Characterization of Transition-Metal Oxide Deposition on Carbon Electrodes of a Supercapacitor
Abstract
:1. Introduction
2. Experimental
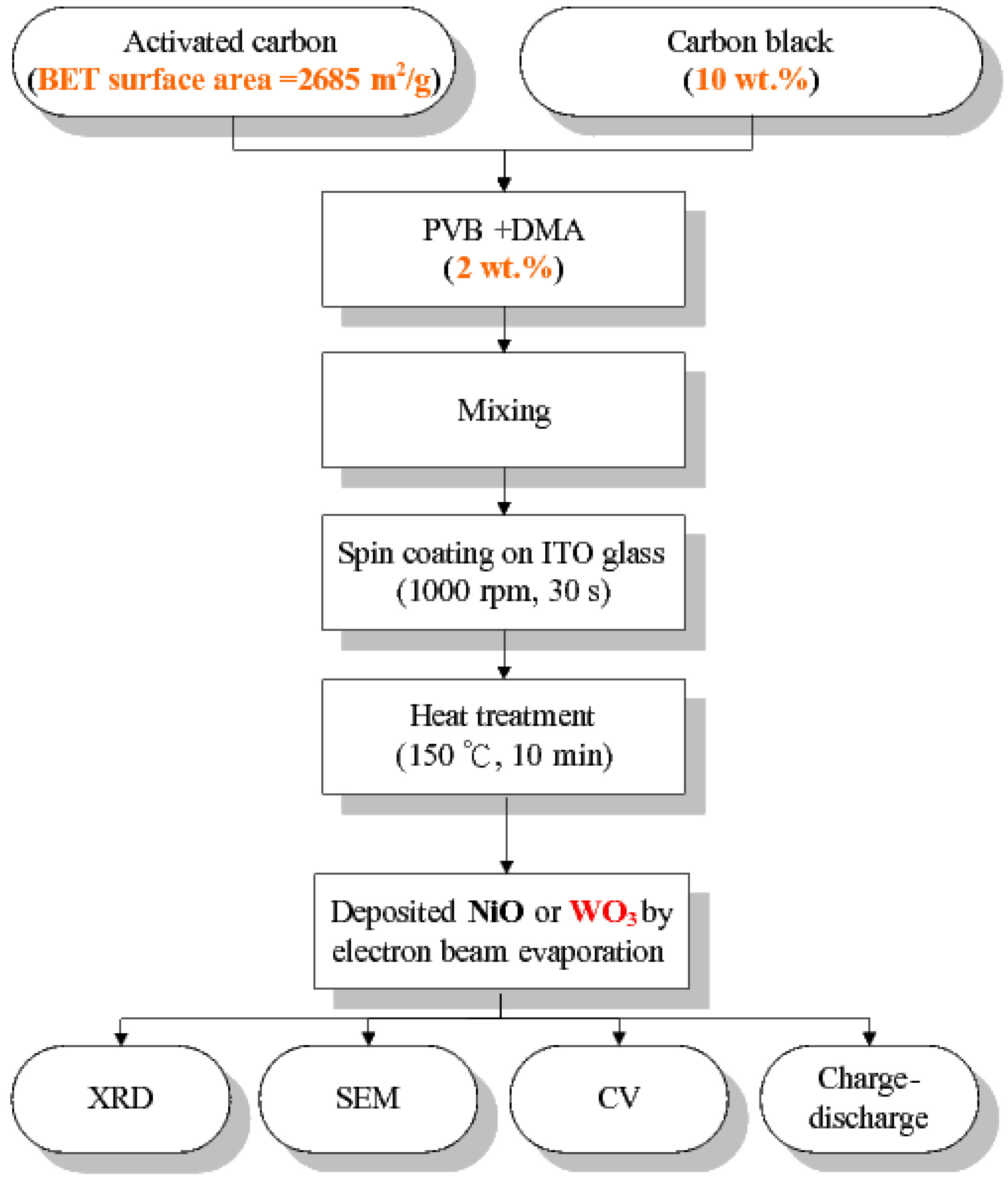
2.1. Preparation of Carbon Electrodes
2.2. Fabrication of Composite Electrodes
2.3. Characterization and Electrochemical Properties
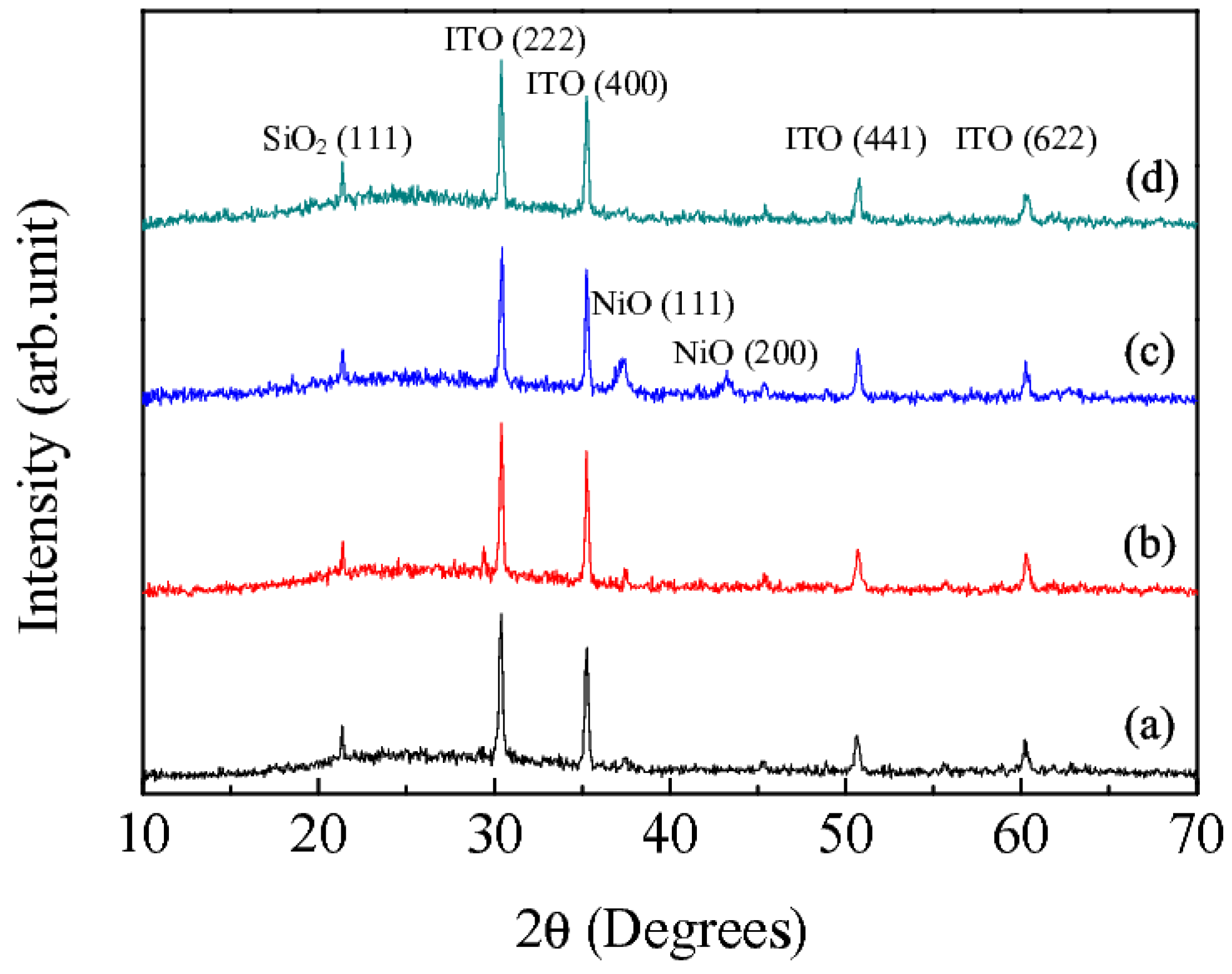
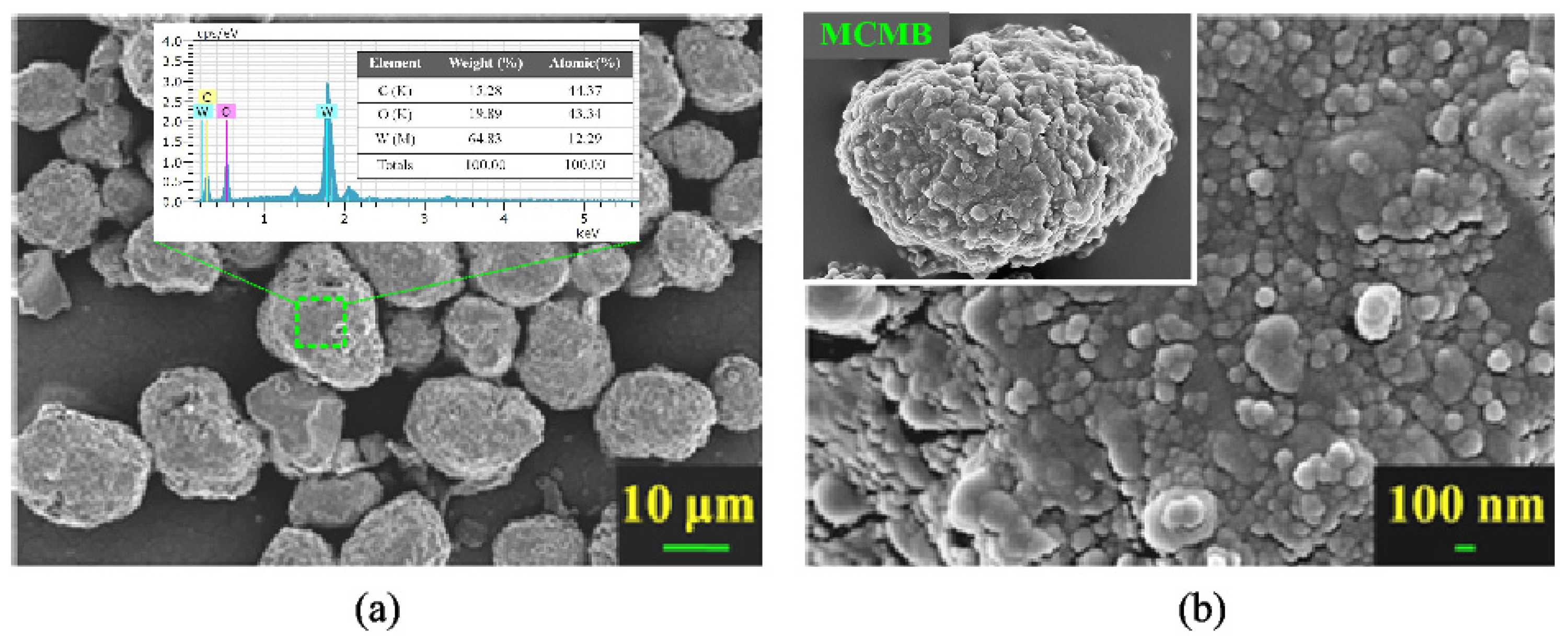
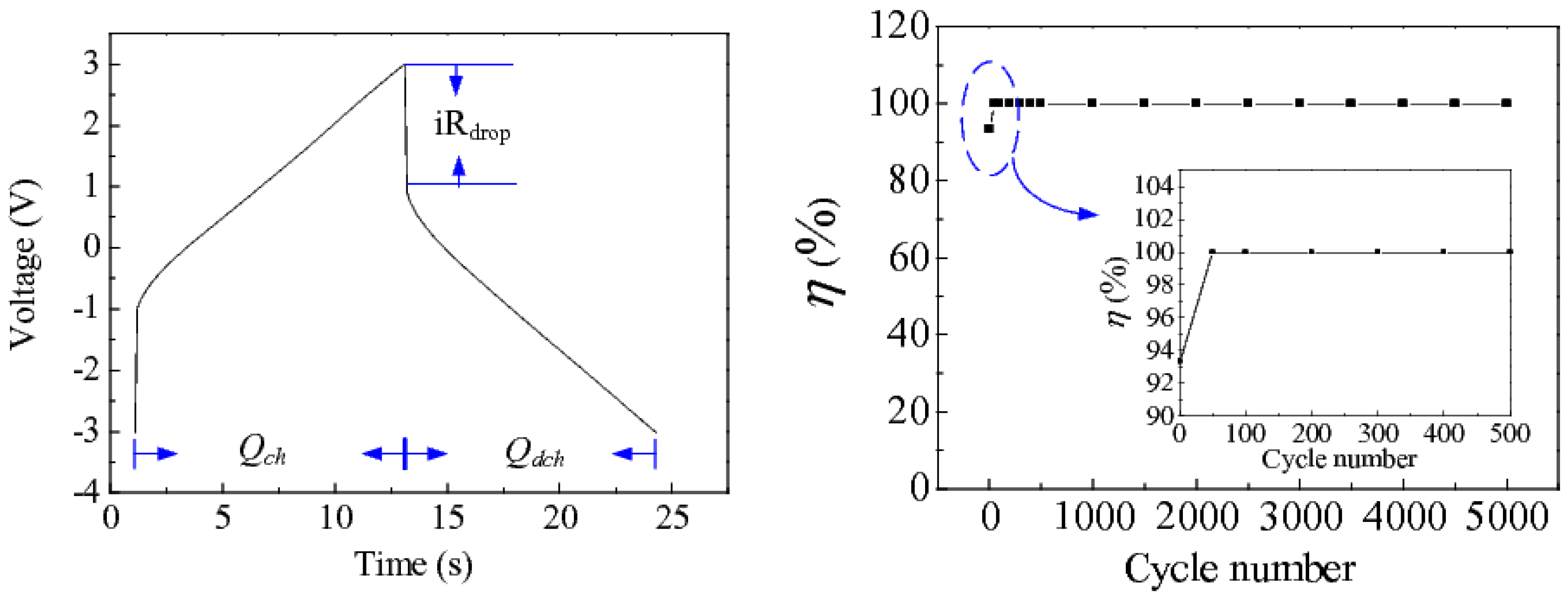
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Source 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Fritts, D.H. An analysis of electrochemical capacitors. J. Electrochem. Soc. 1997, 144, 2233–2241. [Google Scholar] [CrossRef]
