Autofluorescence Imaging and Spectroscopy of Human Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lung Tissue Sample Preparation
2.2. Autofluorescence Imaging and Spectral Measurement
3. Results
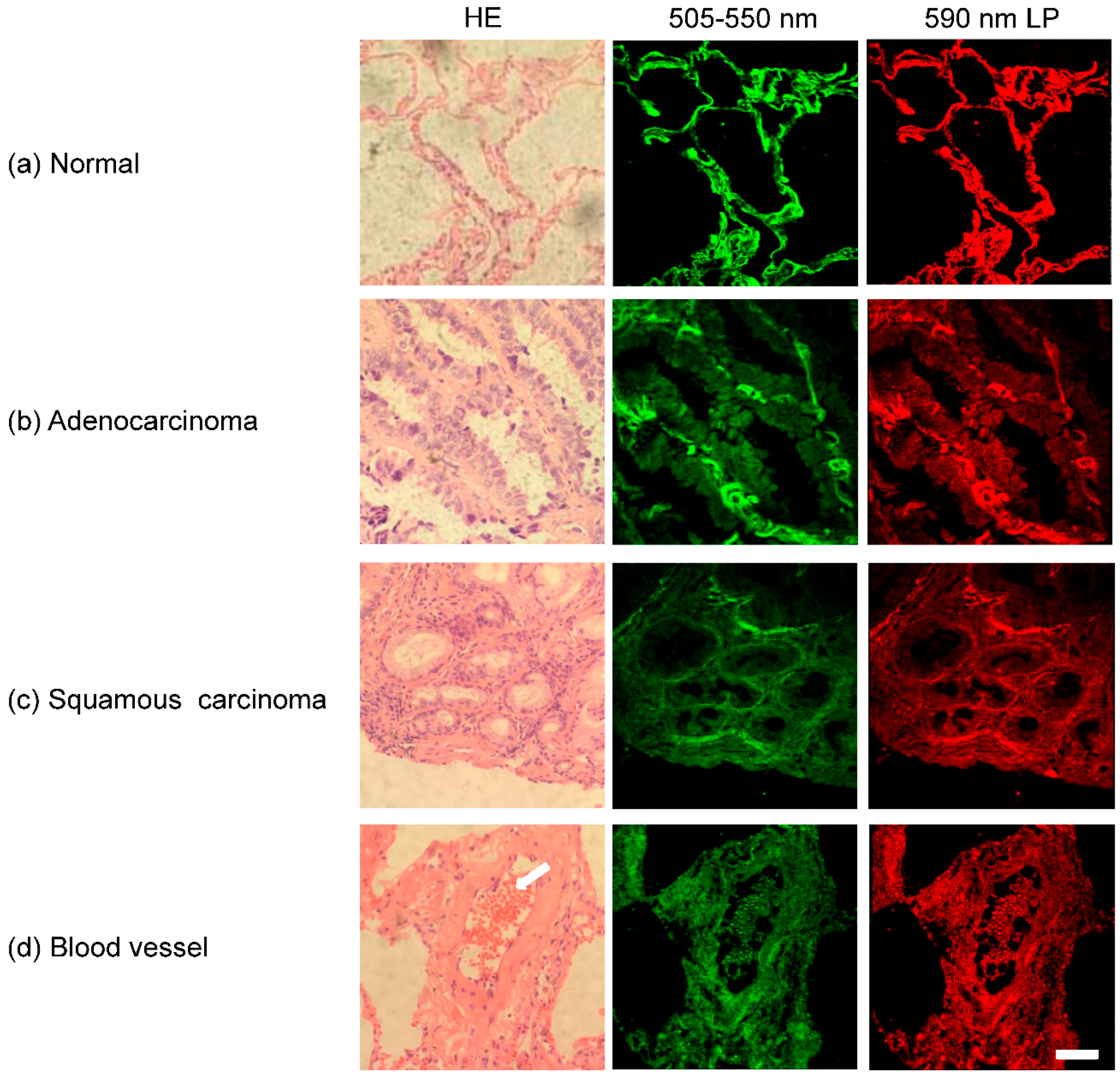
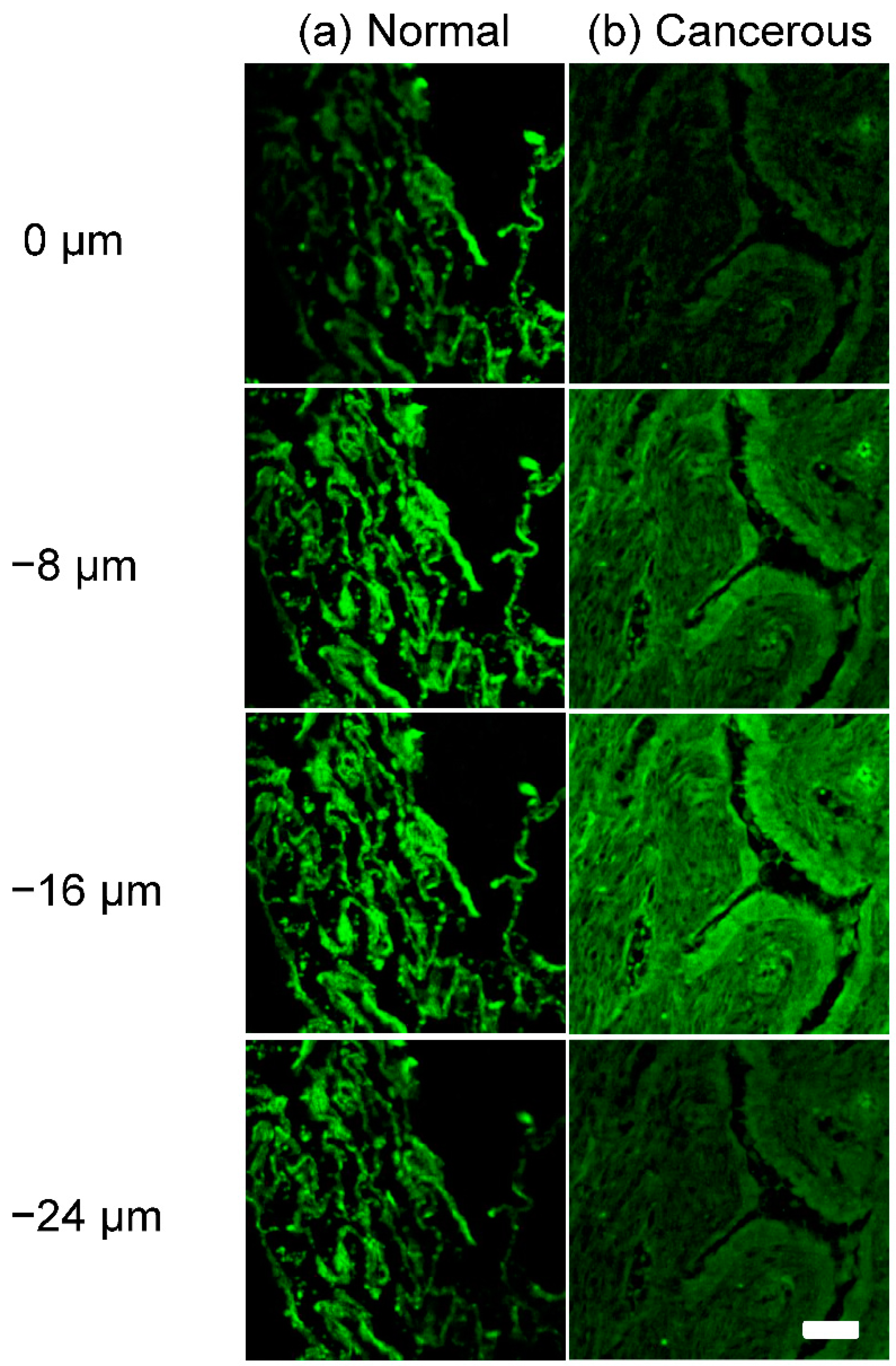
3.1. Autofluorescence Images of Lung Tissues
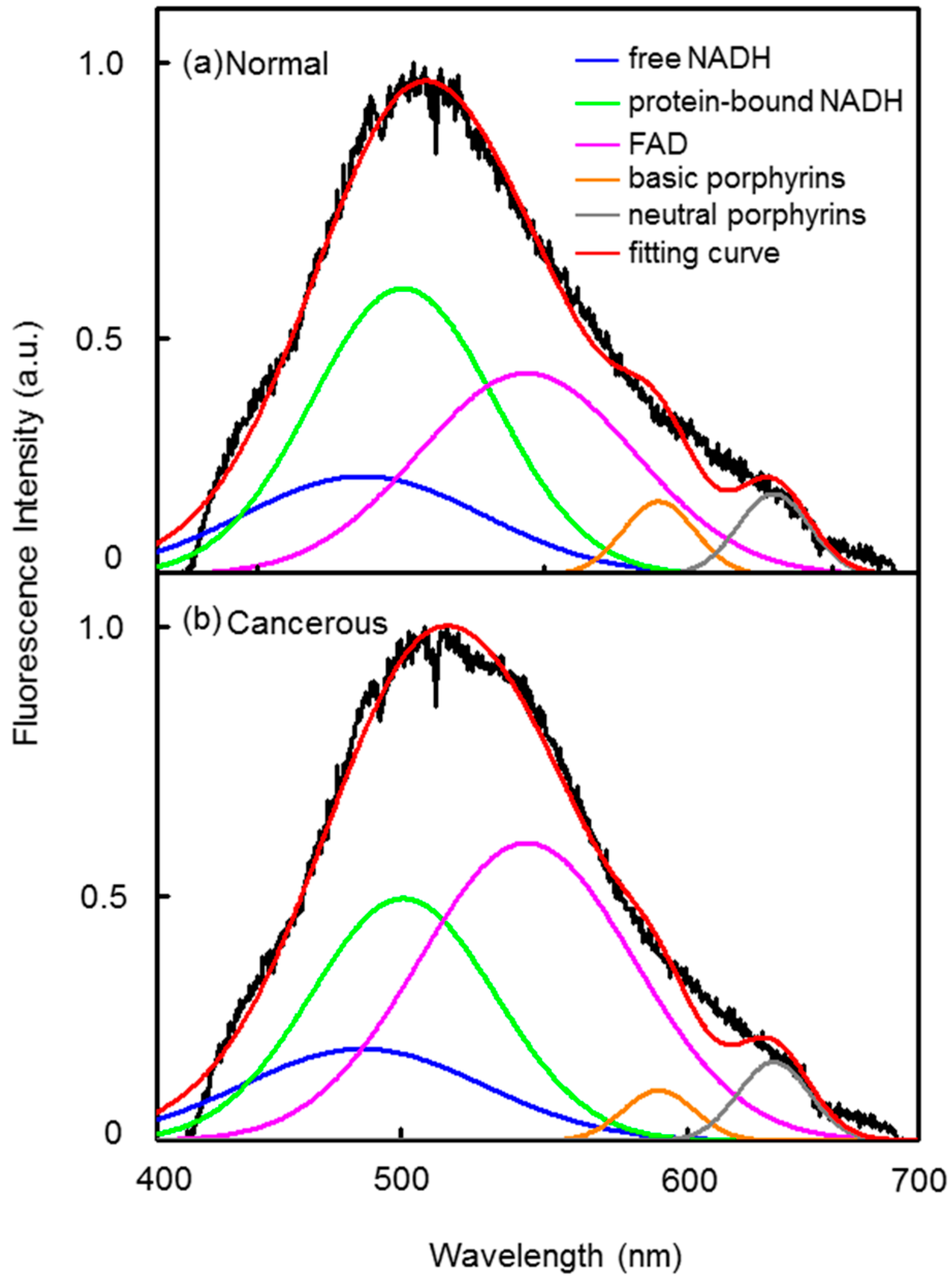
3.2. Autofluorescence Spectra of Lung Tissue Samples
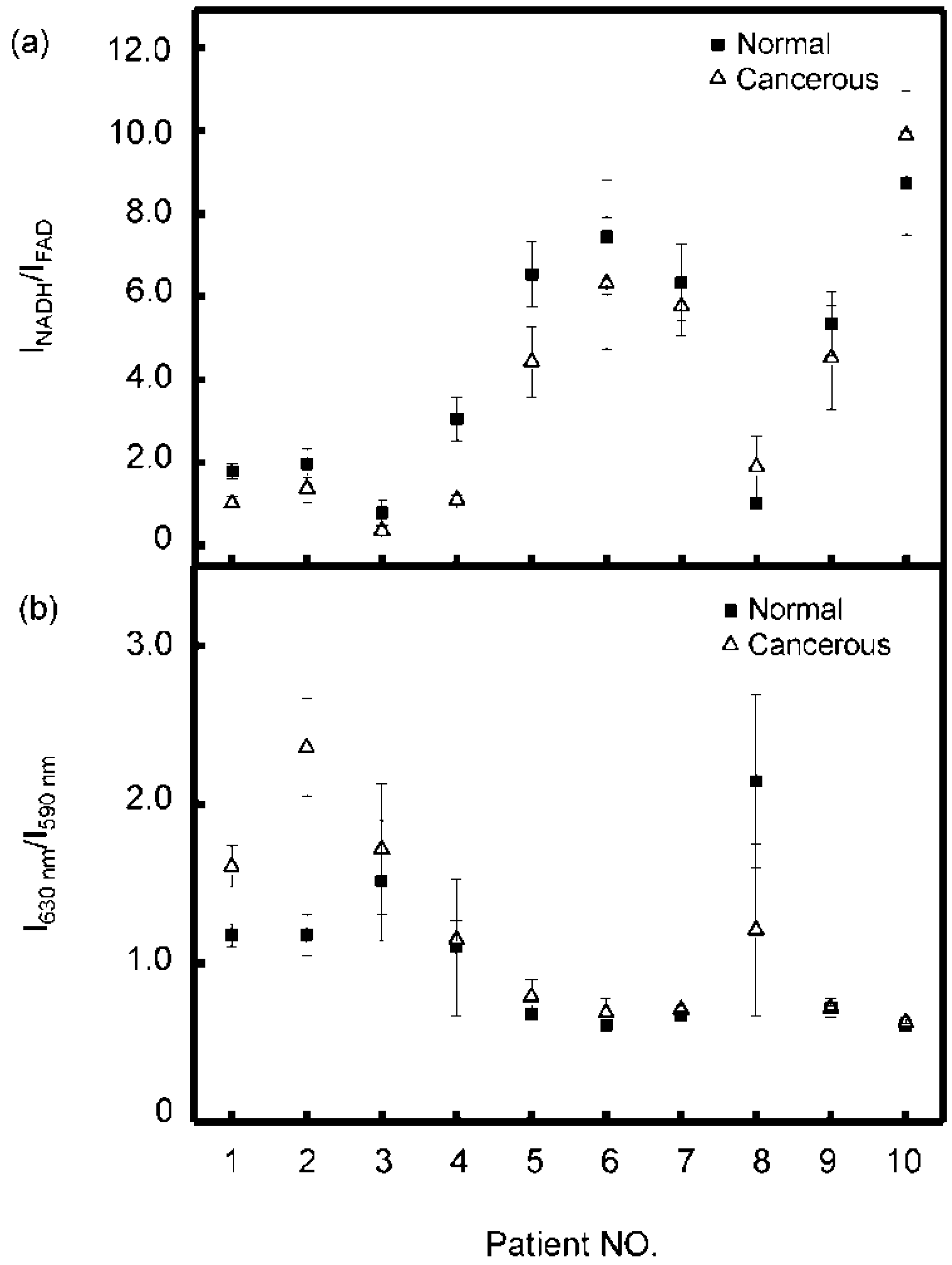
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.J.; Mak, R.H.; Yeap, B.Y.; Cryer, S.K.; Pinnell, N.E.; Christianson, L.W.; Sher, D.J.; Arvold, N.D.; Baldini, E.H.; Chen, A.B.; et al. Aggressive therapy for patients with non-small cell lung carcinoma and synchronous brain-only oligometastatic disease is associated with long-term survival. Lung Cancer 2014, 85, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Mordant, P.; Grand, B.; Cazes, A.; Foucault, C.; Dujon, A.; Barthes, F.L.; Riquet, M. Adenosquamous carcinoma of the lung: Surgical management, pathologic characteristics, and prognostic implications. Ann. Thorac. Surg. 2013, 95, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Gaponova, A.; Nikonova, A.; Deneka, A.; Egleston, B.; Litwin, S.; Duncan, J.; Duncan, K.; Borghaei, H.; Mehra, R.; Proia, D. Preclinical testing demonstrates strong activity of STA-12–8666, an HSP90 inhibitor-SN-38 conjugate, in small cell lung cancer (SCLC). Cancer Res. 2015, 75, 1731. [Google Scholar] [CrossRef]
