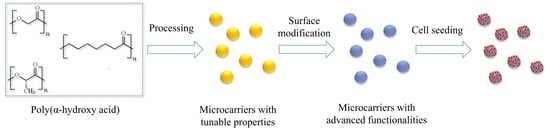
Poly(α-hydroxy Acids)-Based Cell Microcarriers
Abstract
:1. Introduction
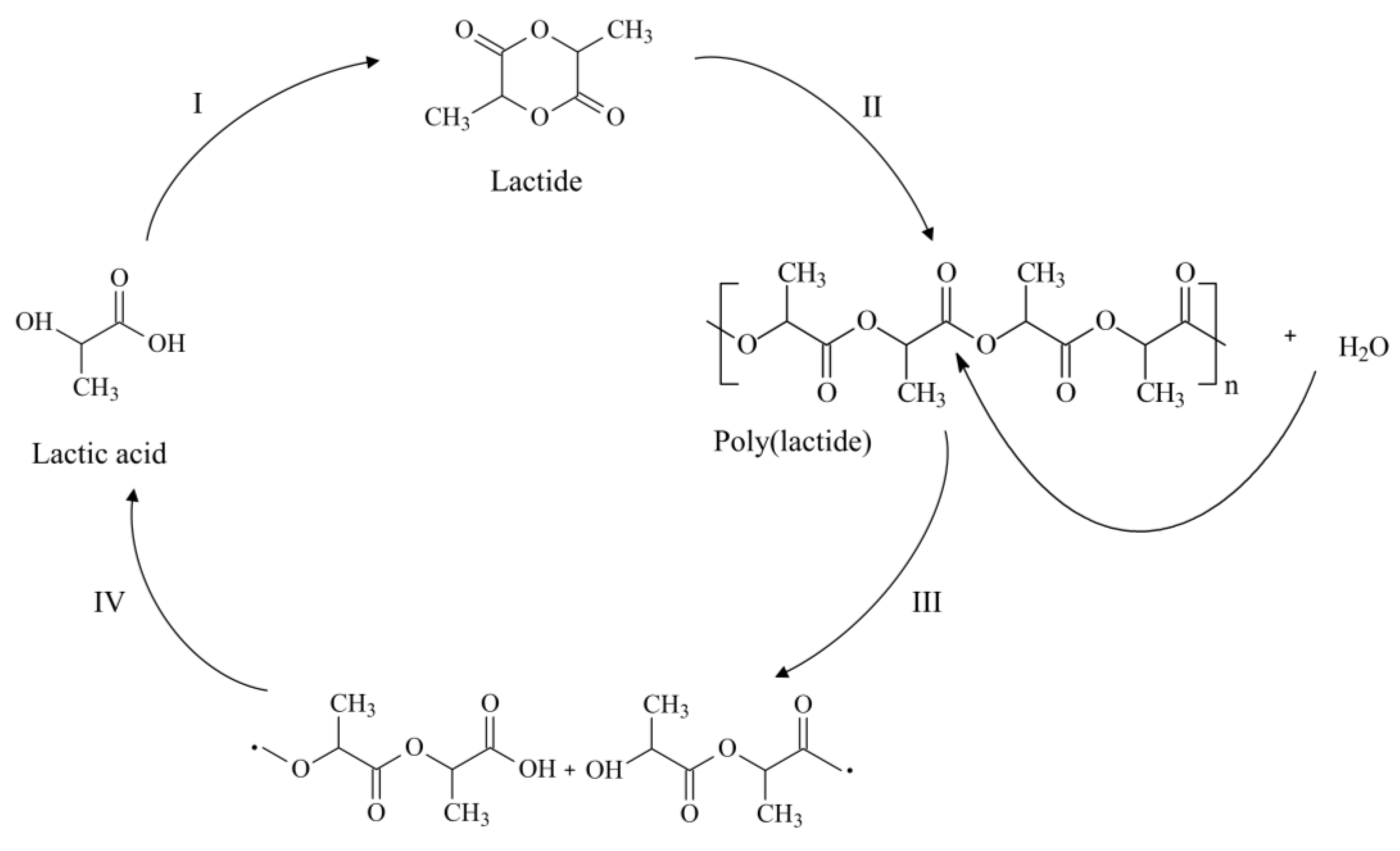
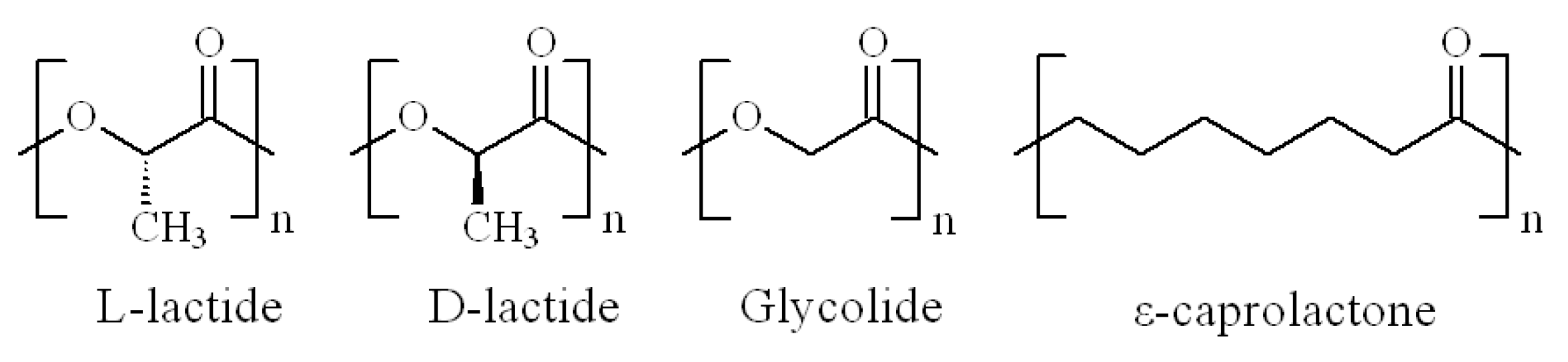
2. Polylactide and Its Copolyesters: Towards Tunable Mechanical Properties and Degradation Rates
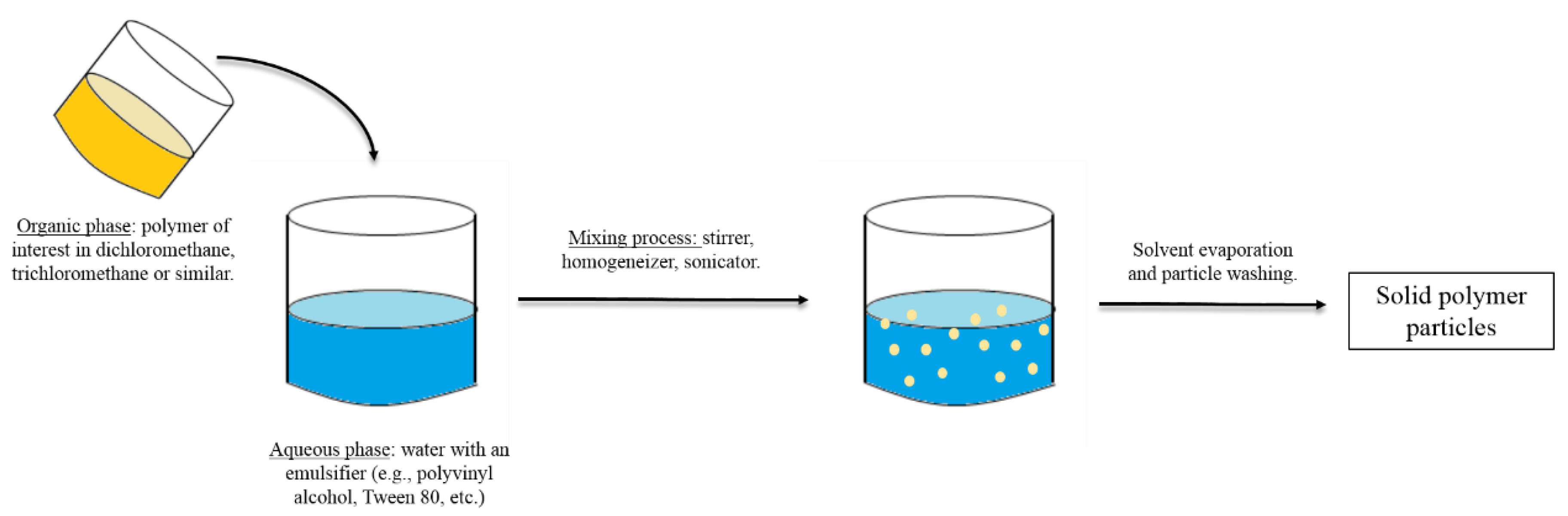
3. Methods for the Fabrication of Microcarriers
4. The Use of Poly(α-hydroxyacids) as Cell Microcarriers
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Van Wezel, A.L. Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nature 1967, 216, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.W.; Wang, D.I.C.; Thilly, W.G. Optimization of growth surface parameters in microcarrier cell culture. Biotechnol. Bioeng. 1979, 21, 821–845. [Google Scholar] [CrossRef]
- Yuan, Y.; Kallos, M.S.; Hunter, C.; Sen, A. Improved expansion of human bone marrow-derived mesenchymal stem cells in microcarrier-based suspension culture. J. Tissue Eng. Regen. Med. 2014, 8, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Melero-Martin, J.M.; Dowling, M.-A.; Smith, M.; Al-Rubeai, M. Expansion of chondroprogenitor cells on macroporous microcarriers as an alternative to conventional monolayer systems. Biomaterials 2006, 27, 2970–2979. [Google Scholar] [CrossRef] [PubMed]
- Stich, S.; Ibold, Y.; Abbas, A.; Ullah, M.; Sittinger, M.; Ringe, J.; Schulze-Tanzil, G.; Müller, C.; Kohl, B.; John, T. Continuous cultivation of human hamstring tenocytes on microcarriers in a spinner flask bioreactor system. Biotechnol. Prog. 2014, 30, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; van Blitterswijk, C.A.; Grojec, M.; Martens, D.E.; Tramper, J.; Riesle, J. Expansion of bovine chondrocytes on microcarriers enhances redifferentiation. Tissue Eng. 2003, 9, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Kreijveld, E.; Temenoff, J.S.; van Blitterswijk, C.A.; Riesle, J. Expansion of human nasal chondrocytes on macroporous microcarriers enhances redifferentiation. Biomaterials 2003, 24, 5153–5161. [Google Scholar] [CrossRef]
- Kodali, A.; Lim, T.C.; Leong, D.T.; Tong, Y.W. Cell–microsphere constructs formed with human adipose-derived stem cells and gelatin microspheres promotes stemness, differentiation, and controlled pro-angiogenic potential. Macromol. Biosci. 2014, 14, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Lecina, M.; Ting, S.; Choo, A.; Reuveny, S.; Oh, S. Scalable platform for human embryonic stem cell differentiation to cardiomyocytes in suspended microcarrier cultures. Tissue Eng. C Methods 2010, 16, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Frauenschuh, S.; Reichmann, E.; Ibold, Y.; Goetz, P.M.; Sittinger, M.; Ringe, J. A microcarrier-based cultivation system for expansion of primary mesenchymal stem cells. Biotechnol. Prog. 2007, 23, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.