- Beliakov, A.I.; Brintsev, A.M. Proceedings of the 9th International Seminar on Double Layer Capacitors and Similar Energy Storage Devices, Deerfield Beach, FL, USA, 6–8 December 1999.
- Ma, Y.; Tai, C.-W.; Gustafsson, T.; Edström, K. Recycled poly(vinyl alcohol) sponge for carbon encapsulation of size-tunable tin dioxide nanocrystalline composites. ChemSusChem 2015, 8, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tai, C.-W.; Younesi, R.; Gustafsson, T.; Lee, J.-Y.; Edström, K. Iron doping in spinel NiMn2O4: stabilization of the mesoporous cubic phase and kinetics activation toward highly reversible Li+ storage. Chem. Mater. 2015, 27, 7698–7709. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Takasu, Y.; Mizutani, S.; Kumagai, M.; Sawaguchi, S.; Murakami, Y. Ti-V-W-O/Ti oxide electrodes as candidates for electrochemical capacitors. Electrochem. Sol. State Lett. 1999, 2, 1–2. [Google Scholar] [CrossRef]
- Jeong, Y.-U.; Manthiram, A. Amorphous tungsten oxide/ruthenium oxide composites for electrochemical capacitors. J. Electrochem. Soc. 2001, 148, A189–A193. [Google Scholar] [CrossRef]
- Chang, K.-H.; Hu, C.-C.; Huang, C.-M.; Liu, Y.-L.; Chang, C.-I. Microwave-assisted hydrothermal synthesis of crystalline WO3–WO3·0.5H2O mixtures for pseudocapacitors of the asymmetric type. J. Power Source 2011, 196, 2387–2392. [Google Scholar] [CrossRef]
- Yuan, G.-H.; Jiang, Z.-H.; Aramata, A.; Gao, Y.Z. Electrochemical behavior of activated-carbon capacitor material loaded with nickel oxide. Carbon 2005, 43, 2913–2917. [Google Scholar] [CrossRef]
