Microporous Titanium through Metal Injection Moulding of Coarse Powder and Surface Modification by Plasma Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Feedstocks
2.2. Differential Scanning Calorimetry Analysis of the Binders
2.3. Rheological Tests of the Feedstocks
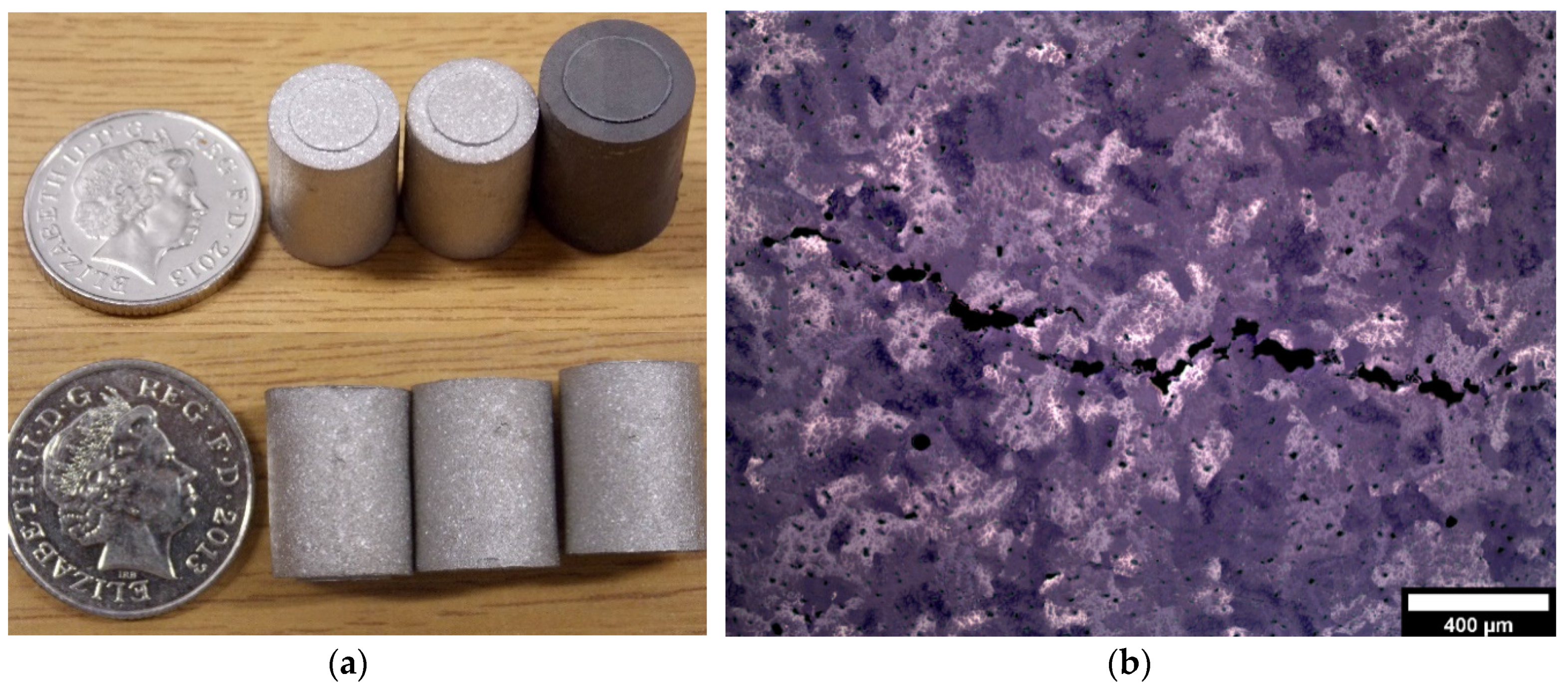
2.4. Sample Preparation and Sintering
2.5. Microstructural and Mechanical Analyses of the Samples
2.6. Plasma Electrolytic Oxidation of Samples
3. Results
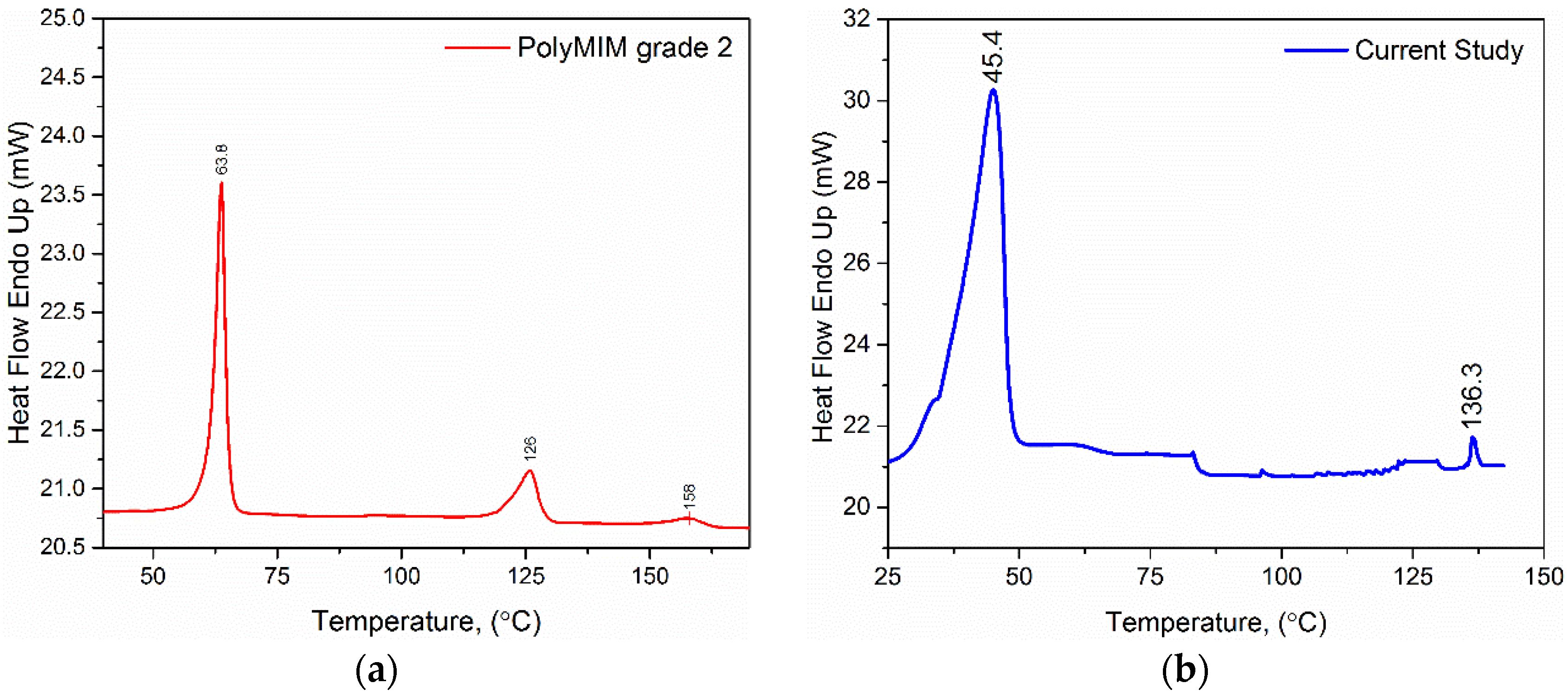
3.1. DSC Analysis Results
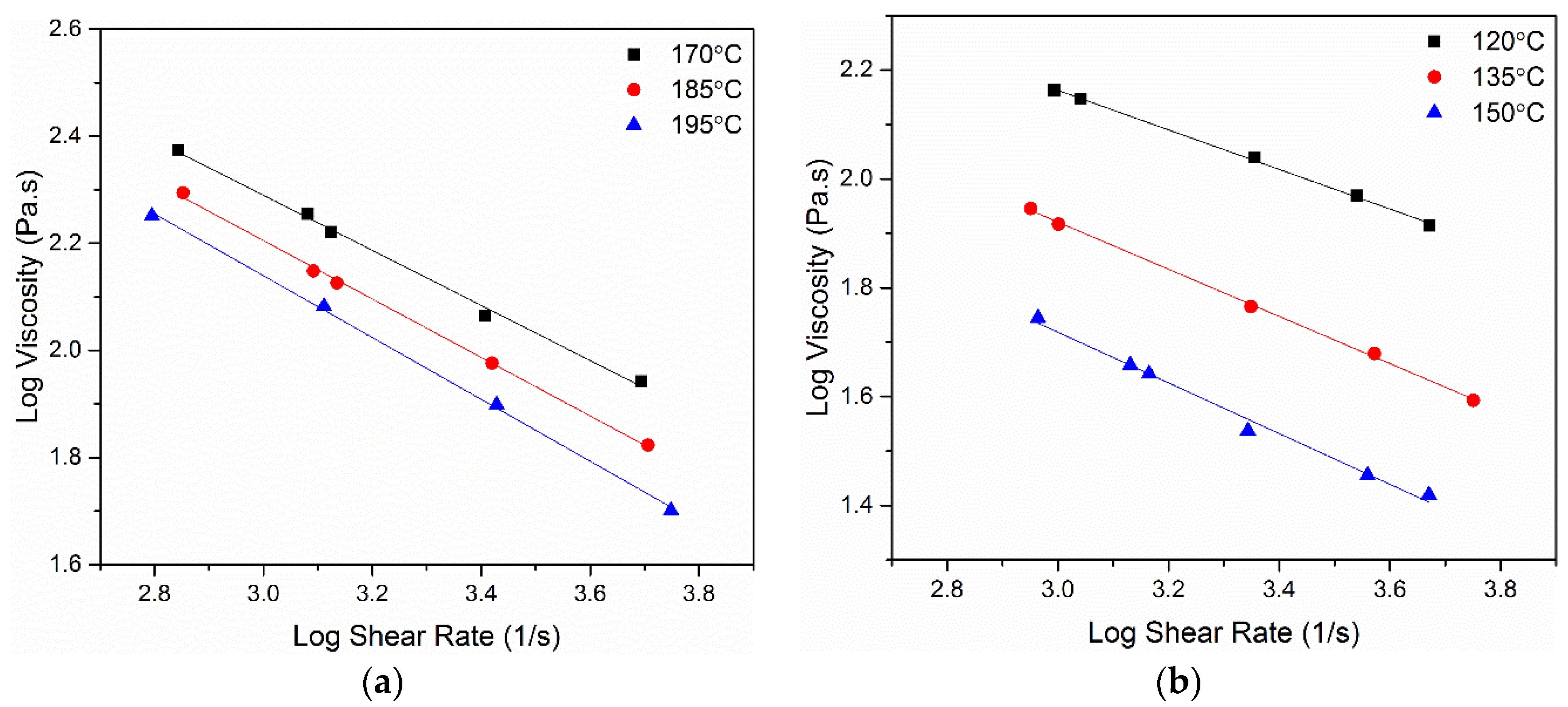
3.2. Rheological Analysis Results
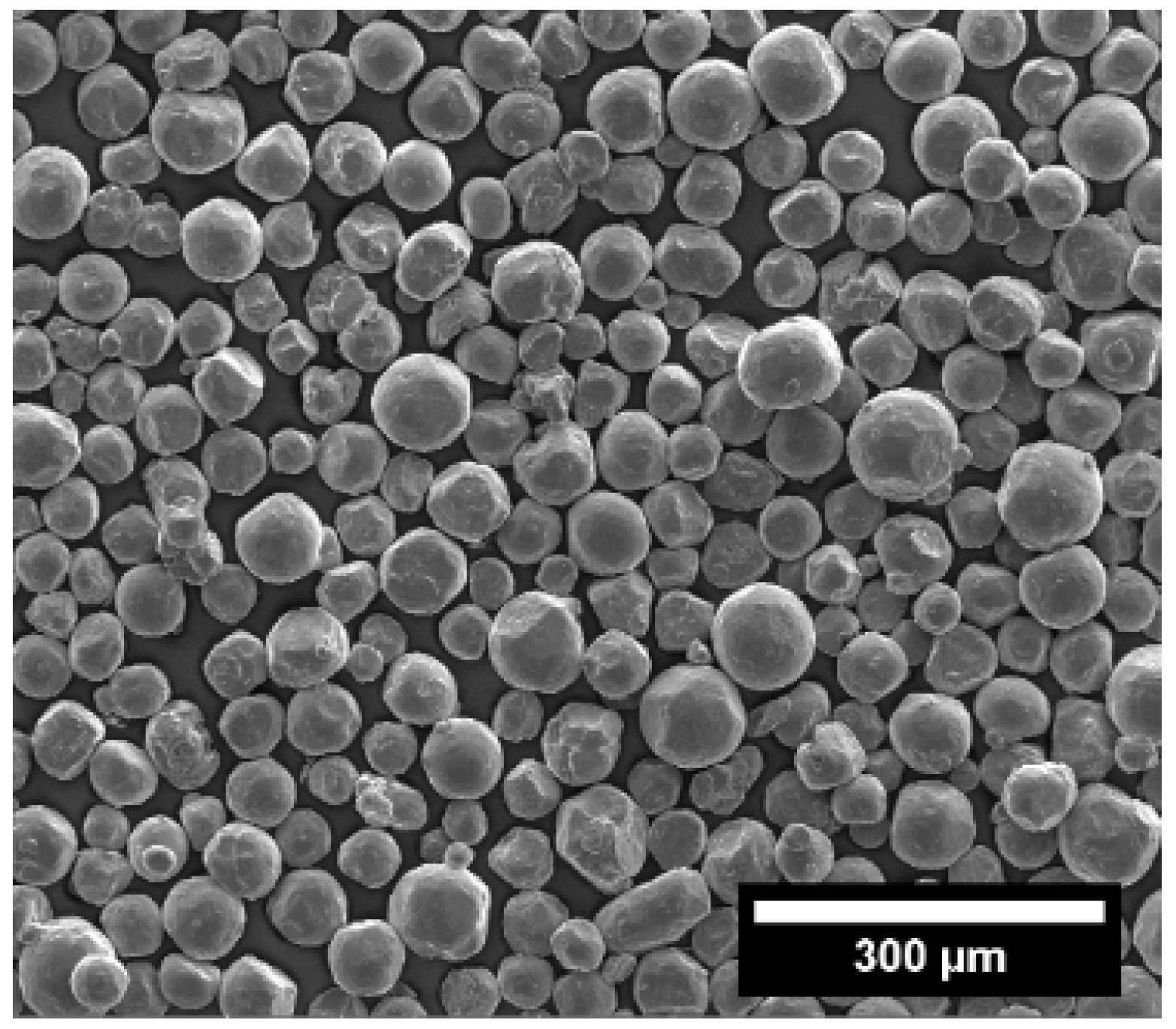
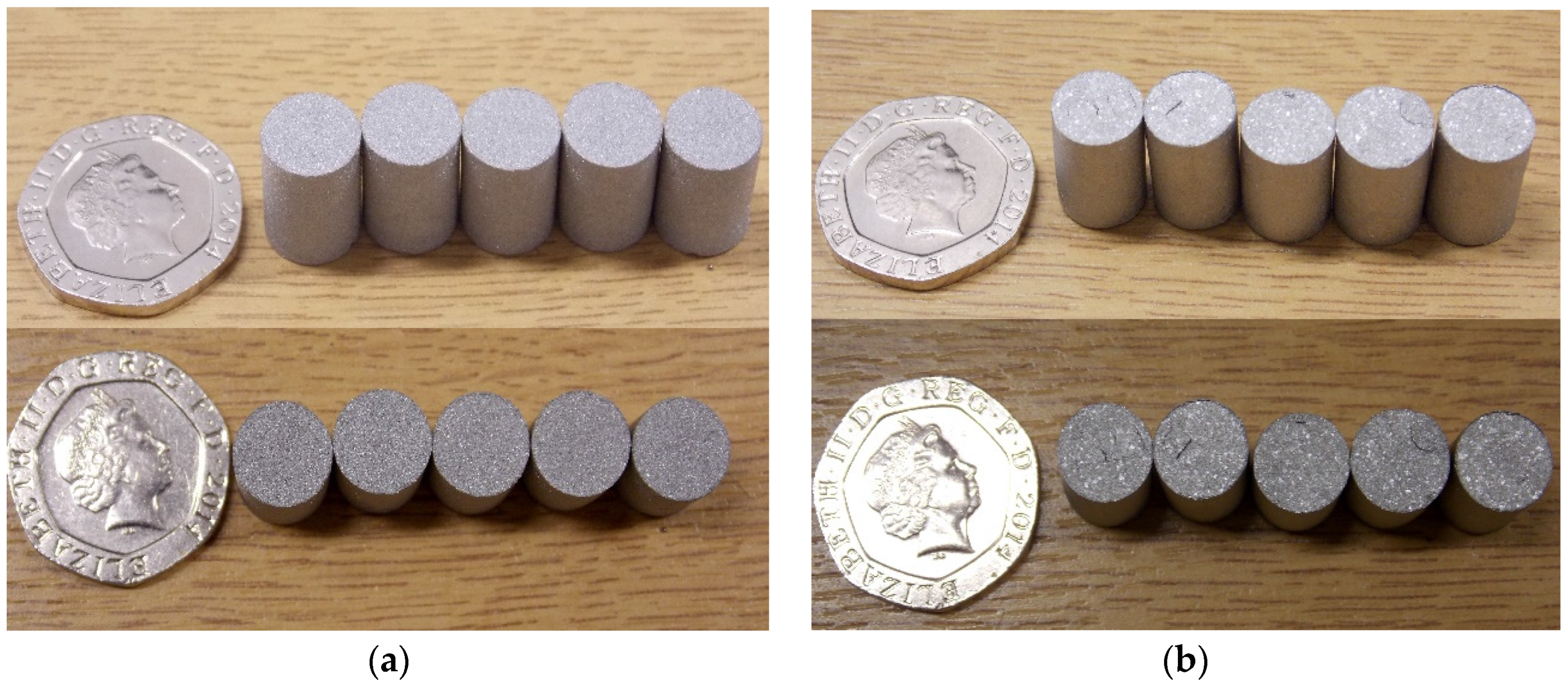
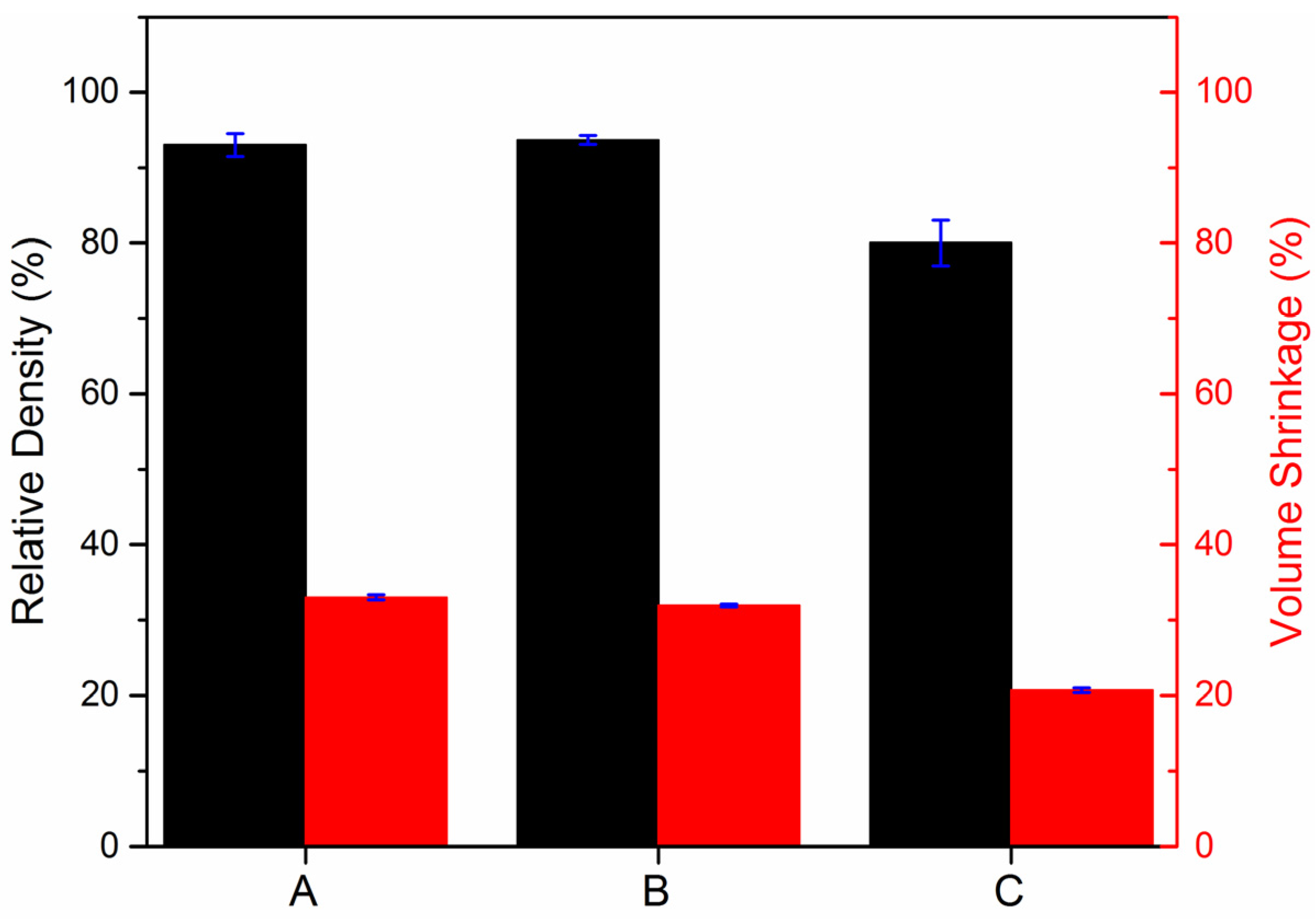
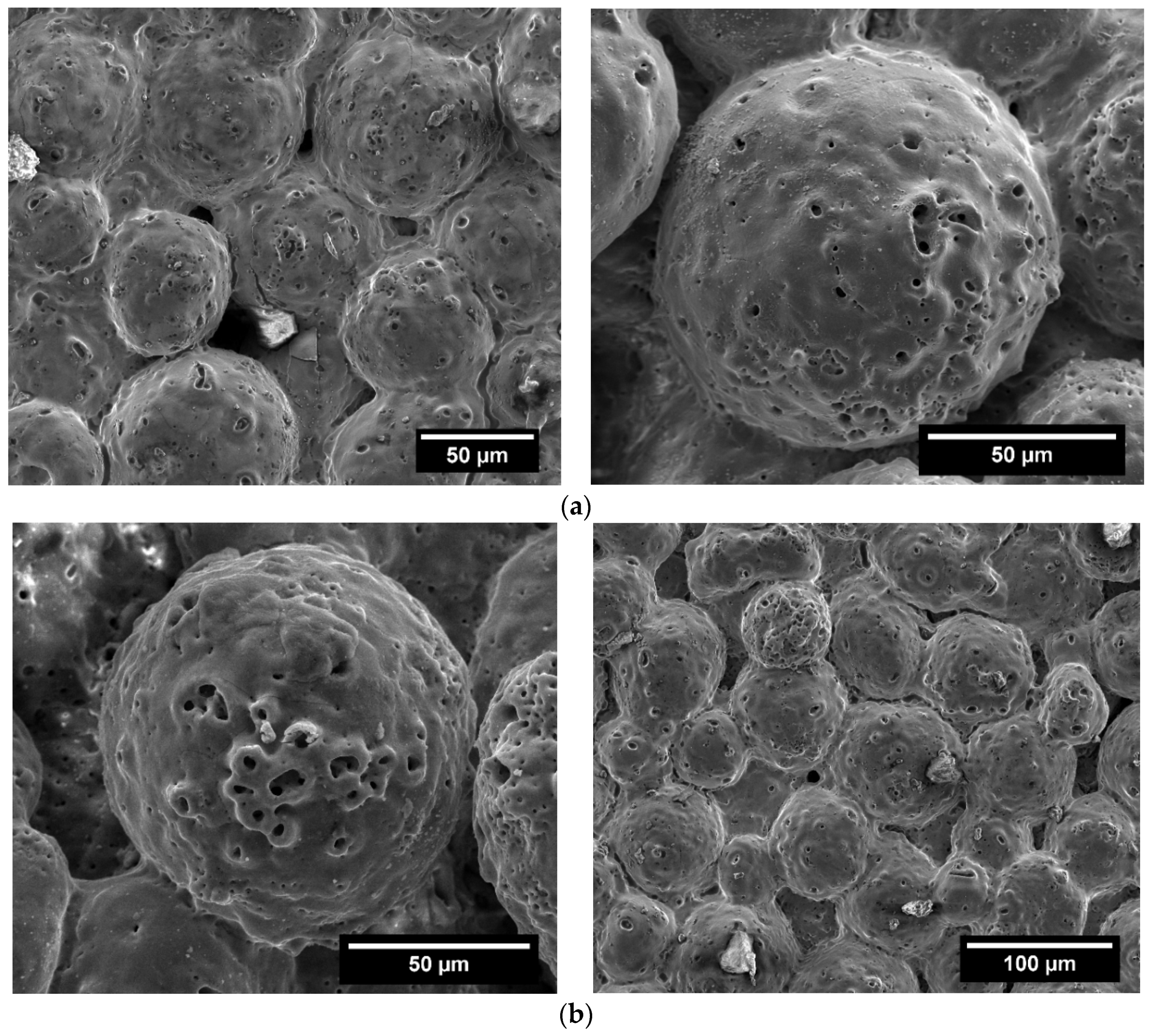
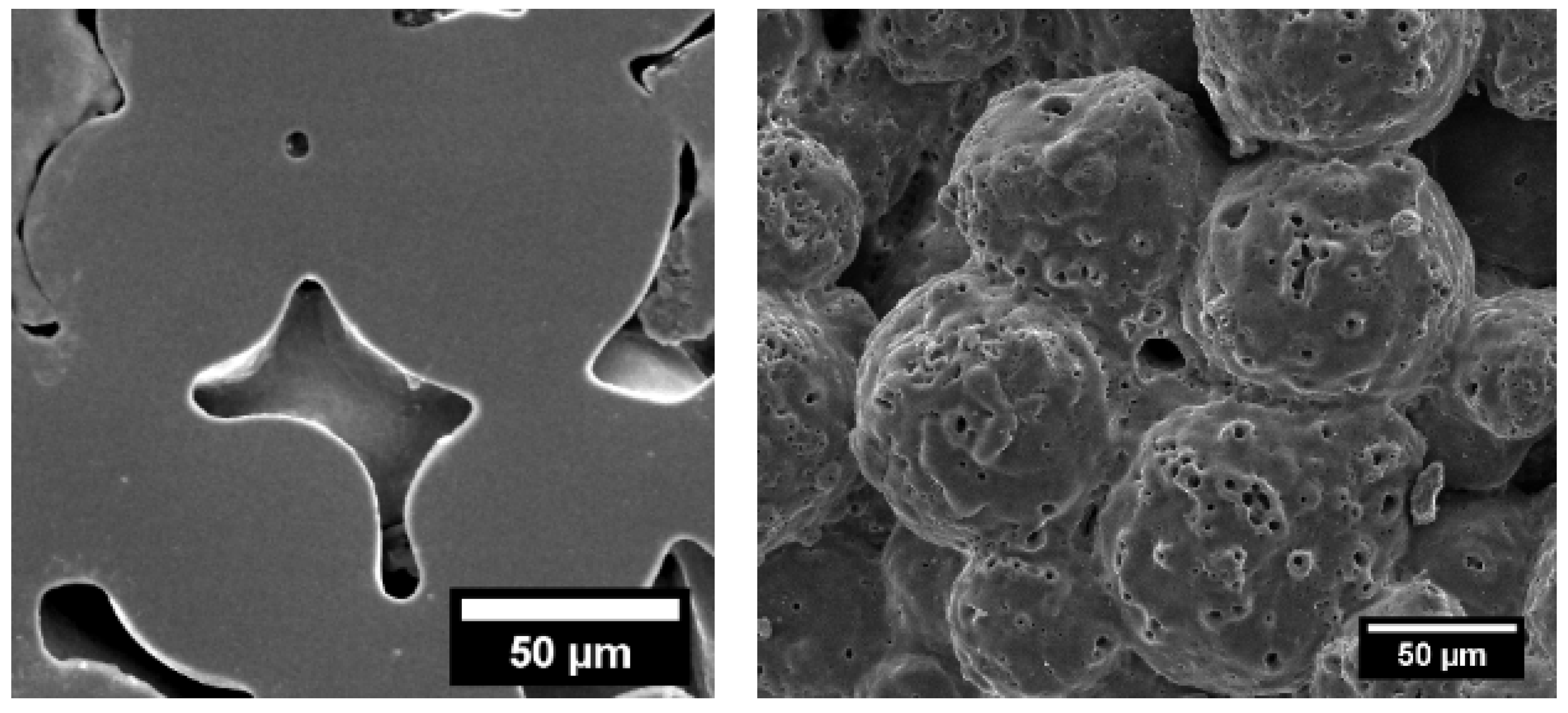
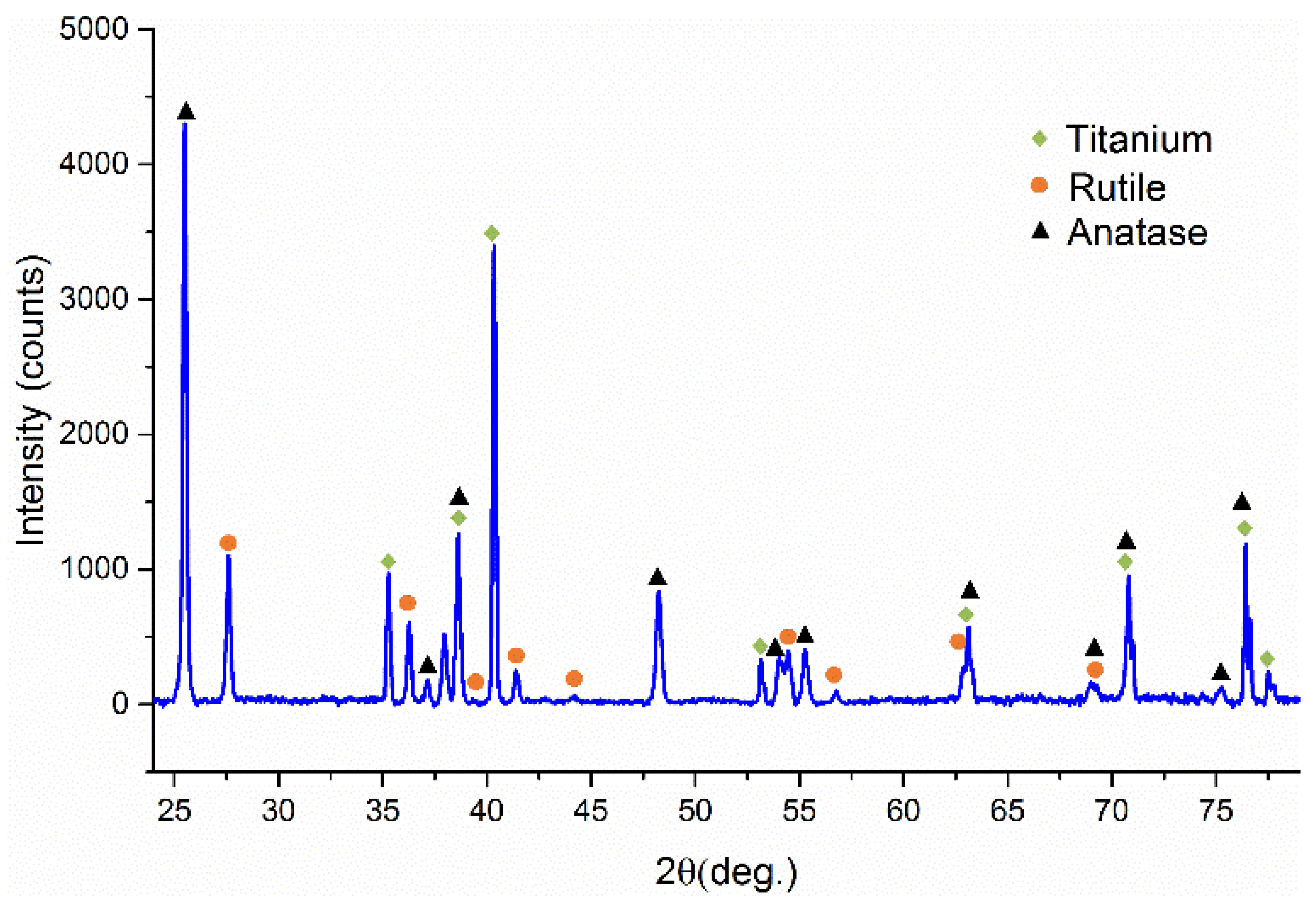
3.3. Structural Characterisation of Green and Sintered Samples
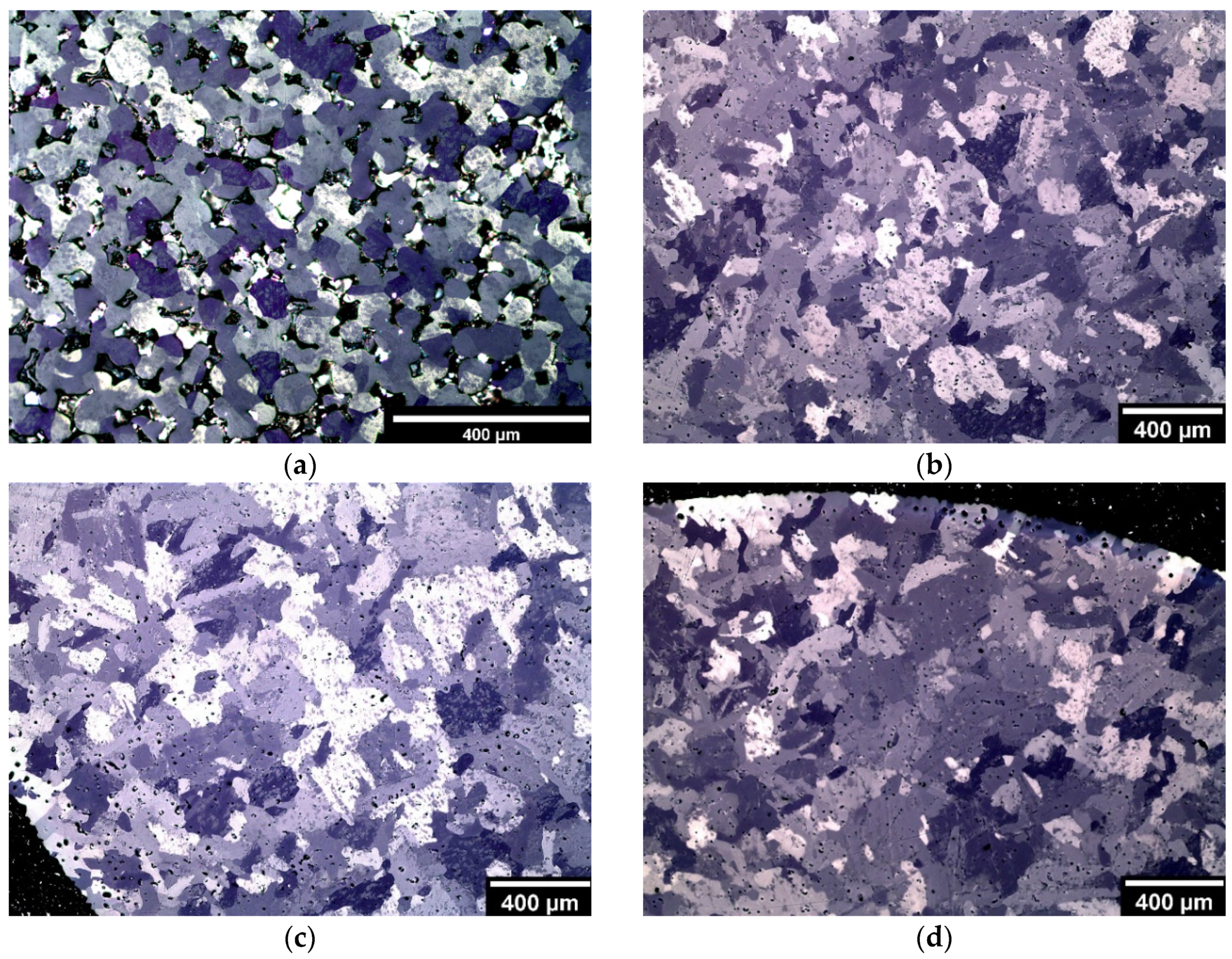
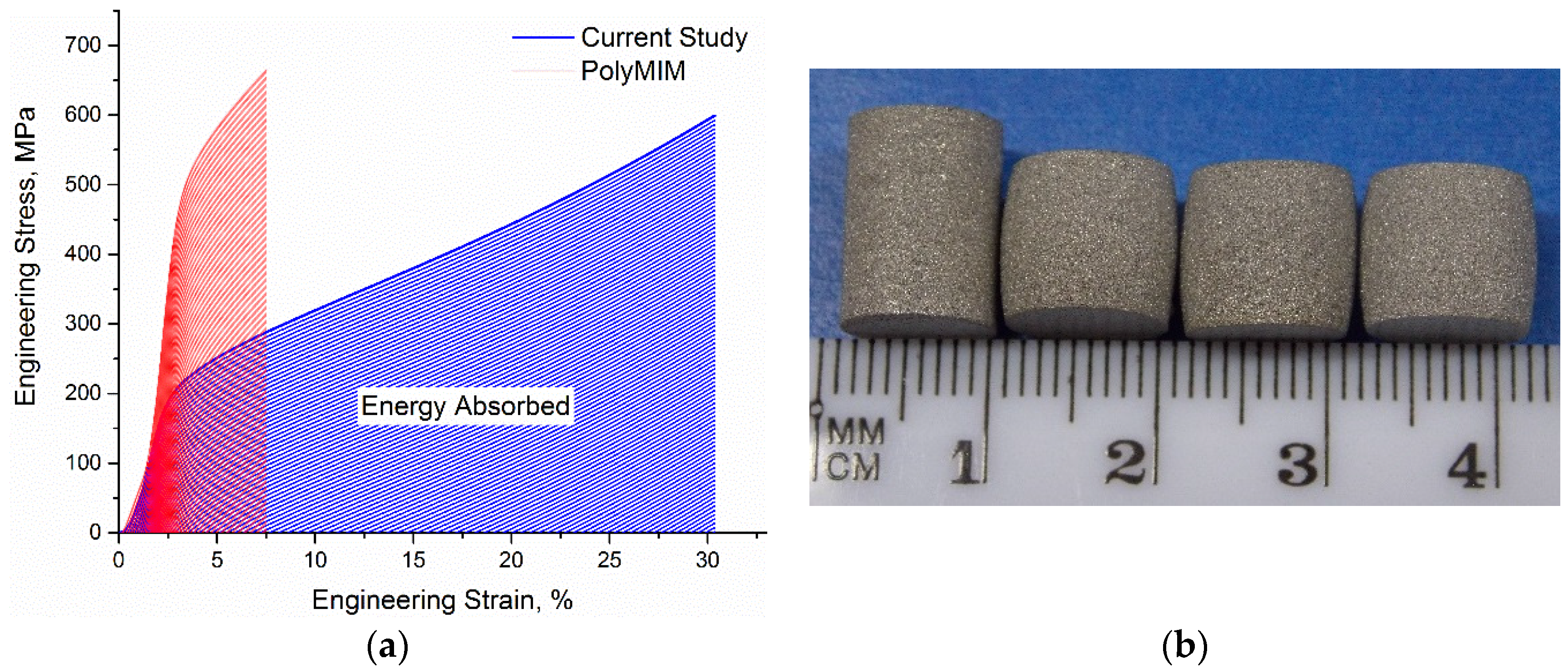
3.4. Microstructural and Mechanical Analysis Results
3.5. Characteristics of PEO-Coated Samples
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heaney, D.F. Handbook of Metal Injection Molding; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- German, R.M. Progress in titanium metal powder injection molding. Materials 2013, 6, 3641–3662. [Google Scholar] [CrossRef]