-Z.; Kim, J.-H.; Park, J.-H.; Choi, S.-J.; Kim, H.-W.; Wall, I. Performance of evacuated calcium phosphate microcarriers loaded with mesenchymal stem cells within a rat calvarium defect. J. Mater. Sci. Mater. Med. 2012, 23, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; El-Fiqi, A.; Park, J.H.; Kim, T.H.; Kim, J.H.; Kim, H.W. Therapeutic bioactive microcarriers: Co-delivery of growth factors and stem cells for bone tissue engineering. Acta Biomater. 2014, 10, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Dame, M.; Beals, T.F.; Wass, J.A. Growth of three established cell lines on glass microcarriers. Biotechnol. Bioeng. 1983, 25, 1359–1372. [Google Scholar] [PubMed]
- Lam, A.T.-L.; Li, J.; Chen, A.K.-L.; Reuveny, S.; Oh, S.K.-W.; Birch, W.R. Cationic surface charge combined with either vitronectin or laminin dictates the evolution of human embryonic stem cells/microcarrier aggregates and cell growth in agitated cultures. Stem Cells Dev. 2014, 23, 1688–1703. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Li, J.; Chen, A.K.-L.; Reuveny, S.; Cool, S.M.; Birch, W.R.; Oh, S.K.-W. Translating human embryonic stem cells from 2-dimensional to 3-dimensional cultures in a defined medium on laminin- and vitronectin-coated surfaces. Stem Cells Dev. 2011, 21, 1701–1715. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Loh, Q.L.; Chong Wong, M.T.; Tan, N.S.; Choong, C. Bioactivated protein-based porous microcarriers for tissue engineering applications. J. Mater. Chem. B 2014, 2, 7795–7803. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, S.; Liang, B.; Liu, Y.; Choong, C.; Pehkonen, S.O. Chitosan microsphere scaffold tethered with rgd-conjugated poly(methacrylic acid) brushes as effective carriers for the endothelial cells. Macromol. Biosci. 2014, 14, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Hydrogels in acellular and cellular strategies for intervertebral disc regeneration. J. Tissue Eng. Regen. Med. 2013, 7, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.R.; Reis, R.L.; Mano, J.F. Multiphasic, multistructured and hierarchical strategies for cartilage regeneration. In Engineering Mineralized and Load Bearing Tissue; Bertassoni, L.E., Coelho, P.G., Eds.; Springer: Cham, Switzerland, 2015; pp. 143–160. [Google Scholar]
- Tam, R.Y.; Fuehrmann, T.; Mitrousis, N.; Shoichet, M.S. Regenerative therapies for central nervous system diseases: A biomaterials approach. Neuropsychopharmacology 2014, 39, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Fontana, G.; Chen, X.; Sanz-Nogues, C.; Zeugolis, D.I.; Dockery, P.; O’Brien, T.; Pandit, A. A shape-controlled tuneable microgel platform to modulate angiogenic paracrine responses in stem cells. Biomaterials 2014, 35, 8757–8766. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.P.; Kumar, V. New emerging trends in synthetic biodegradable polymers—Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Edlund, U.; Albertsson, A.C. Polyesters based on diacid monomers. Adv. Drug Deliv. Rev. 2003, 55, 585–609. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Bergsma, J.E.; de Bruijn, W.C.; Rozema, F.R.; Bos, R.R.M.; Boering, G. Late degradation tissue response to poly(l-lactide) bone plates and screws. Biomaterials 1995, 16, 25–31. [Google Scholar] [CrossRef]
- Tams, J.; Rozema, F.R.; Bos, R.R.M.; Roodenburg, J.L.N.; Nikkels, P.G.J.; Vermey, A. Poly(l-lactide) bone plates and screws for internal fixation of mandibular swing osteotomies. Int. J. Oral Maxillofac. Surg. 1996, 25, 20–24. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikarashi, K. In vitro hydrolysis of poly(l-lactide) crystalline residues as extended-chain crystallites. Part I: Long-term hydrolysis in phosphate-buffered solution at 37 °C. Biomaterials 2004, 25, 5449–5455. [Google Scholar] [CrossRef] [PubMed]
- Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Sarasua, J.-R.; Prud’homme, R.E.; Wisniewski, M.; le Borgne, A.; Spassky, N. Crystallization and melting behavior of polylactides. Macromolecules 1998, 31, 3895–3905. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Crystallization from the melt of poly(lactide)s with different optical purities and their blends. Macromol. Chem. Phys. 1996, 197, 3483–3499. [Google Scholar] [CrossRef]
- Alexis, F.; Venkatraman, S.; Kumar Rath, S.; Gan, L.-H. Some insight into hydrolytic scission mechanisms in bioerodible polyesters. J. Appl. Polym. Sci. 2006, 102, 3111–3117. [Google Scholar] [CrossRef]
- Sabbatier, G.; le Nouën, D.; Chevallier, P.; Durand, B.; Laroche, G.; Dieval, F. Air spun poly(lactic acid) nanofiber scaffold degradation for vascular tissue engineering: A 1H NMR study. Polym. Degrad. Stab. 2012, 97, 1520–1526. [Google Scholar] [CrossRef]
- Saha, S.K.; Tsuji, H. Effects of molecular weight and small amounts of d-lactide units on hydrolytic degradation of poly(l-lactic acid)s. Polym. Degrad. Stab. 2006, 91, 1665–1673. [Google Scholar] [CrossRef]
- Yoon, J.-S.; Jung, H.-W.; Kim, M.-N.; Park, E.-S. Diffusion coefficient and equilibrium solubility of water molecules in biodegradable polymers. J. Appl. Polym. Sci. 2000, 77, 1716–1722. [Google Scholar] [CrossRef]
- Lu, L.; Peter, S.J.; Lyman, M.D.; Lai, H.-L.; Leite, S.M.; Tamada, J.A.; Vacanti, J.P.; Langer, R.; Mikos, A.G. In vitro degradation of porous poly(l-lactic acid) foams. Biomaterials 2000, 21, 1595–1605. [Google Scholar] [CrossRef]
