Assessment of Bacillus pumilus Isolated from Fresh Water Milieu for Bioflocculant Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Processing
2.2. Growth Media for Bioflocculant Production
2.3. Isolation of Bioflocculant-Producing Bacteria
2.4. Determination of Flocculating Activity
2.5. Optimization of Culture Conditions for Bioflocculant Production
2.5.1. Effect of Carbon Sources on Bioflocculant Production
2.5.2. Effect of Nitrogen Sources on Bioflocculant Production
2.5.3. Effect of Inoculum Size on Bioflocculant Production
2.5.4. Effect of Initial pH of Growth Medium on Bioflocculant Production
2.5.5. Effect of Cations of Flocculating Activity of Crude Bioflocculant
2.6. Time Course of Bioflocculant Production
2.7. Extraction and Purification of Bioflocculant
2.8. Chemical Composition Analysis of Bioflocculant
2.9. Fourier Transform Infrared Spectrophotometry (FTIR) Analysis
2.10. Scanning Electron Microscopy Imaging (SEM)
2.11. Optimization of Flocculating Activity of Purified Bioflocculant
2.11.1. Effect of Bioflocculant Dosage on Flocculating Activity
2.11.2. Effect of Temperature on the Flocculating Activity
2.11.3. Effect of pH on the Flocculating Activity
2.11.4. Effect of Cations on Flocculating Activity
2.12. Statistical Analysis
3. Results and Discussion
3.1. Isolation of Bioflocculant-Producing Bacteria
3.2. Optimization of Culture Conditions for Bioflocculant Production
3.2.1. Effect of Carbon Source on Bioflocculant Production
3.2.2. Effect of Nitrogen Source on Bioflocculant Production
3.2.3. Effect of Inoculum Size on Bioflocculant Production
3.2.4. Effect of Initial pH of Production Medium on Bioflocculant Production
3.2.5. Effect of Cations on Flocculating Activity of Crude Bioflocculant
3.3. Time Course of Biofloccculant Production
3.4. Chemical Composition of Purified Bioflocculant
3.5. Fourier Transform Infrared Spectrophotometry (FTIR) Analysis
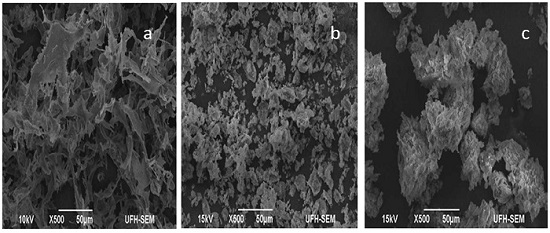
3.6. Scanning Electron Microscopy Imaging (SEM)
3.7. Optimization of Flocculating Activity of Purified Bioflocculant
3.7.1. Effect of Bioflocculant Dosage on Flocculating Activity
3.7.2. Effect of Temperature on the Flocculating Activity
3.7.3. Effect of pH on the Flocculating Activity
3.7.4. Effect of Cations on the Flocculating Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nie, M.; Yin, X.; Jia, J.; Wang, Y.; Liu, S.; Shen, Q.; Li, P.; Wang, Z. Production of a novel bioflocculant MNXY1 by Klebsiella pneumoniae strain NY1 and application in precipitation of cyanobacteria and municipal wastewater treatment. J. Appl. Microbiol. 2011, 111, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.; Farag, S.; Elreesh, G.A.; Elkady, M.; Nosier, M.; Hallem, D.A.E. Characterization of bioflocculants produced by bacteria isolated from crude petroleum oil. Int. J. Environ. Sci. Technol. 2011, 8, 831–840. [Google Scholar] [CrossRef]
- Li, X.M.; Yang, Q.; Huang, K.; Zheng, G.M.; Xiao, D.X.; Liu, J.J.; Long, W.F. Screening and characterization of a bioflocculant produced by Aeromonas. sp. Biomed. Environ. Sci. 2007, 20, 274–278. [Google Scholar] [PubMed]
- Arezoo, C. The potential role of aluminum in Alzheimer’s disease. Nephrol. Dialys. Transplant. 2002, 17, 17–20. [Google Scholar]
- Dearfield, K.L.; Abernathy, C.O.; Ottley, M.S.; Brantner, J.H.; Hayes, P.F. Acrylamide: Its metabolism, developmental and reproductive effects, genotoxicity and carcinogenicity. Mutation Res. 1988, 195, 45–77. [Google Scholar] [CrossRef]
- Zhi, L.; Baoping, H.; Hong, L. Optimum conditions to treat high-concentration microparticle slime water with bioflocculants. Min. Sci. Technol. 2010, 20, 478–484. [Google Scholar]
- Zhang, J.; Wang, R.; Jiang, P.; Liu, Z. Production of an exopolysaccharide bioflocculant by Sorangium cellulosum. Lett. Appl. Microbiol. 2002, 34, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Luo, K.; Liao, D.; Li, X.; Wang, D.; Liu, X.; Zeng, M.; Li, X. A novel bioflocculant produced by Klebsiella sp. and its application to sludge dewatering. Water Environ. J. 2012, 26, 560–566. [Google Scholar] [CrossRef]