- Wen, G.; Cao, P.; Gabbitas, B.; Zhang, D.; Edmonds, N. Development and design of binder systems for titanium metal injection molding: An overview. Metall. Mater. Trans. A 2013, 44, 1530–1547. [Google Scholar] [CrossRef] [Green Version]
- Qian, M.; Froes, F.H. Titanium Powder Metallurgy: Science, Technology and Applications; Butterworth-Heinemann: London, UK, 2015. [Google Scholar]
- Komeya, K.; Inoue, H. Sintering of aluminium nitride: Particle size dependence of sintering kinetics. J. Mater. Sci. 1969, 4, 1045–1050. [Google Scholar] [CrossRef]
- De Vasconcellos, L.M.R.; Oliveira, F.N.; de Oliveira Leite, D.; de Vasconcellos, L.G.O.; do Prado, R.F.; Ramos, C.J.; de Alencastro Graça, M.L.; Cairo, C.A.A.; Carvalho, Y.R. Novel production method of porous surface Ti samples for biomedical application. J. Mater. Sci. Mater. Med. 2012, 23, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Thelen, S.; Barthelat, F.; Brinson, L.C. Mechanics considerations for microporous titanium as an orthopedic implant material. J. Biomed. Mater. Res. A 2004, 69, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Shbeh, M.M.; Goodall, R. Open pore titanium foams via metal injection molding of metal powder with a space holder. Met. Powder Rep. 2016, 71, 450–455. [Google Scholar] [CrossRef]