- Wu, L.; Ding, J. In vitro degradation of three-dimensional porous poly(d,l-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 2004, 25, 5821–5830. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Pan, J. Polymer chain scission, oligomer production and diffusion: A two-scale model for degradation of bioresorbable polyesters. Acta Biomater. 2011, 7, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Etxeberria, A.; Sarasua, J.-R. Synthesis, structure and properties of poly(l-lactide-co-ε-caprolactone) statistical copolymers. J. Mech. Behav. Biomed. Mater. 2012, 9, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Hiljanen-Vainio, M.; Karjalainen, T.; Seppälä, J. Biodegradable lactone copolymers. I. Characterization and mechanical behavior of ε-caprolactone and lactide copolymers. J. Appl. Polym. Sci. 1996, 59, 1281–1288. [Google Scholar] [CrossRef]
- Garkhal, K.; Verma, S.; Jonnalagadda, S.; Kumar, N. Fast degradable poly(l-lactide-co-ε-caprolactone) microspheres for tissue engineering: Synthesis, characterization, and degradation behavior. J. Polym. Sci. A Polym. Chem. 2007, 45, 2755–2764. [Google Scholar] [CrossRef]
- Fernández, J.; Larrañaga, A.; Etxeberría, A.; Sarasua, J.R. Effects of chain microstructures and derived crystallization capability on hydrolytic degradation of poly(l-lactide/ε-caprolactone) copolymers. Polym. Degrad. Stab. 2013, 98, 481–489. [Google Scholar] [CrossRef]
- Qian, H.; Bei, J.; Wang, S. Synthesis, characterization and degradation of aba block copolymer of l-lactide and ε-caprolactone. Polym. Degrad. Stab. 2000, 68, 423–429. [Google Scholar] [CrossRef]
- Grijpma, D.W.; Pennings, A.J. (Co)polymers of l-lactide, 1. Synthesis, thermal properties and hydrolytic degradation. Macromol. Chem. Phys. 1994, 195, 1633–1647. [Google Scholar] [CrossRef]
- Fernández, J.; Etxeberria, A.; Ugartemendia, J.M.; Petisco, S.; Sarasua, J.-R. Effects of chain microstructures on mechanical behavior and aging of a poly(l-lactide-co-caprolactone) biomedical thermoplastic-elastomer. J. Mech. Behav. Biomed. Mater. 2012, 12, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Etxeberría, A.; Sarasua, J.R. Effects of repeat unit sequence distribution and residual catalyst on thermal degradation of poly(l-lactide/ε-caprolactone) statistical copolymers. Polym. Degrad. Stab. 2013, 98, 1293–1299. [Google Scholar] [CrossRef]
- Dobrzynski, P.; Li, S.; Kasperczyk, J.; Bero, M.; Gasc, F.; Vert, M. Structure−property relationships of copolymers obtained by ring-opening polymerization of glycolide and ε-caprolactone. Part 1. Synthesis and characterization. Biomacromolecules 2005, 6, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Kasperczyk, J.; Li, S.; Dobrzynski, P.; Jarzabek, B. Controlled poly(l-lactide-co-trimethylene carbonate) delivery system of cyclosporine a and rapamycine—The effect of copolymer chain microstructure on drug release rate. Int. J. Pharm. 