- Deng, S.B.; Yu, G.; Ting, Y.P. Production of a bioflocculant by Aspergillus parasiticus and its application in dye removal. Colloids Surf. B 2005, 44, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, H.K. Characterization of a novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodinium impudicum KG03. Bioresour. Technol. 2007, 98, 361–367. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Ren, N.; Wang, A.; Ma, F.; Gao, L.; Peng, Y.; Lee, D. Use of waste fermenting liquor to produce bioflocculant with isolated strains. Inter. J. Hydr. Energy. 2008, 33, 3295–3301. [Google Scholar] [CrossRef]
- Wan, C.; Zhao, X.Q.; Guo, S.L.; Alam, M.A.; Bai, F.W. Bioflocculant production from Solibacillus silvestris W01 and its application in cost-effective harvest of marine microalga Nannochloropsis oceanica by flocculation. Bioresour. Technol. 2013, 135, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, H.; Zhou, J. Characterization of a bioflocculant MBF-5 by Klebsiella pneumoniae and its application in Acanthamoeba cysts removal. Bioresour. Technol. 2013, 137, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Chen, L.; Chen, C.; Zhang, W.; Liu, M.; Han, Y.; Zhou, J. Production and characteristics of a bioflocculant by Klebsiella pneumoniae YZ-6 isolated from human saliva. Appl. Biochem. Biotechnol. 2014, 172, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.M.; Lee, S.J.; Park, M.H.; Kim, H.S.; Kim, H.C.; Yoon, J.H.; Kwon, G.S.; Yoon, B.D. Harvesting of Chlorella vulgaris using a bioflocculant from Paenibacillus sp. AM49. Biotechnol. Lett. 2001, 23, 1229–1234. [Google Scholar] [CrossRef]
- Wang, S.; Gong, W.; Liu, X.; Tian, L.; Yue, Q.; Gao, B. Production of a novel bioflocculant by culture of Klebsiella mobilis using dairy wastewater. Biochem. Eng. 2007, 36, 81–86. [Google Scholar] [CrossRef]
- Mandal, A.K.; Yadav, K.K.; Sen, I.K.; Kumar, A.; Chakraborti, S.; Islam, S.S.; Chakraborti, R. Partial characterization and flocculating behavior of an exopolysaccharide produced in nutrient-poor medium by a facultative oligotroph Klebsiella sp. PB12. J. Biosci. Bioeng. 2013, 115, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.R.; Erin, G.; Chrisy, M.; Tracy, J.; Mincer, W.F. Culturable marine actinomycete diversity from tropical Pacific Ocean sediments. Environ. Microbiol. 2005, 7, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Ntsaluba, L.; Nwodo, U.U.; Mabinya, L.V.; Okoh, A.I. Studies on bioflocculant production by a mixed culture of Methylobacteria sp. Obi and Actinobacterium sp. Mayor. BMC Biotechnol. 2013, 13. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Lin, B.; Xia, S.Q.; Wang, X.J.; Yang, A.M. Production and application of a novel bioflocculant by multiple-microorganism consortia using brewery wastewater as carbon source. Environ. Sci. 2007, 19, 667–673. [Google Scholar] [CrossRef]
- Kurane, R.; Hatamochi, K.; Kakuno, T.; Kiyohara, M.; Hirano, M.; Taniguchi, Y. Production of a bioflocculant by Rhodococcus erythropolis S-l grown on alcohols. Biosci. Biotechnol. Biochem. 1994, 2, 428–429. [Google Scholar] [CrossRef]
- Xia, S.Q.; Zhang, Z.Q.; Wang, X.J.; Yang, A.M.; Chen, L.; Zhao, J.F.; Didier, L.; Nicole, J.R. Production and characterization of a bioflocculant by Proteus mirabilis TJ-1. Bioresour. Technol. 2008, 99, 6520–6527. [Google Scholar] [CrossRef] [PubMed]
- Lachhwani, P. Studies on Polymeric Bioflocculant Producing Microorganisms. Master’s Thesis, Deemed University, Patiala, India, 2005. [Google Scholar]