- Yan, W.; Berthe, J.; Wen, C. Numerical investigation of the effect of porous titanium femoral prosthesis on bone remodeling. Mater. Des. 2011, 32, 1776–1782. [Google Scholar] [CrossRef]
- Maetzig, M.; Walcher, H. Moulding equipment for the processing of coarse powders by metal injection moulding. Powder Inject. Mould. Int. 2013, 7, 1, 69–72. [Google Scholar]
- Rebl, H.; Finke, B.; Schmidt, J.; Mohamad, H.S.; Ihrke, R.; Helm, C.A.; Nebe, J.B. Accelerated cell-surface interlocking on plasma polymer-modified porous ceramics. Mater. Sci. Eng. C 2016, 69, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Lampman, S. Characterization and Failure Analysis of Plastics; ASM International: Materials Park, OH, USA, 2003. [Google Scholar]
- Shbeh, M.M.; Goodall, R. Design of water debinding and dissolution stages of metal injection moulded porous Ti foam production. Mater. Des. 2015, 87, 295–302. [Google Scholar] [CrossRef]
- German, R.M.; Bose, A. Injection Molding of Metals and Ceramics; Metal Powder Industries Federation: Princeton, NJ, USA, 1997. [Google Scholar]
- Chhabra, R.P.; Richardson, J.F. Non-Newtonian Flow and Applied Rheology: Engineering Applications; Butterworth-Heinemann: London, UK, 2011. [Google Scholar]