2011, 414, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dobrzynski, P.; Kasperczyk, J.; Bero, M.; Braud, C.; Vert, M. Structure−property relationships of copolymers obtained by ring-opening polymerization of glycolide and ε-caprolactone. Part 2. Influence of composition and chain microstructure on the hydrolytic degradation. Biomacromolecules 2005, 6, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Larrañaga, A.; Etxeberria, A.; Wang, W.; Sarasua, J.R. A new generation of poly(lactide/ε-caprolactone) polymeric biomaterials for application in the medical field. J. Biomed. Mater. Res. A 2014, 102, 3573–3584. [Google Scholar] [CrossRef] [PubMed]
- Mundargi, R.C.; Babu, V.R.; Rangaswamy, V.; Patel, P.; Aminabhavi, T.M. Nano/micro technologies for delivering macromolecular therapeutics using poly(d,l-lactide-co-glycolide) and its derivatives. J. Controll. Release 2008, 125, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.P.; Labhasetwar, V.; Amidon, G.L.; Levy, R.J. Gastrointestinal uptake of biodegradable microparticles: Effect of particle size. Pharm. Res. 1996, 13, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Win, K.Y.; Feng, S.-S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P.; Puisieux, F. Nano- and microparticles for the delivery of polypeptides and proteins. Adv. Drug Deliv. Rev. 1993, 10, 141–162. [Google Scholar] [CrossRef]
- Ibraheem, D.; Iqbal, M.; Agusti, G.; Fessi, H.; Elaissari, A. Effects of process parameters on the colloidal properties of polycaprolactone microparticles prepared by double emulsion like process. Colloids Surf. A Physicochem. Eng. Asp. 2014, 445, 79–91. [Google Scholar] [CrossRef]
- Benoit, M.A.; Baras, B.; Gillard, J. Preparation and characterization of protein-loaded poly(ε-caprolactone) microparticles for oral vaccine delivery. Int. J. Pharm. 1999, 184, 73–84. [Google Scholar] [CrossRef]
- O’Donnell, P.B.; McGinity, J.W. Preparation of microspheres by the solvent evaporation technique. Adv. Drug Deliv. Rev. 1997, 28, 25–42. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual polyvinyl alcohol associated with poly(d,l-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Controll. Release 2002, 82, 105–114. [Google Scholar] [CrossRef]
- Yang, Y.; Bajaj, N.; Xu, P.; Ohn, K.; Tsifansky, M.D.; Yeo, Y. Development of highly porous large PLGA microparticles for pulmonary drug delivery. Biomaterials 2009, 30, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Yoon, J.J.; Lee, D.S.; Park, T.G. Gas foamed open porous biodegradable polymeric microspheres. Biomaterials 2006, 27, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Sun, L.; Jiang, J.; Zhang, X.; Ding, W.; Gan, Z. Biodegradable polymeric microcarriers with controllable porous structure for tissue engineering. Macromol. Biosci. 2009, 9, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Yang, F.; Zhang, Q.; Zhang, T.; Gu, N. Fabrication of nonporous and porous cationic PLGA microspheres. Mater. Lett. 2014, 117, 86–89. [Google Scholar] [CrossRef]