- Taioli, E.; Wolf, A.S.; Camacho-Rivera, M.; Flores, R.M. Women with malignant pleural mesothelioma have a threefold better survival rate than men. Ann. Thorac. Surg. 2014, 98, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Caprioli, R.M. Matrix sublimation/recrystallization for imaging proteins by mass spectrometry at high spatial resolution. Anal. Chem. 2011, 83, 5728–5734. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, F.T.; Zysk, A.M.; Chaney, E.J.; Kotynek, J.G.; Oliphant, U.J.; Bellafiore, F.J.; Rowland, K.M.; Johnson, P.A.; Boppart, S.A. Intraoperative evaluation of breast tumor margins with optical coherence tomography. Cancer Res. 2009, 69, 8790–8796. [Google Scholar] [CrossRef] [PubMed]
- Maygarden, S.J.; Detterbeck, F.C.; Funkhouser, W.K. Bronchial margins in lung cancer resection specimens: Utility of frozen section and gross evaluation. Mod. Pathol. 2004, 17, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Khosroshahi, M.E.; Rahmani, M. Detection and evaluation of normal and malignant cells using laser-induced fluorescence spectroscopy. J. Fluoresc. 2012, 22, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Alchab, L.; Dupuis, G.; Balleyguier, C.; Mathieu, M.C.; Fontaine-Aupart, M.P.; Farcy, R. Towards an optical biopsy for the diagnosis of breast cancer in vivo by endogenous fluorescence spectroscopy. J. Biophotonics 2010, 3, 373–384. [Google Scholar] [CrossRef] [PubMed]
- De Veld, D.; Witjes, M.; Sterenborg, H.; Roodenburg, J. The status of in vivo autofluorescence spectroscopy and imaging for oral oncology. Oral Oncol. 