- Gao, J.; Bao, H.Y.; Xin, M.X.; Liu, Y.X.; Li, Q.; Zhang, Y.F. Characterization of a bioflocculant from a newly isolated Vagococcus sp. W31. J. Zhejiang University Sci. B 2006, 7, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoh, A.I. Characterization of a bioflocculant produced by a consortium of Halomonas sp. Okoh and Micrococcus sp. Leo. Inter. J. Environ. Res. Publ. Health. 2013, 10, 5097–5110. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, M.F.; Kennedy, J.F. Carbohydrate Analysis, 2nd ed.; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- He, J.; Zou, J.; Shao, Z.; Zhang, J. Characteristics and flocculating mechanism of a novel bioflocculant HBF-3 produced by deep-sea bacterium mutant Halomona sp. V3a. World J. Microbiol. Biotechnol. 2010, 26, 1135–1141. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.Y.; Yue, Q.Y.; Wei, J.C.; Zhou, W.Z.; Gu, R. Color removal from textile industry wastewater using composite flocculants. Environ. Technol. 2010, 28, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, F.; Qu, Y.; Sun, D.; Li, A.; Guo, J.; Yu, B. Characterization of a compound bioflocculant produced by mixed culture of Rhizobium radiobacter F2 and Bacillus sphaeicus F6. World J. Microbiol. Biotechnol. 2011, 27, 2559–2565. [Google Scholar] [CrossRef]
- Introduction to the biotechnology of Bacillus. In Biotechnology Handbooks; Harwood, C.R. (Ed.) Pleneum Press: London, UK, 1989; Volume 2, pp. 1–4.
- Handtke, S.; Schroeter, R.; Jurgen, B.; Methling, K.; Schluter, R. Bacillus pumilus reveals a remarkably high resistance to hydrogen peroxide provoked oxidative stress. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Schweder, T.; Hecker, M. Monitoring of stress responses. Adv. Biochem. Eng. Biotechnol. 2004, 89, 47–71. [Google Scholar] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.B.; Kogoma, T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 1991, 55, 561–585. [Google Scholar] [PubMed]
- Xiong, Y.; Wang, Y.; Yu, Y.; Li, Q.; Wang, H.; Chen, R.; He, N. Production and characterization of a novel bioflocculant from Bacillus licheniformis. Appl. Environ. Microbiol. 2010, 76, 2778–2782. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Chen, Y.; Zhao, J.; Teng, H.; Zhu, L.; Yuan, S. Microbial flocculation by Bacillus mucilaginosus: Applications and mechanisms. Bioresour. Technol. 2008, 99, 4825–4831. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.B.; Bai, R.B.; Hu, X.M.; Luo, Q. Characteristics of a bioflocculant produced by Bacillus mucilaginosus and its use in starch wastewater treatment. Appl. Microbiol. Biotechnol. 2003, 60, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, R.; Lei, H.; Shan, Z.; Bai, T.; Yu, Q.; Li, H. Characaterization and flocculating properties of a novel bioflocculant produced by Bacillus circulans. World J. Microbiol. Biotechnol. 2009, 25, 745–752. [Google Scholar] [CrossRef]
- Kaewchai, S.; Prasertsan, P. Biosorption of heavy metal by thermotolerant polymer-producing bacterial cells and the bioflocculant. J. Sci. Technol. 2002, 24, 421–430. [Google Scholar]
- Shadia, M.A.A.; Hoda, A.H.; Foukia, E.M.; Nayera, A.M.A. Extracellular metabolites produced by a novel strain, Bacillus alvei NRC-14: 3. Synthesis of a bioflocculant that has chitosan-like structure. J. Life Sci. 2011, 8, 883–890. [Google Scholar]
- He, N.; Li, Y.; Chen, J. Production of a novel polygalacturonic acid bioflocculant REA-11 by Corynebacteria glutamicum. Bioresour. Technol. 2004, 94, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Cosa, S.; Mabinya, L.V.; Olaniran, A.O.; Okoh, O.O.; Okoh, A.I. Bioflocculant production by Virgibacillus. sp. Rob Isolated from the bottom sediment of Algoa Bay in the Eastern Cape, South Africa. Molecules 2011, 16, 2431–2442. [Google Scholar] [CrossRef] [PubMed]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoh, A.I. Evaluation of the flocculation potential and characterization of bioflocculant produced by Micrococcus sp. Leo. Appl. Biochem. Microbiol. 2014, 50, 601–608. [Google Scholar] [CrossRef]
- Ugbenyen, A.; Cosa, S.; Mabinya, L.V.; Babalola, O.O.; Aghdasi, F.; Okoh, A.I. Thermostable bacterial bioflocculant produced by Cobetia spp. isolated from Algoa Bay (South Africa). Int. J. Environ. Res. Publ. Health. 2012, 9, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Lixi, Y.; Chunling, M.; Zhenming, C. Bioflocculant produced by Klebsiella sp. MYC and its application in the treatment of oil-field produced water. J. Ocean Univ. China 2006, 5, 333–338. [Google Scholar]