- Syvitski, J.P. Principles, Methods and Application of Particle Size Analysis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Roberjot, S.; Auzene, D.; Iordache, L.; Baraldi, U. Water solvent debinding for PIM parts. PIM Int. 2011, 5, 51–54. [Google Scholar]
- Peacock, A.J. The chemistry of polyethylene. J. Macromol. Sci. C Polym. Rev. 2001, 41, 285–323. [Google Scholar] [CrossRef]
- Maier, C.; Calafut, T. Polypropylene: The Definitive User’s Guide and Databook; William Andrew: Norwich, NY, USA, 1998. [Google Scholar]
- Kumar, S.; Singh, R. Recovery of hydrocarbon liquid from waste high density polyethylene by thermal pyrolysis. Braz. J. Chem. Eng. 2011, 28, 659–667. [Google Scholar]
- Stuart, B. Infrared Spectroscopy; Wiley Online Library: Hoboken, NJ, USA, 2005. [Google Scholar]
- Klar, E.; Samal, P.K. Powder Metallurgy Stainless Steels: Processing, Microstructures, and Properties; ASM International: Materials Park, OH, USA, 2007. [Google Scholar]
- Zeng, W.; Li, S.; Chow, W. Preliminary studies on burning behavior of polymethylmethacrylate (PMMA). J. Fire Sci. 2002, 20, 297–317. [Google Scholar] [CrossRef]
- Petunina, Y.V. Effect of high oxygen and nitrogen contents on the mechanical properties of titanium. Met. Sci. Heat Treat. 1961, 3, 276–279. [Google Scholar] [CrossRef]