2005, 41, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Langhout, G.C.; Spliethoff, J.W.; Schmitz, S.J.; Aalbers, A.; van Velthuysen, M.L.; Hendriks, B.H.; Ruers, T.J.; Kuhlmann, K.F. Differentiation of healthy and malignant tissue in colon cancer patients using optical spectroscopy: A tool for image-guided surgery. Laser Surg. Med. 2015, 47, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.D.; Hyman, J.M.; Borzhemskaya, L.; Bowen, A.; McKellar, C.; Ullery, M.; Mathias, E.; Ronsick, C.; Link, J.; Wilson, M. Rapid intrinsic fluorescence method for direct identification of pathogens in blood cultures. mBio 2013, 4, e00865-13. [Google Scholar] [CrossRef] [PubMed]
- Palero, J.A.; Bader, A.N.; de Bruijn, H.S.; Sterenborg, H.J.; Gerritsen, H.C. In vivo monitoring of protein-bound and free nadh during ischemia by nonlinear spectral imaging microscopy. Biomed. Opt. Express 2011, 2, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R.; Szmacinski, H.; Nowaczyk, K.; Johnson, M.L. Fluorescence lifetime imaging of free and protein-bound nadh. Proc. Natl. Acad. Sci. USA 1992, 89, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Moesta, K.T.; Ebert, B.; Handke, T.; Nolte, D.; Nowak, C.; Haensch, W.E.; Pandey, R.K.; Dougherty, T.J.; Rinneberg, H.; Schlag, P.M. Protoporphyrin IX occurs naturally in colorectal cancers and their metastases. Cancer Res. 2001, 61, 991–999. [Google Scholar] [PubMed]
- Hung, J.; Lam, S.; Leriche, J.C.; Palcic, B. Autofluorescence of normal and malignant bronchial tissue. Laser Surg. Med. 1991, 11, 99–105. [Google Scholar] [CrossRef]
