Valorization of Agri-Food Waste via Fermentation: Production of l-lactic Acid as a Building Block for the Synthesis of Biopolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. LAB Strains and Chemicals
2.2. Preparation of Raw Materials
2.3. LAB Screening Conditions
2.4. 1L-Scale Batch Fermentation Conditions
2.5. Analytical Methods
3. Results and Discussion
3.1. LAB Screening and Selection
3.2. Waste Chemical Characterization and Fermentation Mash Preparation
3.3. 1L Scale Batch Fermentation
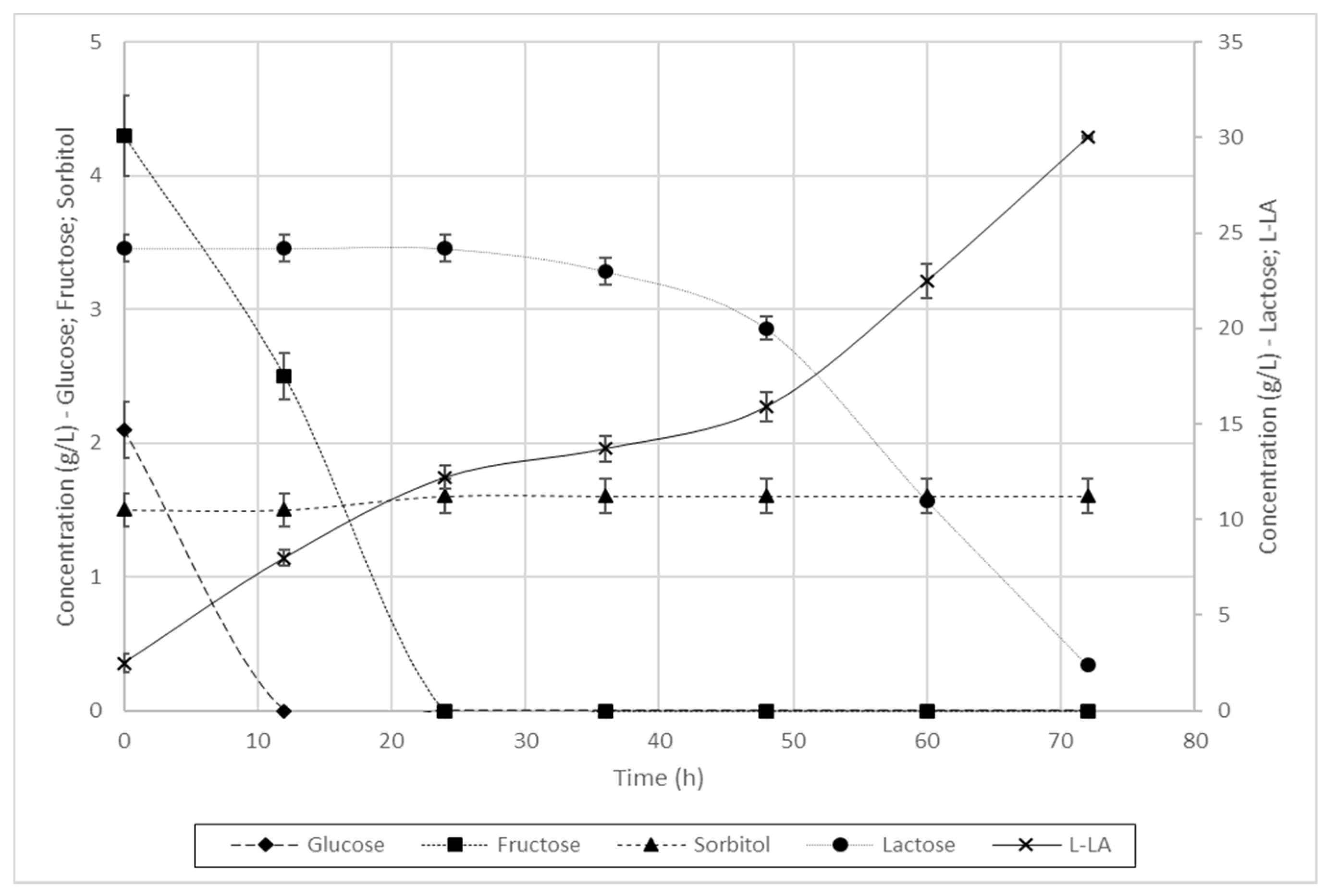
3.3.1. Fermentation of L. casei
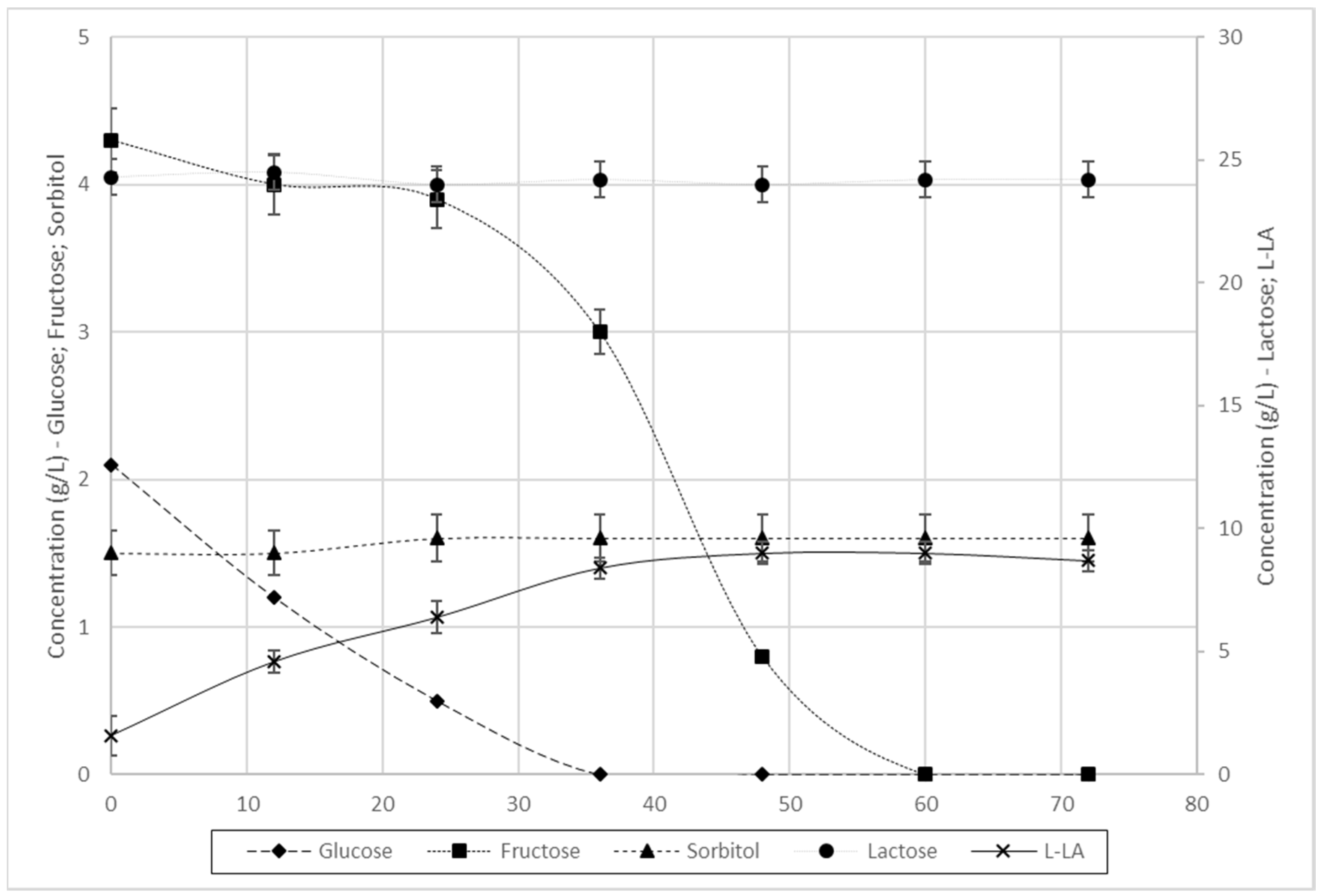
3.3.2. Fermentation of L. farciminis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pleissner, D.; Qi, Q.; Gao, C.; Rivero, C.P.; Webb, C.; Lin, C.S.K.; Venus, J. Valorization of organic residues for the production of added value chemicals: A contribution to the bio-based economy. Biochem. Eng. J. 2015, 116, 3–16. [Google Scholar] [CrossRef]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, J.E.; de Klerk-Engels, B.; Struik, P.C.; Rabbinge, R. Securing renewable resource supplies for changing market demands in a bio-based economy. Ind. Crops Prod. 2005, 21, 129–144. [Google Scholar] [CrossRef]