- Gong, W.; Wang, S.; Sun, X.; Liu, X.; Yue, Q.; Gao, B. Bioflocculant production by culture of Serratia ficaria and its application in wastewater treatment. Bioresour. Technol. 2008, 99, 4668–4674. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, K.; Li, B.; Yuan, H.; Yang, J. Production and characterization of an intracellular biofloculant by Chryseobacterium daeguense W6 cultured in low nutrition medium. Bioresour. Technol. 2010, 101, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Cosa, S.; Ugbenyen, M.A.; Mabinya, L.V.; Okoh, I.A. Characterization of a thermostable polysaccharide bioflocculant produced by Virgibacillus species isolated from Algoa bay. Afr. J. Microbiol. Res. 2013, 7, 2925–2938. [Google Scholar]
- Salehizadeh, H.; Shojaosadati, S.A. Extracellular biopolymeric flocculants: Recent trends and biotechnological importance. Biotechnol. Adv. 2001, 19, 371–385. [Google Scholar] [CrossRef]
- Mabinya, L.V.; Cosa, S.; Mkwetshana, N.; Okoh, A.I. Halomonas. sp. OKOH—A marine bacterium isolated from the bottom sediment of Algoa Bay—Produces a polysaccharide bioflocculant: Partial characterization and biochemical analysis of its properties. Molecules 2011, 16, 4358–4370. [Google Scholar] [CrossRef] [PubMed]
- Aljuboori, R.A.H.; Idris, A.; Abdullah, N.; Mohamed, R. Production and characterization of a bioflocculant produced by Aspergillus flavus. Bioresour. Technol. 2013, 127, 48–493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Cui, Y.N.; Wang, Y. Bioflocculants produced by Gram-positive Bacillus xn12 and Streptomyces xn17 for swine wastewater application. Chem. Biochem. Eng. Q. 2013, 27, 245–250. [Google Scholar]
- Li, Y.; Li, Q.; Hao, D.; Hu, Z.; Song, D.; Yang, M. Characterization and flocculation mechanism of an alkali-activated polysaccharide flocculant from Arthrobacter sp. B4. Bioresour. Technol. 2014, 170, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Zufarzaana, Z.; Ahmad, Z.A.; Zulkifli, H.S.; Mohd, K.Y. Cation dependence, pH tolerance, and dosage requirement of a bioflocculant produced by Bacillus spp. UPMB13: Flocculation performance optimization through Kaolin assays. Sci. World J. 2012. [Google Scholar] [CrossRef]
- Wu, J.Y.; Ye, H.F. Characterization and flocculanting properties of an extracelluar biopolymer produced from a Bacillus subtilis DYU1 isolate. Proc. Biochem. 2007, 42, 1114–1123. [Google Scholar] [CrossRef]
- Ugbenyen, A.M.; Okoh, A.I. Characteristics of a bioflocculant produced by a consortium of Cobetia and Bacillus species and its application in the treatment of wastewaters. Water SA 2014, 40, 139–144. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, S.; Lei, H.; Chen, R.; Yu, Q.; Li, H. Production of a novel bioflocculant by Bacillus licheniformis X14 and its application to low temperature drinking water treatment. Bioresour. Technol. 2009, 100, 3650–3656. [Google Scholar] [CrossRef] [PubMed]
- Elkady, M.F.; Farag, S.; Sahar, Z.; Gadallah, A.; Desouky, A. Bacillus mojavensis strain 32A, a bioflocculant-producing bacteria isolated from an Egyptian salt production pond. Bioresour. Technol. 2011, 102, 8143–8151. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Y.; Zhang, T.; Zhang, D.Y.; Li, C.H.; Wen, J.P.; Du, L.X. A novel bioflocculant produced by Enterobacter aerogenes and its use in defaecating the trona suspension. Biochem. Eng. J. 2005, 27, 1–7. [Google Scholar] [CrossRef]
- Batta, N.; Subudhi, S.; Lal, B.; Devi, A. Isolation of a lead tolerant novel bacterial species, Achromobacter sp. TL-3: Assessment of bioflocculant activity. Indian J. Experimental. Biol. 2013, 51, 1004–1011. [Google Scholar]
- Vatansever, A. Bioflocculation of Activated Sludge in Relation to Calcium Ion Concentration. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2005. [Google Scholar]