- Huang, Z.; McWilliams, A.; Lui, H.; McLean, D.I.; Lam, S.; Zeng, H. Near-infrared raman spectroscopy for optical diagnosis of lung cancer. Int. J. Cancer 2003, 107, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Bard, M.P.; Amelink, A.; Hegt, V.N.; Graveland, W.J.; Sterenborg, H.J.; Hoogsteden, H.C.; Aerts, J.G. Measurement of hypoxia-related parameters in bronchial mucosa by use of optical spectroscopy. Am. J. Respir. Crit. Care 2005, 171, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, Y.; Zeng, H. Intrinsic fluorescence spectroscopy for endoscopic detection and localization of the endobronchial cancerous lesions. J. Biomed. Opt. 2008, 13, 064022–064028. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, H. Early detection of lung cancer and the role of endoscopic fluorescence imaging. Med. Laser Appl. 2003, 18, 20–26. [Google Scholar] [CrossRef]
- Gabrecht, T.; Radu, A.; Zellweger, M.; Lovisa, B.; Goujon, D.; Grosjean, P.; van den Bergh, H.; Monnier, P.; Wagnières, G. Autofluorescence bronchoscopy: Clinical experience with an optimized system in head and neck cancer patients. Med. Laser Appl. 2007, 22, 185–192. [Google Scholar] [CrossRef]
- Gabrecht, T.; Glanzmann, T.; Freitag, L.; Weber, B.-C.; van den Bergh, H.; Wagnieres, G. Optimized autofluorescence bronchoscopy using additional backscattered red light. J. Biomed. Opt. 2007, 12, 064016–064019. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, A.; Mayo, J.; MacDonald, S.; leRiche, J.C.; Palcic, B.; Szabo, E.; Lam, S. Lung cancer screening: A different paradigm. Am. J. Resp. Crit. Care 2003, 168, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Madhuri, S.; Vengadesan, N.; Aruna, P.; Koteeswaran, D.; Venkatesan, P.; Ganesan, S. Native fluorescence spectroscopy of blood plasma in the characterization of oral malignancy. Photochem. Photobiol. 2003, 78, 197–204. [Google Scholar] [CrossRef]