- Paes, B.G.; Almeida, J.R. Genetic improvement of microorganisms for applications in biorefineries. Chem. Biol. Technol. Agric. 2014, 1, 1–21. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.U. Systems strategies for developing industrial microbial strains. Nat. Biotechnol. 2015, 33, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q. From a co-production design to an integrated single-cell biorefinery. Biotechnol. Adv. 2014, 32, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T. Biotechnological potential of agro-industrial residues. I: Sugarcane bagasse. Bioresour. Technol. 2000, 74, 69–80. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T.; Vandenberghe, L.P.; Mohan, R. Biotechnological potential of agro-industrial residues. II: Cassava bagasse. Bioresour. Technol. 2000, 74, 81–87. [Google Scholar] [CrossRef]
- Xue, M.; Liu, D.; Zhang, H.; Qi, H.; Lei, Z. A pilot process of solid state fermentation from sugar beet pulp for the production of microbial protein. J. Ferment. Bioeng. 1997, 73, 203–205. [Google Scholar] [CrossRef]
- Antier, P.; Minjares, A.; Roussos, S.; Raimbault, M.; Viniegra-Gonzalez, G. Pectinase-hyperproducing mutants of Aspergillus niger C28B25 for solid-state fermentation of coffee pulp. Enzyme Microb. Technol. 1993, 15, 254–260. [Google Scholar] [CrossRef]