- Jin, G.-Z.; Park, J.-H.; Seo, S.-J.; Kim, H.-W. Dynamic cell culture on porous biopolymer microcarriers in a spinner flask for bone tissue engineering: A feasibility study. Biotechnol. Lett. 2014, 36, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-J.; Yu, H.-S.; Kim, H.-W. Tissue engineering polymeric microcarriers with macroporous morphology and bone-bioactive surface. Macromol. Biosci. 2009, 9, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Chung, H.J.; Park, T.G. Biodegradable polymeric microspheres with “open/closed” pores for sustained release of human growth hormone. J. Controll. Release 2006, 112, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.A.; Chang, Z. Microfluidic-assisted synthesis of polymer particles. Chem. Eng. Technol. 2008, 31, 1099–1115. [Google Scholar] [CrossRef]
- Xu, Q.; Hashimoto, M.; Dang, T.T.; Hoare, T.; Kohane, D.S.; Whitesides, G.M.; Langer, R.; Anderson, D.G. Preparation of monodisperse biodegradable polymer microparticles using a microfluidic flow-focusing device for controlled drug delivery. Small 2009, 5, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lam, A.T.-L.; Toh, J.P.W.; Reuveny, S.; Oh, S.K.-W.; Birch, W.R. Fabrication of uniform-sized poly-ɛ-caprolactone microspheres and their applications in human embryonic stem cell culture. Biomed. Microdevices 2015, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Li, S.W.; Tostado, C.; Lan, W.J.; Luo, G.S. Preparation of monodispersed chitosan microspheres and in situ encapsulation of bsa in a co-axial microfluidic device. Biomed. Microdevices 2009, 11, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Paik, D.C.; Choi, S.W. Entrapment of protein using electrosprayed poly(d,l-lactide-co-glycolide) microspheres with a porous structure for sustained release. Macromol. Rapid Commun. 2014, 35, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Bock, N.; Woodruff, M.A.; Hutmacher, D.W.; Dargaville, T.R. Electrospraying, a reproducible method for production of polymeric microspheres for biomedical applications. Polymers 2011, 3, 131. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wong, J.S.-P.; Hu, M.; Li, W.; Li, R.K.Y. Facile preparation of hierarchically porous polymer microspheres for superhydrophobic coating. Nanoscale 2014, 6, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Senuma, Y.; Franceschin, S.; Hilborn, J.G.; Tissières, P.; Bisson, I.; Frey, P. Bioresorbable microspheres by spinning disk atomization as injectable cell carrier: From preparation to in vitro evaluation. Biomaterials 2000, 21, 1135–1144. [Google Scholar] [CrossRef]