- Masilamani, V.; Vijmasi, T.; Al Salhi, M.; Govindaraj, K.; Vijaya-Raghavan, A.P.; Antonisamy, B. Cancer detection by native fluorescence of urine. J. Biomed. Opt. 2010, 15, 057003–057009. [Google Scholar] [CrossRef] [PubMed]
- Hariri, L.P.; Tumlinson, A.R.; Besselsen, D.G.; Utzinger, U.; Gerner, E.W.; Barton, J.K. Endoscopic optical coherence tomography and laser-induced fluorescence spectroscopy in a murine colon cancer model. Laser Surg. Med. 2006, 38, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.M.; Keely, P.J.; Breslin, T.M.; Ramanujam, N. Autofluorescence spectroscopy of normal and malignant human breast cell lines. Photochem. Photobiol. 2003, 78, 462–469. [Google Scholar] [CrossRef]
- Drezek, R.; Brookner, C.; Pavlova, I.; Boiko, I.; Malpica, A.; Lotan, R.; Follen, M.; Richards-Kortum, R. Autofluorescence microscopy of fresh cervical-tissue sections reveals alterations in tissue biochemistry with dysplasia. Photochem. Photobiol. 2001, 73, 636–641. [Google Scholar] [CrossRef]
- Pavlova, I.; Sokolov, K.; Drezek, R.; Malpica, A.; Follen, M.; Richards-Kortum, R. Microanatomical and biochemical origins of normal and precancerous cervical autofluorescence using laser-scanning fluorescence confocal microscopy. Photochem. Photobiol. 2003, 77, 550–555. [Google Scholar] [CrossRef]