- Joshi, V.K.; Parmar, M.; Rana, N.S. Pectin esterase production from apple pomace in solid-state and submerged fermentations. Food Technol. Biotechnol. 2006, 44, 213–217. [Google Scholar]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications—A review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Chen, H. Single cell oil production in solid-state fermentation by Microsphaeropsis sp. from steam-exploded wheat straw mixed with wheat bran. Bioresour. Technol. 2008, 99, 3885–3889. [Google Scholar] [CrossRef] [PubMed]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass—Volume I; U.S. Department of Energy: Oak Ridge, TN, USA, 2004.
- Martinez, F.A.C.; Balciunas, E.M.; Salgado, J.M.; González, J.M.D.; Converti, A.; de Souza Oliveira, R.P. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Ghaffar, T.; Irshad, M.; Anwar, Z.; Aqil, T.; Zulifqar, Z.; Tariq, A.; Mehmood, S. Recent trends in lactic acid biotechnology: A brief review on production to purification. J. Radiat. Res. Appl. Sci. 2014, 7, 222–229. [Google Scholar] [CrossRef]
- Global Lactic Acid and Poly Lactic Acid (PLA) Market by Application (Packaging, Agriculture, Transport, Electronics, Textiles) Expected to Reach USD 4,312.2 Million and USD 2,169.6 Million Respectively by 2020: Grand View Research, Inc. Available online: https://www.grandviewresearch.com/press-release/global-lactic-acid-and-poly-lactic-acid-market (accessed on 25 July 2016).