- Levato, R.; Mateos-Timoneda, M.A.; Planell, J.A. Preparation of biodegradable polylactide microparticles via a biocompatible procedure. Macromol. Biosci. 2012, 12, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.W.; Yoo, H.S.; Yoon, J.J.; Park, T.G. Biodegradable PLGA microcarriers for injectable delivery of chondrocytes: Effect of surface modification on cell attachment and function. Biotechnol. Prog. 2004, 20, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Planell, J.A.; Mateos-Timoneda, M.A.; Engel, E. Role of ecm/peptide coatings on sdf-1α triggered mesenchymal stromal cell migration from microcarriers for cell therapy. Acta Biomater. 2015, 18, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Gao, C.; Xie, Y.; Gong, Y.; Shen, J. Collagen-coated polylactide microspheres as chondrocyte microcarriers. Biomaterials 2005, 26, 6305–6313. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Curran, S.J.; Curran, J.M.; Hunt, J.A. The use of poly(l-lactide) and rgd modified microspheres as cell carriers in a flow intermittency bioreactor for tissue engineering cartilage. Biomaterials 2006, 27, 4453–4460. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Huang, D.; Lao, L.; Gao, C. RGD modified PLGA/gelatin microspheres as microcarriers for chondrocyte delivery. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91B, 228–238. [Google Scholar] [CrossRef] [PubMed]
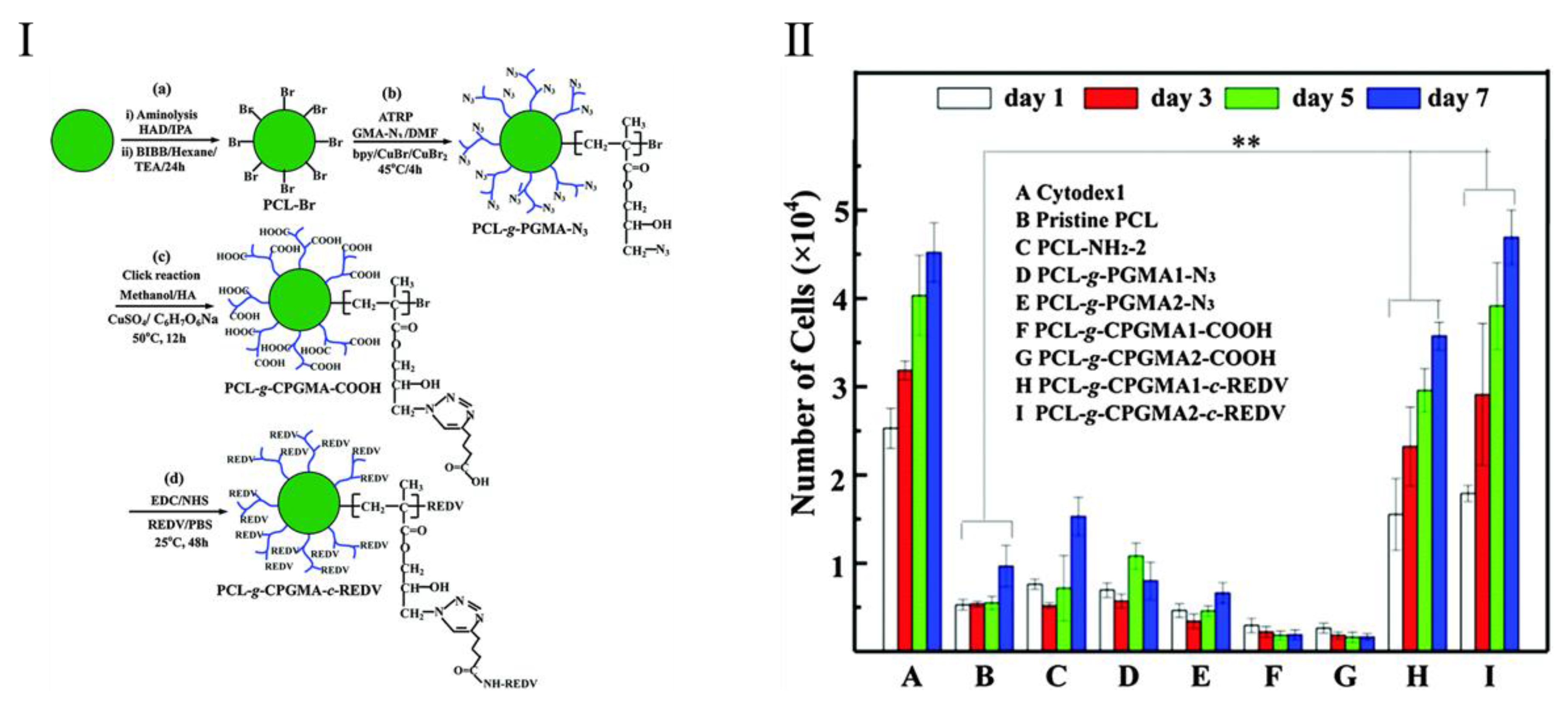
- Yuan, S.; Xiong, G.; He, F.; Jiang, W.; Liang, B.; Pehkonen, S.; Choong, C. PCL microspheres tailored with carboxylated poly(glycidyl methacrylate)-REDV conjugates as conducive microcarriers for endothelial cell expansion. J. Mater. Chem. B 2015, 3, 8670–8683. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, N.; Wang, Z.; Wang, Y.; Liu, Y.; Ito, Y.; Zhang, P. Biodegradable microcarriers of poly(lactide-co-glycolide) and nano-hydroxyapatite decorated with IGF-1 via polydopamine coating for enhancing cell proliferation and osteogenic differentiation. Macromol. Biosci. 2015, 15, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Yague, M.A.; Larrañaga, A.; Gladkovskaya, O.; Stanley, A.; Tadayyon, G.; Guo, Y.; Sarasua, J.-R.; Tofail, S.A.M.; Zeugolis, D.I.; Pandit, A.; et al. Effects of polydopamine functionalization on boron nitride nanotube dispersion and cytocompatibility. Bioconjug. Chem. 2015, 26, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Larrañaga, A.; Ramos, D.; Amestoy, H.; Zuza, E.; Sarasua, J.-R. Coating of bioactive glass particles with mussel-inspired polydopamine as a strategy to improve the thermal stability of poly(l-lactide)/bioactive glass composites. RSC Adv. 2015, 5, 65618–65626. [Google Scholar] [CrossRef]
- Newman, K.D.; McBurney, M.W. Poly(d,l lactic-co-glycolic acid) microspheres as biodegradable microcarriers for pluripotent stem cells. Biomaterials 2004, 25, 5763–5771. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Park, T.G. Injectable cellular aggregates prepared from biodegradable porous microspheres for adipose tissue engineering. Tissue Eng. A 2009, 15, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Jeon, O.; Kim, B.S. Poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for cartilage tissue engineering. Tissue Eng. 2005, 11, 438–447. [Google Scholar] [CrossRef] [PubMed]