- Evers, D.; Hendriks, B.; Lucassen, G.; Ruers, T. Optical spectroscopy: Current advances and future applications in cancer diagnostics and therapy. Future Oncol. 2012, 8, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.J.; Howington, J.; Feigenberg, S.; Movsas, B.; Pisters, K. Treatment of non-small cell lung cancer stage I and stage II: Accp evidence-based clinical practice guidelines. Chest 2007, 132 (Suppl. 3), 234S–242S. [Google Scholar] [CrossRef] [PubMed]
- Proietti, A.; Alì, G.; Pelliccioni, S.; Lupi, C.; Sensi, E.; Boldrini, L.; Servadio, A.; Chella, A.; Ribechini, A.; Cappuzzo, F. Anaplastic lymphoma kinase gene rearrangements in cytological samples of non–small cell lung cancer: Comparison with histological assessment. Cancer Cytopathol. 2014, 122, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Berezin, M.Y.; Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, R.; Masilamani, V.; Alsalhi, M.S.; Devanesan, S.; Ramamurthy, P.; Palled, S.R.; Ganesh, K.M. Cervical cancer detection by time-resolved spectra of blood components. J. Biomed. Opt. 2014, 19, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Koziol, B.; Markowicz, M.; Kruk, J.; Plytycz, B. Riboflavin as a source of autofluorescence in eisenia fetida coelomocytes. Photochem. Photobiol. 2006, 82, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Tang, G.; Wang, W.; Savage, H.; Schantz, S.; Alfano, R. Native fluorescence spectroscopic evaluation of chemotherapeutic effects on malignant cells using nonnegative matrix factorization analysis. Technol. Cancer Res. Treat. 2011, 10, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Song, C.; Ma, J.; Mi, L. Diagnosis of cervical cancer by auto-fluorescence spectrum. In Proceedings of the International Conference Innovative Optical Health Science, Shanghai, China, 10–12 October 2016.