- Wang, Y.; Tashiro, Y.; Sonomoto, K. Fermentative production of lactic acid from renewable materials: Recent achievements, prospects, and limits. J. Biosci. Bioeng. 2015, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Makino, M.; Kaku, N.; Koyama, M.; Nakamura, K.; Sasano, K. Fermentative l-lactic acid production from non-sterilized rice washing drainage containing rice bran by a newly isolated lactic acid bacteria without any additions of nutrients. J. Biosci. Bioeng. 2013, 115, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Srikanth, K.; Limaye, A.M.; Sivaprakasam, S. Homo-fermentative production of d-lactic acid by Lactobacillus sp. employing casein whey permeate as a raw feed-stock. Biotechnol. Lett. 2014, 36, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- McCaskey, T.A.; Zhou, S.D.; Britt, S.N.; Strickland, R. Bioconversion of municipal solid waste to lactic acid by Lactobacillus species. Appl. Biochem. Biotechnol. 1994, 45, 555–563. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Kim, J.S.; Hwang, H.J.; Park, M.S.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Kim, J.C. Production of l-lactic acid from a green microalga, Hydrodictyon reticulum, by Lactobacillus paracasei LA104 isolated from the traditional Korean food, makgeolli. Bioresour. Technol. 2012, 110, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Qiao, H.; Zheng, Z.; Chu, Q.; Li, X.; Yong, Q.; Ouyang, J. Lactic acid production from pretreated hydrolysates of corn stover by a newly developed bacillus coagulans strain. PLoS ONE 2016, 11, e0149101. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Idris, A. Simultaneous saccharification and fermentation of lactic acid from empty fruit bunch at high solids loading. BioResources 2016, 11, 3799–3812. [Google Scholar] [CrossRef]
- Mazzoli, R.; Bosco, F.; Mizrahi, I.; Bayer, E.A.; Pessione, E. Towards lactic acid bacteria-based biorefineries. Biotechnol. Adv. 2014, 32, 1216–1236. [Google Scholar] [CrossRef] [PubMed]
- Kandler, O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 1983, 49, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Van de Guchte, M.; Serror, P.; Chervaux, C.; Smokvina, T.; Ehrlich, S.D.; Maguin, E. Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 2002, 82, 187–216. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.; Naveena, B.J.; Reddy, G. Use of inexpensive nitrogen sources and starch for l (+) lactic acid production in anaerobic submerged fermentation. Bioresour. Technol. 2007, 98, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xie, N.; Guo, L.; Wang, L.; Yu, B.; Ma, Y. Efficient open fermentative production of polymer-grade l-lactate from sugarcane bagasse hydrolysate by thermotolerant Bacillus sp. strain P38. PLoS ONE 2014, 9, e107143. [Google Scholar] [CrossRef] [PubMed]
- Sansonetti, S.; Curcio, S.; Calabrò, V.; Iorio, G. Bio-ethanol production by fermentation of ricotta cheese whey as an effective alternative non-vegetable source. Biomass Bioenergy 2009, 33, 1687–1692. [Google Scholar] [CrossRef]
- Silva, J.; Carvalho, A.S.; Ferreira, R.; Vitorino, R.; Amado, F.; Domingues, P.; Gibbs, P.A. Effect of the pH of growth on the survival of Lactobacillus delbrueckii subsp. bulgaricus to stress conditions during spray-drying. J. Appl. Bacteriol. 2005, 98, 775–782. [Google Scholar] [CrossRef] [PubMed]
- DeMan, C.J.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Jones, D.B. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentages of Proteins; US Department of Agriculture: Washington, DC, USA, 1941; pp. 1–22.
