Photoacoustic Tomography Imaging of the Adult Zebrafish by Using Unfocused and Focused High-Frequency Ultrasound Transducers
Abstract
:1. Introduction
2. Methods and Materials
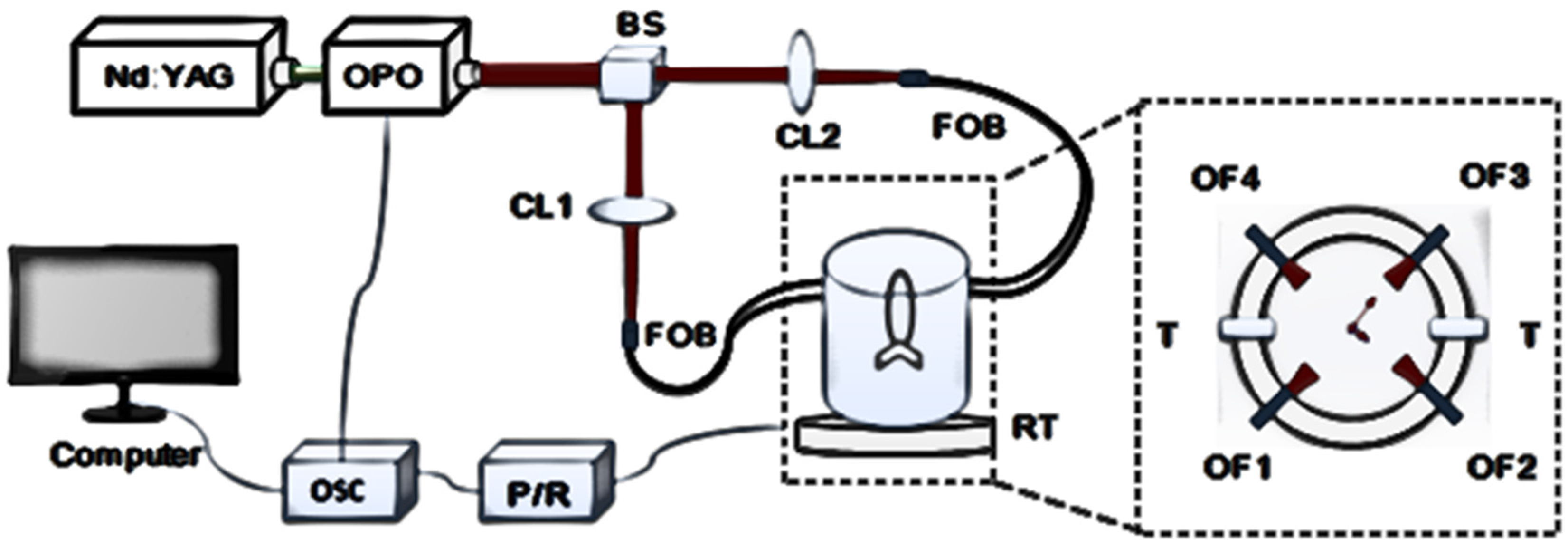
2.1. System Configuration
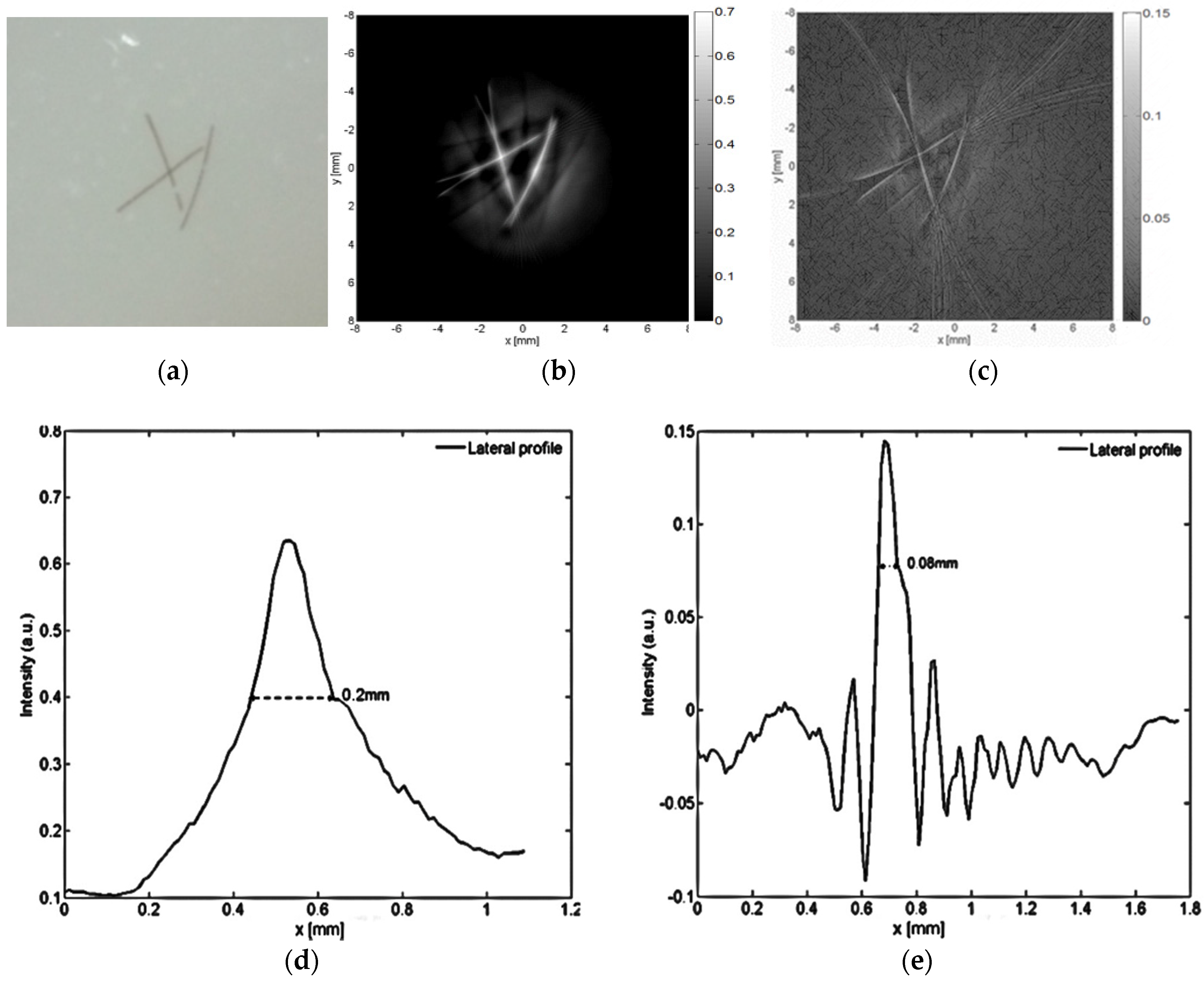
2.2. Phantom Expreimental Test
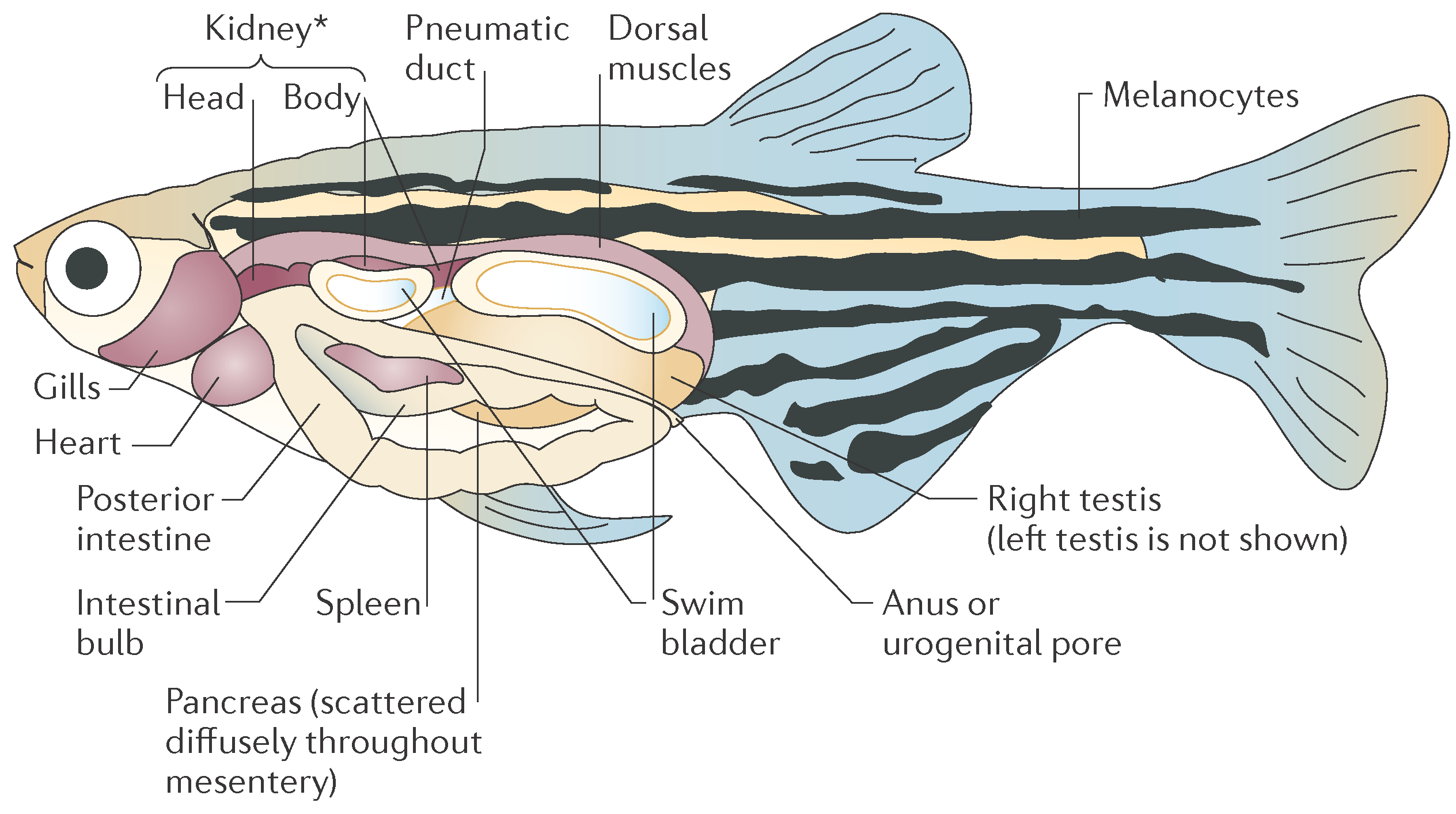
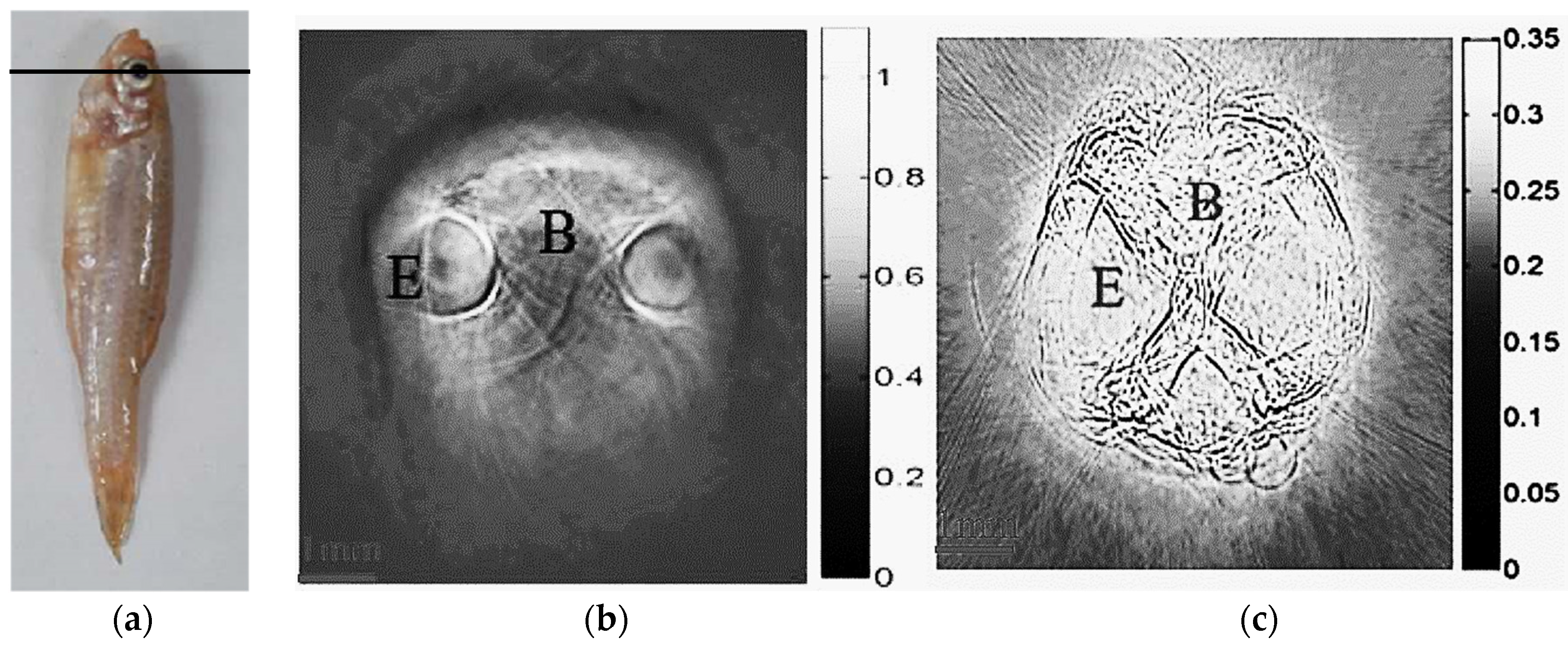
2.3. Photoacoustic Imaging of Adult Zebrafish
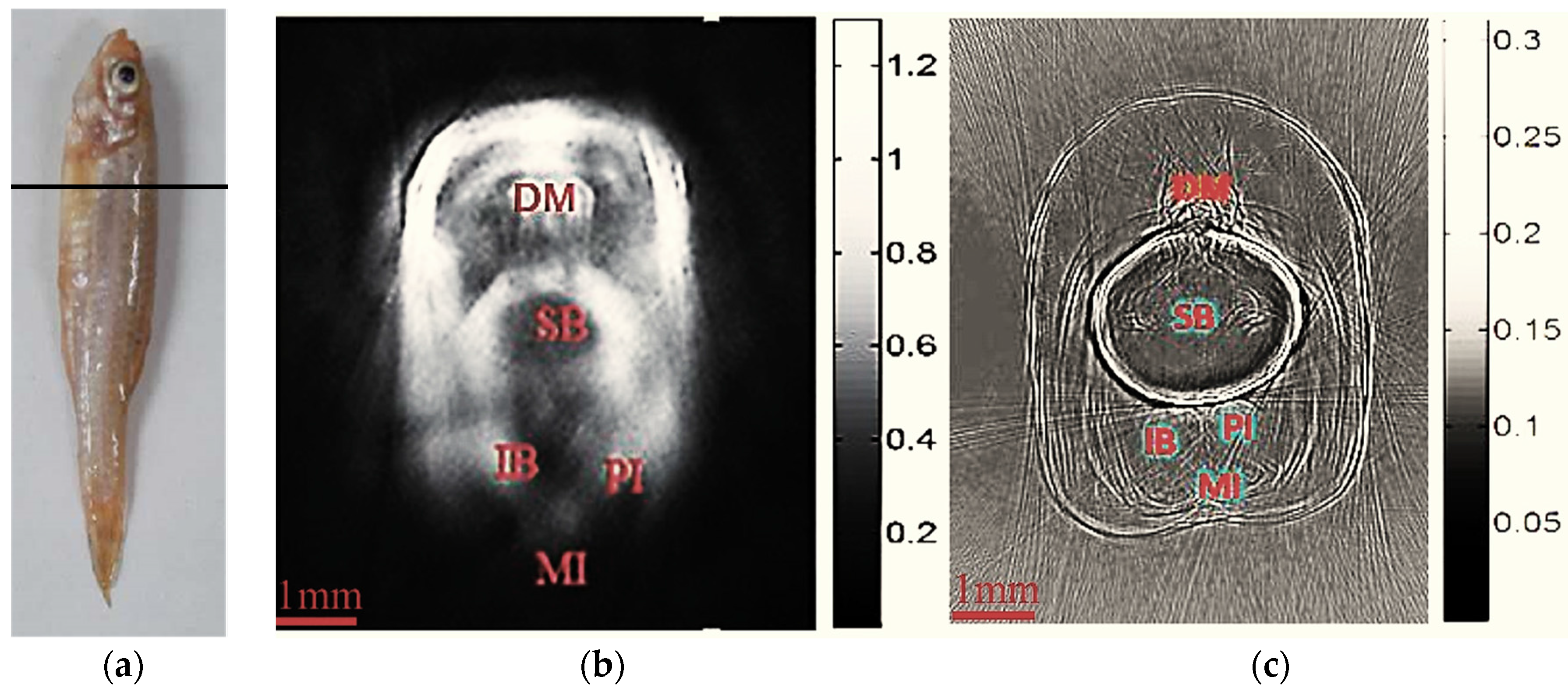
3. Results and Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ye, S.; Yang, R.; Xiong, J.; Shung, K.K.; Zhou, Q.; Li, C.; Ren, Q. Label-free imaging of zebrafish larvae in vivo by photoacoustic microscopy. Biomed. Opt. Express 2012, 3, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Distel, M.; Deán-Ben, X.L.; Ntziachristos, V.; Razansky, D. Non-invasive whole-body imaging of adult zebrafish with optoacoustic tomography. Phys. Med. Biol. 2012, 57, 7227–7237. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, A.; Shcherbakova, D.M.; Xia, J.; Wang, L.V.; Verkhusha, V.V. Multicontrast photoacoustic in vivo imaging using near-infrared fluorescent proteins. Sci. Rep. 2014, 4, 3939. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Guo, H.; Qi, W.; Zhang, Z.; Rong, J.; Yuan, Z.; Ge, W.; Jiang, H.; Xi, L. Whole-body multispectral photoacoustic imaging of adult zebrafish. Biomed. Opt. Express 2016, 7, 3543–3550. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, L.; Zhu, L.; Xia, J.; Wang, L.V. Multiview Hilbert transformation for full-view photoacoustic computed tomography using a linear array. J. Biomed. Opt. 2015, 20, 066010. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Sessa, A.; Burke, C. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, G.S.; Rieger, S.; Martin, S.M.; Cavanaugh, A.M.; Portera-Cailliau, C.; Sagasti, A. Two-photon axotomy and time-lapse confocal imaging in live zebrafish embryos. J. Vis. Exp. 2009, 24. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Yuan, Z. Two schemes for quantitative photoacoustic tomography based on Monte Carlo simulation. Med. Phys. 2016, 43, 3987–3997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Wang, L.M.; Li, Z.Y.; Yuan, Z. Retroreflective-type Janus microspheres as a novel contrast agent for enhanced optical coherence tomography. J. Biophotonics 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Z.W.; Ge, W.; Yuan, Z. Long-term in vivo monitoring of injury induced brain regeneration of the adult zebrafish by using spectral domain optical coherence tomography. Chin. Opt. Lett. 2016, 14, 081702. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Z. Multi-spectral photoacoustic elasticity tomography. Biomed. Opt. Express 2016, 7, 3323–3334. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, Z. Dual-modality imaging of the human finger joint systems by using combined multi-spectral photoacoustic computed tomography and ultrasound computed tomography. Biomed. Res. Int. 2016. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Zhou, T.; Wang, L.; Gao, D.; Zhang, X.; Liu, Y.; Wu, C.; Yuan, Z. A PIID-DTBT based semiconducting polymer dot with broad and strong optical absorption in the visible-light region as a highly-effective contrast agent for multiscale and multi-spectral photoacoustic imaging. Nano Res. 2016. [Google Scholar] [CrossRef]
- Liu, M.; Schmitner, N.; Sandrian, M.G. In vivo three-dimensional dual wavelength photoacoustic tomography imaging of the far red fluorescent protein E2-Crimson expressed in adult zebrafish. Biomed. Opt. Express 2013, 4, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhang, X.; Chiu, C.T.; Chen, R.; Shung, K.K.; Zhou, Q.; Jiao, S. Systematic study of high-frequency ultrasonic transducer design for laser-scanning photoacoustic ophthalmoscopy. J. Biomed. Opt. 2014, 19, 016015. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhang, Q.; Sobel, E.; Jiang, H. Image-guided optical spectroscopy in diagnosis of osteoarthritis: A clinical study. Biomed. Opt. Express 2010, 1, 74–86. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Rose, K.; Zon, L. Zebrafish cancer: The state of the art and the path forward. Nat. Rev. Cancer 2013, 13, 624–636. [Google Scholar] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, D.; Yuan, Z. Photoacoustic Tomography Imaging of the Adult Zebrafish by Using Unfocused and Focused High-Frequency Ultrasound Transducers. Appl. Sci. 2016, 6, 392. https://doi.org/10.3390/app6120392
Liu Y, Li D, Yuan Z. Photoacoustic Tomography Imaging of the Adult Zebrafish by Using Unfocused and Focused High-Frequency Ultrasound Transducers. Applied Sciences. 2016; 6(12):392. https://doi.org/10.3390/app6120392
Chicago/Turabian StyleLiu, Yubin, Dongliang Li, and Zhen Yuan. 2016. "Photoacoustic Tomography Imaging of the Adult Zebrafish by Using Unfocused and Focused High-Frequency Ultrasound Transducers" Applied Sciences 6, no. 12: 392. https://doi.org/10.3390/app6120392