- Peng, L.; Yang, C.; Zeng, G.; Wang, L.; Dai, C.; Long, Z.; Liu, H.; Zhang, Y. Characterization and application of bioflocculant prepared by Rhodococcus erythropolis using sludge and livestock wastewater as cheap culture media. Appl. Microbiol. Biotechnol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yuan, H.; Yang, J.; Li, B. Characterization of bioflocculants from biologically aerated filter backwashed sludge and its application in dying wastewater treatment. Bioresour. Technol. 2009, 100, 2629–2632. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Qi, S.; Li, Z.; An, Q.; Xie, M.; Yang, B.; Wang, Y. Production, purification and application of polysaccharide-based bioflocculant by Paenibacillus mucilaginosus. Carbohydr. Polym. 2014, 113, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kurane, R.; Toeda, K.; Takeda, T.; Suzuki, T. Culture Conditions for Production of Microbial Flocculant by Rhodococcus erythropolis. Agricult. Biol. Chem. 1986, 50, 2309–2313. [Google Scholar]
- Salehizadeh, H.; Shojaosadati, S.A. Isolation and characterization of a bioflocculant produced by Bacillus firmus. Biotechnol. Lett. 2002, 24, 35–40. [Google Scholar] [CrossRef]
- Pan, Y.Z.; Shi, B.; Zhang, Y. Research on flocculation property of bioflocculant PG.a21 Ca. Modern Appl. Sci. 2009, 3, 106–112. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoh, A.I. Bacillus toyonensis Strain AEMREG6, a bacterium isolated from South African marine environment sediment samples produces a glycoprotein bioflocculant. Molecules 2015, 20, 5239–5259. [Google Scholar] [CrossRef] [PubMed]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoli, A.S.; Okoh, A.I. Characterization of a bioflocculant (MBF-UFH) produced by Bacillus sp. AEMREG7. Inter. J. Molecul. Sci. 2015, 16, 12986–13003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Wang, S.; Jiang, P. Characterization of a bioflocculant produced by the marine Myxobacterium nannocystis sp. NU-2. Appl. Microbiol. Biotechnol. 2002, 59, 517–522. [Google Scholar] [PubMed]
- Zheng, Y.; Ye, Z.L.; Fang, X.L.; Li, Y.H.; Cai, W.M. Production and characteristics of a bioflocculant produced by Bacillus sp. F19. Bioresour. Technol. 2008, 99, 7686–7691. [Google Scholar] [CrossRef] [PubMed]
- Okaiyeto, K.; Nwodo, U.U.; Okoli, S.A.; Mabinya, L.V.; Okoh, A.I. Implications for public health demands alternatives to inorganic and synthetic flocculants: bioflocculants as important candidates. Microbiologyopen 2016, 5, 177–211. [Google Scholar] [CrossRef] [PubMed]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoli, A.S.; Okoh, A.I. Evaluation of flocculating performance of a thermostable bioflocculant produced by marine Bacillus sp. Environ. Technol. 2016, 37, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.L.; Xu, S.H. Characterization of bioflocculant MBF3-3 produced by an isolated Bacillus sp. World J. Microbiol. Biotechnol. 2008, 24, 1627–1632. [Google Scholar] [CrossRef]












| Component | Percentage (%) |
|---|---|
| Total sugar | 75.4 |
| Protein | 5.3 |
| Uronic acid | 15.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makapela, B.; Okaiyeto, K.; Ntozonke, N.; Nwodo, U.U.; Green, E.; Mabinya, L.V.; Okoh, A.I. Assessment of Bacillus pumilus Isolated from Fresh Water Milieu for Bioflocculant Production. Appl. Sci. 2016, 6, 211. https://doi.org/10.3390/app6080211
Makapela B, Okaiyeto K, Ntozonke N, Nwodo UU, Green E, Mabinya LV, Okoh AI. Assessment of Bacillus pumilus Isolated from Fresh Water Milieu for Bioflocculant Production. Applied Sciences. 2016; 6(8):211. https://doi.org/10.3390/app6080211
Chicago/Turabian StyleMakapela, Busisiwe, Kunle Okaiyeto, Ncedo Ntozonke, Uchechukwu U. Nwodo, Ezekiel Green, Leonard V. Mabinya, and Anthony I. Okoh. 2016. "Assessment of Bacillus pumilus Isolated from Fresh Water Milieu for Bioflocculant Production" Applied Sciences 6, no. 8: 211. https://doi.org/10.3390/app6080211