- Condon, S. Aerobic metabolism of lactic acid bacteria. Irish J. Food Sci. Technol. 1983, 7, 15–25. [Google Scholar]
- Condon, S. Responses of lactic acid bacteria to oxygen. FEMS Microbiol. Rev. 1987, 3, 269–280. [Google Scholar] [CrossRef]
- Hofvendahl, K.; Hahn-Hagerdal, B. Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb. Technol. 2000, 26, 87–107. [Google Scholar] [CrossRef]
- Sheeladevi, A. Lactic acid production using lactic acid bacteria under optimized conditions. Int. J. Pharm. Biol. Arch. 2011, 2, 1686–1691. [Google Scholar]
- Reuter, G. Lactobacillus alimentarius sp. nov., nom rev. and Lactobacillus farciminis sp. nov., nom. rev. Syst. Appl. Microbiol. 1983, 4, 277–279. [Google Scholar] [CrossRef]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of poly (lactic acid): A review. J. Macromol. Sci. C Polym. Rev. 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Highly efficient l-lactic acid production from xylose in cell recycle continuous fermentation using Enterococcus mundtii QU 25. RSC Adv. 2016, 6, 17659–17668. [Google Scholar] [CrossRef]
- Bai, D.M.; Wei, Q.; Yan, Z.H.; Zhao, X.M.; Li, X.G.; Xu, S.M. Fed-batch fermentation of Lactobacillus lactis for hyper-production of l-lactic acid. Biotechnol. Lett. 2003, 25, 1833–1835. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.K.; Wee, Y.J.; Choi, G.W. A novel lactic acid bacterium for the production of high purity l-lactic acid, Lactobacillus paracasei subsp. paracasei CHB2121. J. Biosci. Bioeng. 2012, 114, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Yebra, M.J.; Pérez-Martínez, G. Cross-talk between the l-sorbose and d-sorbitol (d-glucitol) metabolic pathways in Lactobacillus caseia. Microbiology 2002, 148, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Van Dedem, G.; Moo-Young, M. A model for diauxic growth. Biotechnol. Bioeng. 1975, 17, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.-H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Okano, K.; Zhang, Q.; Shinkawa, S.; Yoshida, S.; Tanaka, T.; Fukuda, H.; Kondo, A. Efficient production of optically pure d-lactic acid from raw corn starch by using a genetically modified l-lactate dehydrogenase gene-deficient and α-amylase-secreting Lactobacillus plantarum strain. Appl. Environ. Microbiol. 2009, 75, 462–467. [Google Scholar] [CrossRef] [PubMed]
| # | Genus | Species | DSMZ Code | ATCC Code |
|---|---|---|---|---|