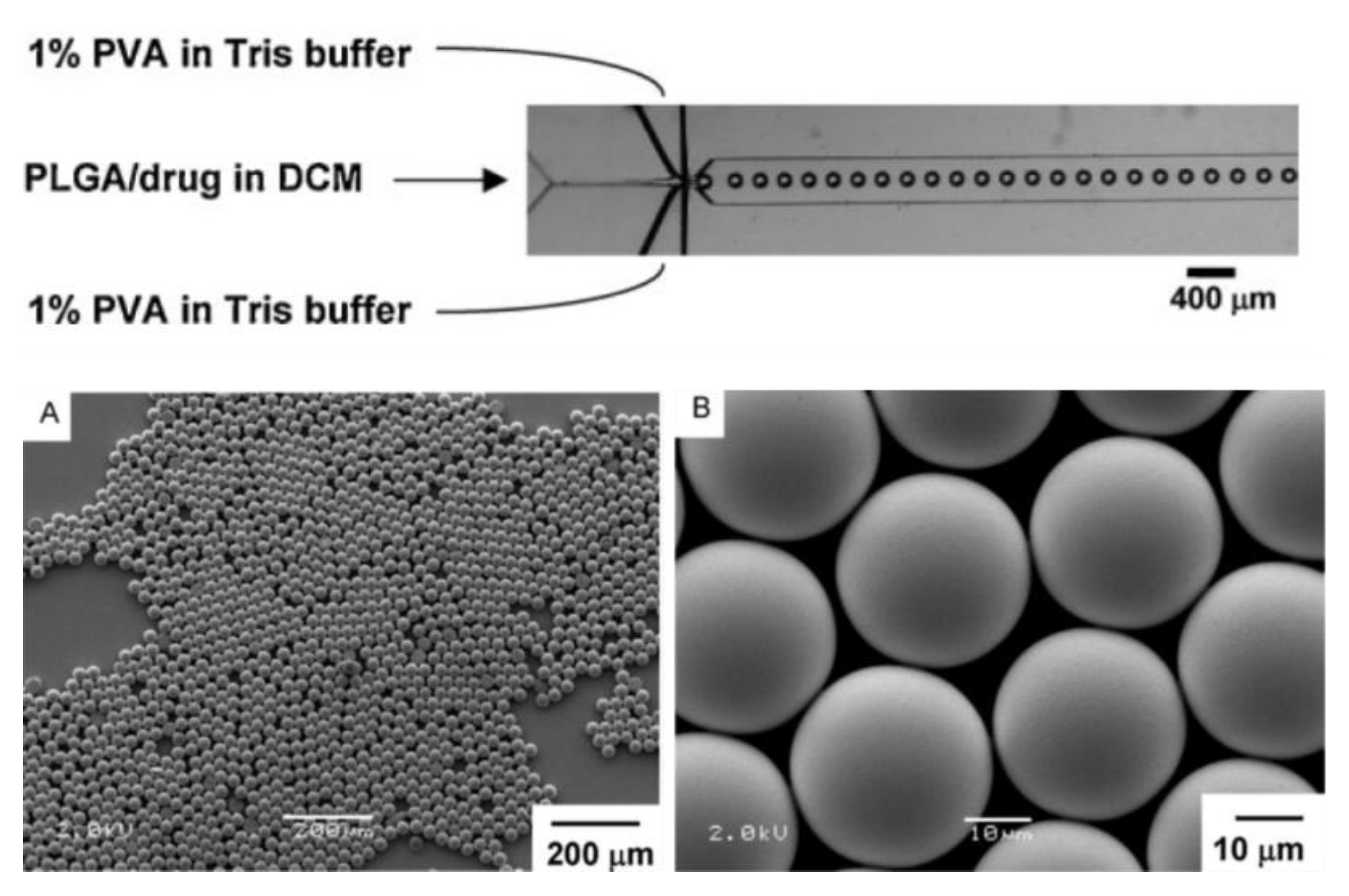
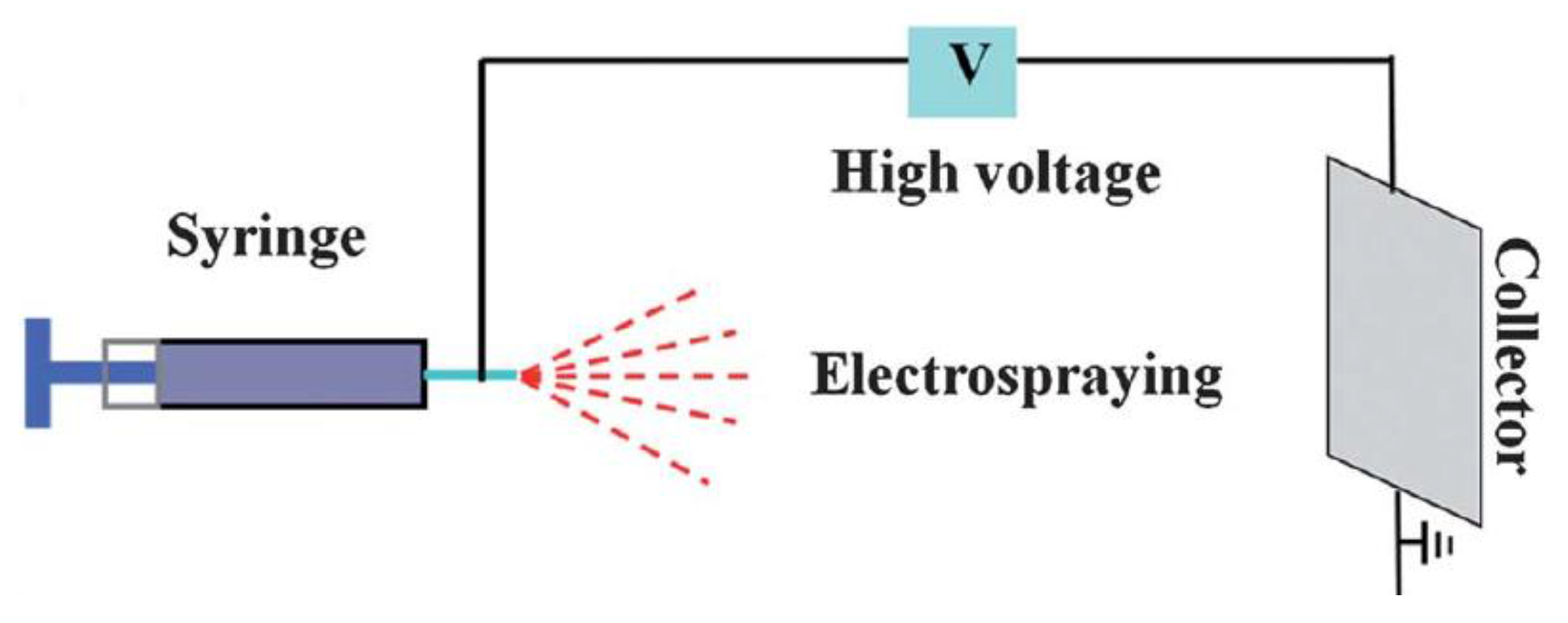
| Commercial Name | Shape | Dimensions | Matrix | Surface | Surface Area (m2/g) | Manufacturer | Performance of Cells |
|---|---|---|---|---|---|---|---|
| Cytodex 1 | Spherical | ~190 µm | Cross-linked dextran | Hydrophilic diethylaminoethyl (DEAE)/Positive charge | 0.44 | GE Healthcare Life Sciences | Study by Lecina et al. [9] about the culture and differentiation toward cardiomyocites (CM) of human embryonic stem cells (hESCs):
Study by Frauenschuh et al. [10] into the expansion of bone-marrow-derived mesenchymal stem cells (MSCs) on Cytodex 1 and 3:
|
| Cytodex 3 | Spherical | ~175 µm | Cross-linked dextran | Pig skin gelatin | 0.27 | GE Healthcare Life Sciences | |
| Cytopore 1 | Spherical | ~230 µm | Cross-linked cotton cellulose | Hydrophilic DEAE/Positive charge | 1.1 | GE Healthcare Life Sciences | |
| Cultispher | Spherical | 130–380 µm | Cross-linked porcine gelatin | N/A | 1.5 | Percell Biolytica | |
| DE-53 | Cylindrical | l: 150–400 µm × d: 35–50 µm | Cellulose | Hydrophilic DEAE/Positive charge | N/A | Whatman | |
| FACT | Spherical | 125–212 µm | Polystyrene | Type I collagen | 0.036 | SoloHill | |
| Tosoh-10 | Spherical | ~10 µm | Hydroxylated methacrylate | Protamine sulphate | N/A | Tosoh | |
| Rapid Cell P | Spherical | 150–210 µm | Polymeric | N/A | 0.037 | Valeant Pharmaceuticals | |
| Hillex | Spherical | 160–200 µm | Polystyrene | N/A | 0.050 | SoloHill |
| Polymer | Composition | Degradation Rate (Day−1) | Reference |
|---|---|---|---|
| poly(l-lactide) (PLLA) | N/A | 0.0009 | [31] |
| N/A | 0.00043 | [33] | |
| poly(d,l-lactide) (PDLLA) | (DL: 0.2%) | 0.0008 | [33] |
| (DL: 1.2%) | 0.00142 | [33] | |
| (DL: 50%) | 0.0088 | [31] | |
| (DL: not reported) | 0.00386 | [36] | |
| poly(l-lactide-co-glycolide) (PLGA)/poly(d,l-lactide-co-glycolide) (PDLGA) | (LA: 80/GA: 20) | 0.0127 | [31] |
| (DL: 53/GA: 47) | 0.0506 | [31] | |
| (LA: 75/GA: 25) | 0.0219 | [36] | |
| (LA: 85/GA: 15) | 0.0106 | [36] | |
| poly(l-lactide-co-ε-caprolactone) (PLCL) | (LA: 74/CL: 26) (random) | 0.034 | [41] |
| (LA: 74/CL: 26) (blocky) | 0.022 | [41] | |
| (LA: 62/CL: 38) | 0.036 | [41] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larrañaga, A.; Sarasua, J.-R. Poly(α-hydroxy Acids)-Based Cell Microcarriers. Appl. Sci. 2016, 6, 436. https://doi.org/10.3390/app6120436
Larrañaga A, Sarasua J-R. Poly(α-hydroxy Acids)-Based Cell Microcarriers. Applied Sciences. 2016; 6(12):436. https://doi.org/10.3390/app6120436
Chicago/Turabian StyleLarrañaga, Aitor, and Jose-Ramon Sarasua. 2016. "Poly(α-hydroxy Acids)-Based Cell Microcarriers" Applied Sciences 6, no. 12: 436. https://doi.org/10.3390/app6120436