- Saito, T.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Murakami, T.; Miyaji, F.; Nakamura, T. Quantitative comparison of osteoconduction between porousapatite and wollastonite-containing glass-ceramics with 5 different pore sizes. Bioceram. Dev. Appl. 2011, 1, 1–3. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F.; Harley, B.A. Cellular Materials in Nature and Medicine; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Shbeh, M.M.; Goodall, R. Single- and multi-layered porous titanium via metal injection moulding. Adv. Mater. Lett. 2016. [Google Scholar] [CrossRef]
- Matykina, E.; Berkani, A.; Skeldon, P.; Thompson, G.E. Real-time imaging of coating growth during plasma electrolytic oxidation of titanium. Electrochim. Acta 2007, 53, 1987–1994. [Google Scholar] [CrossRef]
- Khan, R.; Yerokhin, A.; Li, X.; Dong, H.; Matthews, A. Influence of current density and electrolyte concentration on DC PEO titania coatings. Surf. Eng. 2014, 30, 102–108. [Google Scholar] [CrossRef]
- Barbosa, A.P.C.; Bram, M.; Stöver, D.; Buchkremer, H.P. Realization of a titanium spinal implant with a gradient in porosity by 2-component-metal injection moulding. Adv. Eng. Mater. 2013, 15, 510–521. [Google Scholar] [CrossRef]