| 1 | Lactobacillus | alimentarius | 20249 | 29643 |
| 2 | Lactobacillus | bifermentans | 20003 | 35409 |
| 3 | Lactobacillus | casei | 20011 | 393 |
| 4 | Lactobacillus | cellobiosus | 20055 | 11739 |
| 5 | Lactobacillus | collinoides | 20515 | 27612 |
| 6 | Lactobacillus | farciminis | 20184 | 29644 |
| 7 | Lactobacillus | curvatus | 20019 | 25601 |
| 8 | Lactobacillus | fructosus | 20349 | 13162 |
| 9 | Lactobacillus | viridescens | 20190 | 35410 |
| 10 | Lactobacillus | hilgardii | 20051 | - |
| 11 | Lactobacillus | kefiri | 20587 | 35411 |
| 12 | Lactobacillus | jensenii | 20557 | 25258 |
| 13 | Lactobacillus | fermentum | 20049 | - |
| 14 | Lactobacillus | fermentum | 20052 | 14931 |
| 15 | Lactobacillus | kandleri | 20593 | 51149 |
| 16 | Lactobacillus | reuteri | 20015 | - |
| 17 | Lactobacillus | reuteri | 20016 | 23272 |
| 18 | Lactobacillus | salivarius | 20555 | 11741 |
| 19 | Lactobacillus | sakei | 20017 | 15521 |
| Strain | Aerobic | Microaerophilic | Anaerobic | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YLA/S | μ | Suptake | qS | qLA | YLA/S | μ | Suptake | qS | qLA | YLA/S | μ | Suptake | qS | qLA | |
| (%) | (h−1) | (%) | (g/g·h) | (g/g·h) | (%) | (h−1) | (%) | (g/g·h) | (g/g·h) | (%) | (h−1) | (%) | (g/g·h) | (g/g·h) | |
| L. alimentarius | 0% | 0.012 | 100% | 0.23 | 0.00 | 0% | 0.011 | 100% | 0.24 | 0.00 | 68% | 0.010 | 80% | 0.21 | 0.14 |
| L. bifermentans | 16% | 0.009 | 89% | 0.25 | 0.04 | 8% | 0.002 | 98% | 0.37 | 0.03 | 27% | 0.001 | 100% | 0.40 | 0.11 |
| L. casei | 80% | 0.015 | 99% | 0.22 | 0.18 | 80% | 0.018 | 99% | 2.25 | 0.20 | 98% | 0.024 | 100% | 0.13 | 0.13 |
| L. cellobiosus | 15% | 0.002 | 23% | 0.09 | 0.01 | 21% | 0.018 | 99% | 0.18 | 0.04 | 44% | 0.018 | 96% | 0.17 | 0.08 |
| L. collinoides | 10% | 0.001 | 87% | 1.70 | 0.17 | 9% | 0.004 | 97% | 0.34 | 0.03 | 38% | 0.001 | 96% | 0.44 | 0.17 |
| L. farciminis | 71% | 0.016 | 100% | 0.18 | 0.13 | 78% | 0.001 | 99% | 1.84 | 1.45 | 92% | 0.016 | 99% | 0.19 | 0.18 |
| L. curvatus | 0% | 0.001 | 20% | 0.46 | 0.00 | 0% | 0.001 | 50% | 1.39 | 0.00 | 0% | 0.001 | 50% | 2.52 | 0.00 |
| L. fructosus | 0% | 0.001 | 20% | 0.40 | 0.00 | 0% | 0.001 | 50% | 0.45 | 0.00 | 19% | 0.001 | 27% | 1.60 | 0.30 |
| L. viridescens | 36% | 0.006 | 90% | 0.28 | 0.10 | 41% | 0.002 | 99% | 0.38 | 0.15 | 9% | 0.001 | 88% | 0.35 | 0.03 |
| L. hilgardii | 11% | 0.008 | 87% | 0.25 | 0.03 | 12% | 0.002 | 98% | 0.37 | 0.04 | 20% | 0.001 | 98% | 0.43 | 0.09 |