- Wu, M.-S.; Wang, M.-J.; Jow, J.-J. Fabrication of porous nickel oxide film with open macropores by electrophoresis and electrodeposition for electrochemical capacitors. J. Power Source 2010, 195, 3950–3955. [Google Scholar] [CrossRef]
- Huang, C.-C.; Xing, W.; Zhuo, S.-P. Capacitive performances of amorphous tungsten oxide prepared by microwave irradiation. Scr. Mater. 2009, 61, 985–987. [Google Scholar] [CrossRef]
- Miyake, K.; Kaneko, H.; Sano, M.; Suedomi, N. Physical and electrochromic properties of the amorphous and crystalline tungsten oxide thick films prepared under reducing atmosphere. J. Appl. Phys. 1984, 55, 2747–2753. [Google Scholar] [CrossRef]
- Wu, N.-L.; Wang, S.-Y. Conductivity percolation in carbon–carbon supercapacitor electrodes. J. Power Source 2002, 110, 233–236. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Principles, Methods, and Applications; Oxford University: Oxford, UK, 1996. [Google Scholar]
- Landgrebe, A.R. Power Sources for Transportation Applications: Proceedings of the International Symposium; The Electrochemical Society: Pennington, NJ, USA, 2004; 2003-24; pp. 1–252. [Google Scholar]
- Zheng, J.-P.; Jow, T.-R. A new charge storage mechanism for electrochemical capacitors. J Electrochem. Soc. 1995, 142, L6–L8. [Google Scholar] [CrossRef]
- Srinivasan, V.; Weidner, J.W. An electrochemical route for making porous nickel oxide electrochemical capacitors. J Electrochem. Soc. 1997, 144, L210–L213. [Google Scholar] [CrossRef]