- Loukili, A. Self-Compacting Concrete; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Li, Y.M.; Huang, B.Y.; Qu, X.H. Viscosity and melt rheology of metal injection moulding feedstocks. Powder Metall. 2013, 42, 86–90. [Google Scholar] [CrossRef]
- Krauss, V.A.; Pires, E.N.; Klein, A.N.; Fredel, M.C. Rheological properties of alumina injection feedstocks. Mater. Res. 2005, 8, 187–189. [Google Scholar] [CrossRef]
- Smith, Y. High speed mixing of MIM feedstock. Met. Powder Rep. 1991, 46, 34–37. [Google Scholar]
- Srikanth, V.; Upadhyaya, G. Effect of tungsten particle size on sintered properties of heavy alloys. Powder Technol. 1984, 39, 61–67. [Google Scholar] [CrossRef]
- Seetharaman, S. Fundamentals of Metallurgy; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Tandon, R. Net-shaping of Co-Cr-Mo (F-75) via metal injection molding. In Cobalt-Base Alloys for Biomedical Applications; ASTM International: Materials Park, OH, USA, 1999. [Google Scholar]
- Demangel, C.; Auzène, D.; Vayssade, M.; Duval, J.-L.; Vigneron, P.; Nagel, M.-D.; Puippe, J.-C. Cytocompatibility of titanium metal injection molding with various anodic oxidation post-treatments. Mater. Sci. Eng. C 2012, 32, 1919–1925. [Google Scholar] [CrossRef]
- Zhang, J.; Gungor, M.; Lavernia, E. The effect of porosity on the microstructural damping response of 6061 aluminium alloy. J. Mater. Sci. 1993, 28, 1515–1524. [Google Scholar] [CrossRef]