| L. kefiri | 13% | 0.008 | 75% | 0.22 | 0.03 | 13% | 0.009 | 75% | 0.21 | 0.03 | 1% | 0.001 | 75% | 0.40 | 0.00 |
| L. jensenii | 26% | 0.004 | 98% | 0.33 | 0.09 | 13% | 0.009 | 75% | 0.21 | 0.03 | 28% | 0.015 | 99% | 0.20 | 0.06 |
| L. fermentum 20049 | 61% | 0.001 | 24% | 0.16 | 0.10 | 12% | 0.009 | 99% | 0.27 | 0.03 | 0% | 0.001 | 50% | 2.61 | 0.00 |
| L. fermentum 20052 | 19% | 0.006 | 99% | 0.31 | 0.06 | 28% | 0.005 | 98% | 0.32 | 0.09 | 18% | 0.001 | 99% | 0.44 | 0.08 |
| W. kandleri | 11% | 0.001 | 100% | 0.97 | 0.35 | 10% | 0.001 | 100% | 1.14 | 0.11 | 57% | 0.001 | 79% | 0.45 | 0.25 |
| L. reuteri 20015 | 0% | 0.001 | 12% | 0.27 | 0.00 | 0% | 0.001 | 25% | 0.78 | 0.00 | 0% | 0.001 | 31% | 0.62 | 0.00 |
| L. reuteri 20016 | 32% | 0.022 | 99% | 0.14 | 0.05 | 13% | 0.016 | 98% | 0.19 | 0.03 | 14% | 0.013 | 99% | 0.22 | 0.03 |
| L. salivarius | 0% | 0.003 | 61% | 0.22 | 0.00 | 0% | 0.001 | 16% | 0.22 | 0.00 | 0% | 0.001 | 96% | 1.29 | 0.00 |
| L. sakei | 0% | 0.001 | 20% | 1.11 | 0.00 | 0% | 0.001 | 20% | 1.16 | 0.00 | 0% | 0.001 | 20% | 1.19 | 0.00 |
| Strain | Aerobic | Microaerophilic | Anaerobic | ||||||
|---|---|---|---|---|---|---|---|---|---|
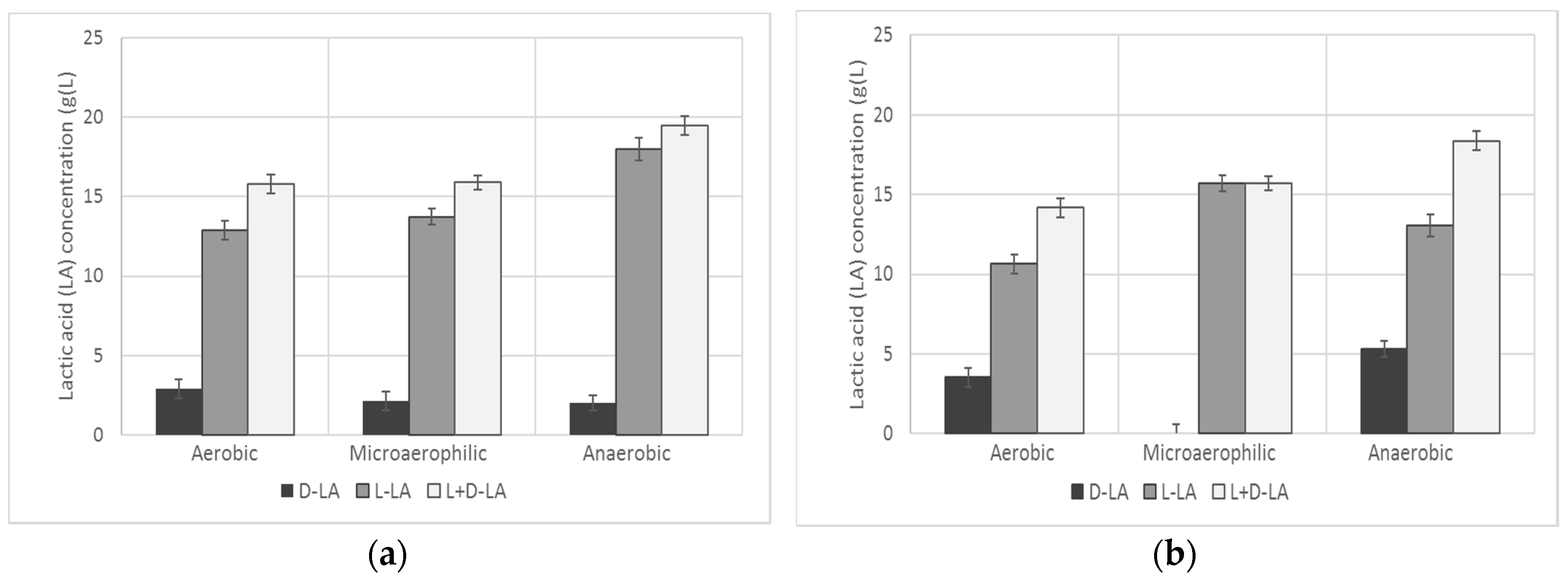
| d-LA | l-LA | l + d-LA | d-LA | l-LA | l + d-LA | d-LA | l-LA | l + d-LA | |
| (g/L) | (g/L) | (g/L) | (g/L) | (g/L) | (g/L) | (g/L) | (g/L) | (g/L) | |
| L. alimentarius | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 10.99 | 10.99 |
| L. bifermentans | 0.00 | 2.84 | 2.84 | 0.00 | 1.68 | 1.68 | 3.91 | 1.55 | 5.46 |
| L. casei | 2.92 | 12.89 | 15.81 | 2.15 | 13.74 | 15.89 | 2.02 | 18.00 | 19.50 |
| L. cellobiosus | 0.00 | 0.71 | 0.71 | 0.00 | 4.07 | 4.07 | 3.65 | 4.85 | 8.50 |
| L. collinoides | 0.00 | 1.75 | 1.75 | 0.00 | 1.68 | 1.68 | 5.26 | 2.39 | 7.65 |
| L. farciminis | 3.52 | 10.66 | 14.18 | 0.00 | 15.71 | 15.71 | 5.32 | 13.05 | 18.37 |
| L. curvatus | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| L. fructosus | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.03 | 0.00 | 1.03 |
| W. halotolerans | 4.77 | 1.84 | 6.62 | 4.33 | 3.75 | 8.07 | 0.00 | 1.75 | 1.75 |
| L. hilgardii | 0.00 | 1.94 | 1.94 | 0.00 | 2.33 | 2.33 | 2.82 | 1.16 | 3.98 |
| L. kefiri | 0.00 | 2.00 | 2.00 | 0.50 | 1.50 | 2.00 | 0.01 | 0.00 | 0.01 |
| L. jensenii | 5.19 | 0.00 | 5.19 | 0.49 | 1.51 | 2.00 | 5.58 | 0.00 | 5.58 |
