PVDF-g-PHEA Synthesized via ATRP" Applied Sciences 5, no. 4: 1992-2008. https://doi.org/10.3390/app5041992