- Thorwarth, G.; Mändl, S.; Rauschenbach, B. Rutile formation and oxygen diffusion in oxygen PIII-treated titanium. Surf. Coat. Technol. 2001, 136, 236–240. [Google Scholar] [CrossRef]
- Chu, P.-J.; Wu, S.-Y.; Chen, K.-C.; He, J.-L.; Yerokhin, A.; Matthews, A. Nano-structured TiO2 films by plasma electrolytic oxidation combined with chemical and thermal post-treatments of titanium, for dye-sensitised solar cell applications. Thin Solid Films 2010, 519, 1723–1728. [Google Scholar] [CrossRef]
- Aliasghari, S.; Skeldon, P.; Thompson, G. Plasma electrolytic oxidation of titanium in a phosphate/silicate electrolyte and tribological performance of the coatings. Appl. Surf. Sci. 2014, 316, 463–476. [Google Scholar] [CrossRef]
- Novaes, A.B., Jr.; Souza, S.L.; Barros, R.R.; Pereira, K.K.; Iezzi, G.; Piattelli, A. Influence of implant surfaces on osseointegration. Braz. Dent. J. 2010, 21, 471–481. [Google Scholar] [PubMed]
- Yang, B.; Uchida, M.; Kim, H.-M.; Zhang, X.; Kokubo, T. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 2004, 25, 1003–1010. [Google Scholar] [CrossRef]



| Wavenumber (cm−1) | Type of Vibration |
|---|---|
| 2950/2915 | C–H Stretching |
| 2883 | CH3 and CH2 Stretching |
| 2849 | C–H Stretching |
| 718 | CH2 Rocking (Methylene Rocking) |