| L. fermentum 20049 | 0.00 | 2.97 | 2.97 | 1.54 | 0.84 | 2.38 | 0.00 | 0.00 | 0.00 |
| L. fermentum 20052 | 1.09 | 2.72 | 3.80 | 1.79 | 3.81 | 5.61 | 1.41 | 2.26 | 3.67 |
| W. kadleri | 2.34 | 0.00 | 2.34 | 1.57 | 0.39 | 1.96 | 7.81 | 1.23 | 9.04 |
| L. reuteri 20015 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| L. reuteri 20016 | 2.69 | 3.75 | 6.44 | 1.86 | 0.84 | 2.70 | 1.54 | 1.42 | 2.96 |
| L. salivarius | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.19 | 0.00 |
| L. sakei | 0.00 | 000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Parameter | Pear Pomace (g/L) | RCW (g/L) |
|---|---|---|
| Dry matter | - | 93.89 ± 0.85 (% w/v) |
| TFS * | 85.36 ± 2.78 | 47.50 ± 1.22 |
| Glucose | 18.68 ± 1.15 | - |
| Fructose | 64.43 ± 1.51 | - |
| Lactose | - | 47.50 ± 1.22 |
| Sorbitol | 2.25 ± 0.10 | - |
| N | 0.496 ± 0.200 | 0.976 ± 0.19 |
| Protein | 3.1 | 6.1 |
| Na | 0.075 ± 0.020 (mg/L) | 0.166 ± 0.088 (mg/L) |
| K | 8.130 ± 0.700 (mg/L) | 0.858 ± 0.099 (mg/L) |
| Ca | 0.323 ± 0.092 (mg/L) | 0.040 ± 0.011 (mg/L) |
| Fe | <0.001 | <0.001 |
| Mg | 0.370 ± 0.017 (mg/L) | 0.160 ± 0.022 (mg/L) |
| pH | 4.08 ± 0.02 | 5.10 ± 0.02 |
| Microrganism | YLA/S | YLA/Si | YX/S | SupGLU | SupFRU | SupLAC | QsGLU | QsFRU | QsLAC | qLA | QLA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (g/L·h) | (g/L·h) | (g/L·h) | (g/g·h) | (g/L·h) | |||||||
| L. casei | 90% | 87% | 8% | 100% | 100% | 100% | 0.11 | 0.18 | 0.50 | 0.17 | 0.42 |
| L. farciminis | 98% | 18% | 15% | 100% | 100% | 0% | 0.11 | 0.09 | 0.00 | 0.14 | 0.13 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dedenaro, G.; Costa, S.; Rugiero, I.; Pedrini, P.; Tamburini, E. Valorization of Agri-Food Waste via Fermentation: Production of l-lactic Acid as a Building Block for the Synthesis of Biopolymers. Appl. Sci. 2016, 6, 379. https://doi.org/10.3390/app6120379
Dedenaro G, Costa S, Rugiero I, Pedrini P, Tamburini E. Valorization of Agri-Food Waste via Fermentation: Production of l-lactic Acid as a Building Block for the Synthesis of Biopolymers. Applied Sciences. 2016; 6(12):379. https://doi.org/10.3390/app6120379
Chicago/Turabian StyleDedenaro, Giovanni, Stefania Costa, Irene Rugiero, Paola Pedrini, and Elena Tamburini. 2016. "Valorization of Agri-Food Waste via Fermentation: Production of l-lactic Acid as a Building Block for the Synthesis of Biopolymers" Applied Sciences 6, no. 12: 379. https://doi.org/10.3390/app6120379