- Lin, C.; Ritter, J.A.; Popov, B.N. Development of carbon-metal oxide supercapacitors from Sol-Gel derived carbon-ruthenium xerogels. J. Electrochem. Soc. 1999, 146, 3155–3160. [Google Scholar] [CrossRef]
- Meng, C.; Liu, C.; Chen, L.; Hu, C.; Fan, S. Highly flexible and all-solid-state paperlike polymer supercapacitors. Nano Lett. 2010, 10, 4025–4031. [Google Scholar] [CrossRef] [PubMed]

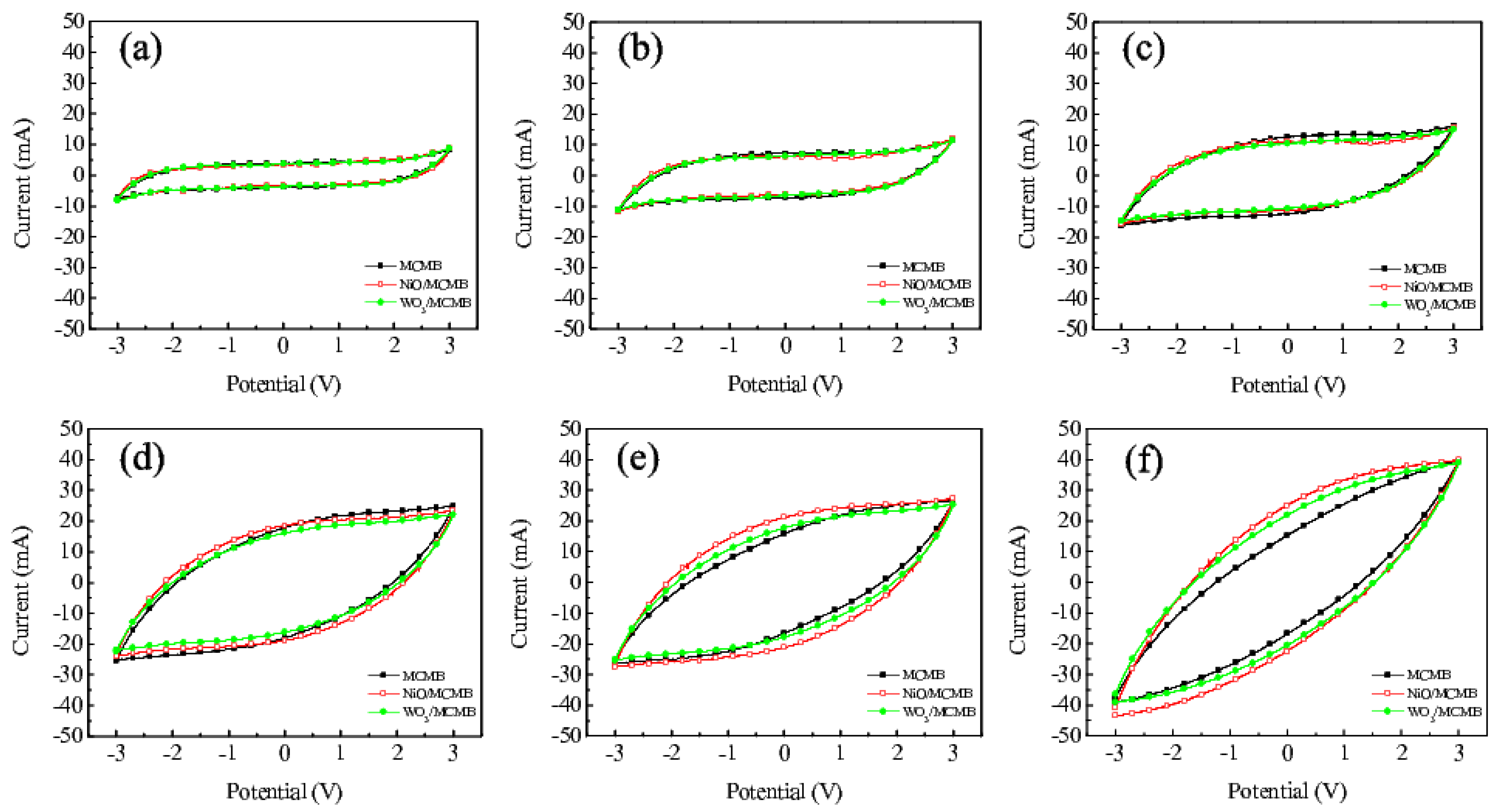
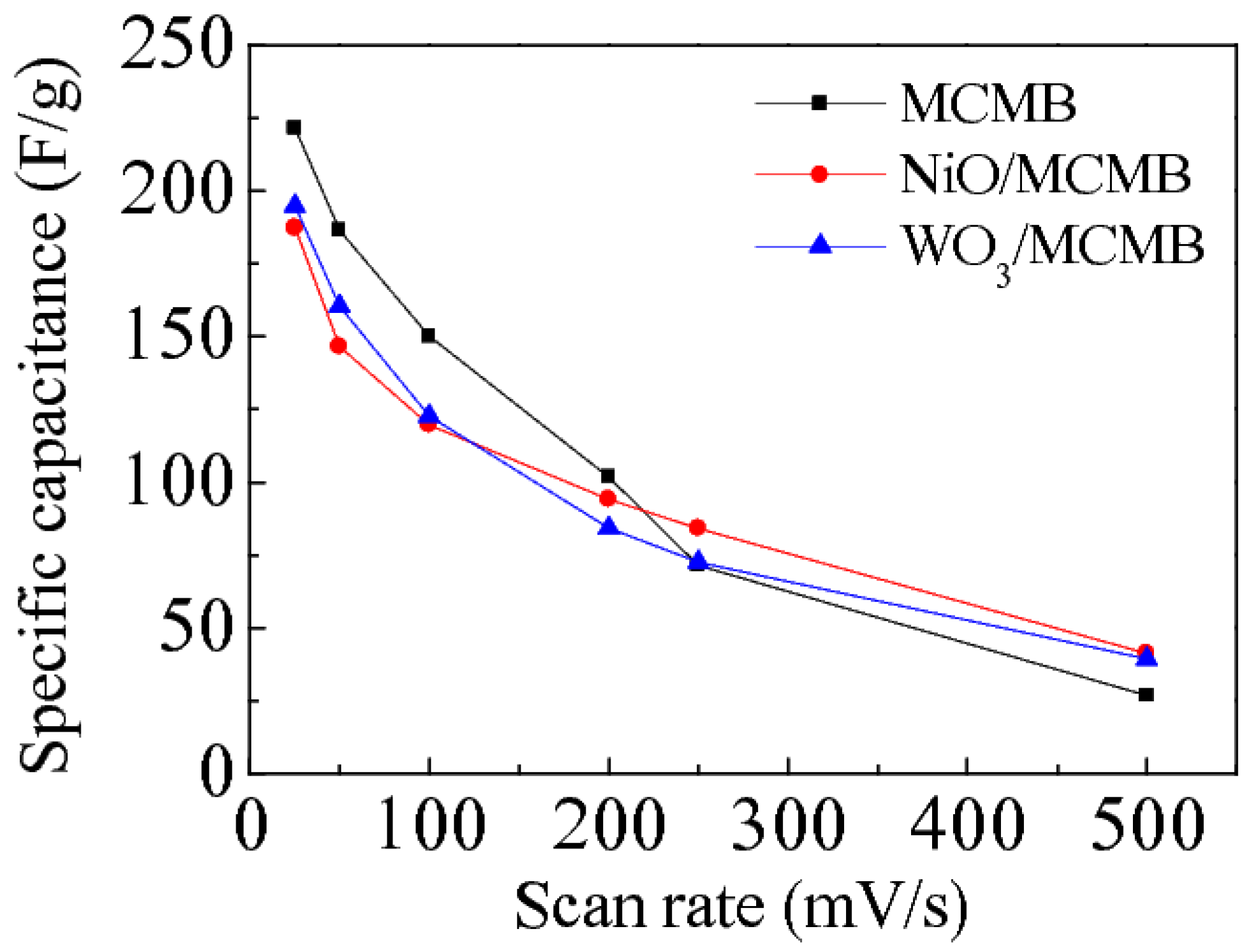
| Scan rates | MCMB | NiO/MCMB | WO3/MCMB |
|---|---|---|---|
| 25 mV/s | 221.5 | 187.2 | 194.8 |
| 50 mV/s | 186.4 | 146.5 | 160.4 |
| 100 mV/s | 150.1 | 119.6 | 122.7 |
| 200 mV/s | 101.7 | 94.1 | 84.3 |
| 250 mV/s | 71.4 | 84.2 | 72.5 |
| 500 mV/s | 26.7 | 41.2 | 39.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Wen, C.-Y.; Wang, C.-M.; Ho, C.-W.; Lin, S.-Y.; Chen, Y.-L. Characterization of Transition-Metal Oxide Deposition on Carbon Electrodes of a Supercapacitor. Appl. Sci. 2016, 6, 413. https://doi.org/10.3390/app6120413
Chen Y-C, Wen C-Y, Wang C-M, Ho C-W, Lin S-Y, Chen Y-L. Characterization of Transition-Metal Oxide Deposition on Carbon Electrodes of a Supercapacitor. Applied Sciences. 2016; 6(12):413. https://doi.org/10.3390/app6120413
Chicago/Turabian StyleChen, Ying-Chung, Chih-Yu Wen, Chih-Ming Wang, Chia-Wei Ho, Shih-Yuan Lin, and Ying-Lin Chen. 2016. "Characterization of Transition-Metal Oxide Deposition on Carbon Electrodes of a Supercapacitor" Applied Sciences 6, no. 12: 413. https://doi.org/10.3390/app6120413