| 1466 | C–H Scissoring (Methylene Scissoring) [21] |
| Feedstock | Temperature (°C) | Yield Stress (kPa) | n |
|---|---|---|---|
| Commercial | 170 | 135 | 0.49 |
| 185 | 118 | 0.45 | |
| 195 | 107 | 0.42 | |
| Current Study | 120 | 79 | 0.63 |
| 135 | 42 | 0.56 | |
| 150 | 35 | 0.53 |
| Contaminant | Sintered Sample from Commercial Feedstock (wt %) | Sintered Sample in the Current Study (wt %) | Ti Powder (wt %) |
|---|---|---|---|
| C | 0.044 | 0.079 | 0.006 |
| O | 0.273 | 0.277 | 0.093 |
| N | 0.033 | 0.023 | 0.010 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menhal Shbeh, M.; Yerokhin, A.; Goodall, R. Microporous Titanium through Metal Injection Moulding of Coarse Powder and Surface Modification by Plasma Oxidation. Appl. Sci. 2017, 7, 105. https://doi.org/10.3390/app7010105
Menhal Shbeh M, Yerokhin A, Goodall R. Microporous Titanium through Metal Injection Moulding of Coarse Powder and Surface Modification by Plasma Oxidation. Applied Sciences. 2017; 7(1):105. https://doi.org/10.3390/app7010105
Chicago/Turabian StyleMenhal Shbeh, Mohammed, Aleksey Yerokhin, and Russell Goodall. 2017. "Microporous Titanium through Metal Injection Moulding of Coarse Powder and Surface Modification by Plasma Oxidation" Applied Sciences 7, no. 1: 105. https://doi.org/10.3390/app7010105