- Masilamani, V.; Al-Zhrani, K.; Al-Salhi, M.; Al-Diab, A.; Al-Ageily, M. Cancer diagnosis by autofluorescence of blood components. J. Lumin. 2004, 109, 143–154. [Google Scholar]
- Zhuo, S.; Yan, J.; Chen, G.; Chen, J.; Liu, Y.; Lu, J.; Zhu, X.; Jiang, X.; Xie, S. Label-free monitoring of colonic cancer progression using multiphoton microscopy. Biomed. Opt. Express 2011, 2, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Ostrander, J.H.; McMahon, C.M.; Lem, S.; Millon, S.R.; Brown, J.Q.; Seewaldt, V.L.; Ramanujam, N. Optical redox ratio differentiates breast cancer cell lines based on estrogen receptor status. Cancer Res. 2010, 70, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Spliethoff, J.W.; Evers, D.J.; Klomp, H.M.; Sandick, J.W.V.; Wouters, M.W.; Nachabe, R.; Lucassen, G.W.; Hendriks, B.H.W.; Wesseling, J.; Ruers, T.J.M. Improved identification of peripheral lung tumors by using diffuse reflectance and fluorescence spectroscopy. Lung Cancer 2013, 80, 165–171. [Google Scholar] [CrossRef] [PubMed]
- DaCosta, R.; Andersson, H.; Cirocco, M.; Marcon, N.; Wilson, B. Autofluorescence characterisation of isolated whole crypts and primary cultured human epithelial cells from normal, hyperplastic, and adenomatous colonic mucosa. J. Clin. Pathol. 2005, 58, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, C.; Wang, M.; Xie, Y. Rapid, label-free, and highly sensitive detection of cervical cancer with fluorescence lifetime imaging microscopy. IEEE J. Sel. Top. Quantum Electron. 2015, 22, 6801307. [Google Scholar] [CrossRef]
- Mujat, C.; Greiner, C.; Baldwin, A.; Levitt, J.M.; Tian, F.; Stucenski, L.A.; Hunter, M.; Kim, Y.L.; Backman, V.; Feld, M. Endogenous optical biomarkers of normal and human papillomavirus immortalized epithelial cells. Int. J. Cancer 2008, 122, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Richards-Kortum, R.; Sevick-Muraca, E. Quantitative optical spectroscopy for tissue diagnosis. Annu. Rev. Phys. Chem. 1996, 47, 555–606. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Ikeda, N.; Nonoguchi, N.; Kajimoto, Y.; Miyatake, S.-I.; Hagiya, Y.; Ogura, S.-I.; Nakagawa, H.; Ishikawa, T.; Kuroiwa, T. Enhanced expression of coproporphyrinogen oxidase in malignant brain tumors: CPOX expression and 5-ALA–induced fluorescence. Neuro Oncol. 2011, 13, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-J.; Shieh, M.-J.; Lin, F.-H.; Lou, P.-J.; Peng, C.-L.; Wei, M.-F.; Yao, C.-J.; Lai, P.-S.; Young, T.-H. Colorectal cancer cell detection by 5-aminolaevulinic acid-loaded chitosan nano-particles. Cancer Lett. 2009, 273, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, R.; Masilamani, V.; Sivaji, K.; Elangovan, M.; Selvaraj, V.; Balamurugan, S.; Al-Salhi, M. Fluorescence spectra of blood components for breast cancer diagnosis. Photomed. Laser Surg. 2008, 26, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, V.; AlSalhi, M.S.; Vijmasi, T.; Govindarajan, K.; Rai, R.R.; Atif, M.; Prasad, S.; Aldwayyan, A.S. Fluorescence spectra of blood and urine for cervical cancer detection. J. Biomed. Opt. 2012, 17, 0980011–0980016. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Machiels, J.-P.; Coulie, P.G.; Berliere, M.; Poncelet, A.J.; Tombal, B.; Stainier, A.; Legrand, C.; Canon, J.-L.; Kremer, Y. Neutrophil: Lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann. Surg. Oncol. 2013, 20, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Cedres, S.; Torrejon, D.; Martinez, A.; Martinez, P.; Navarro, A.; Zamora, E.; Mulet-Margalef, N.; Felip, E. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin. Transl. Oncol. 2012, 14, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Domagala-Kulawik, J. Effects of cigarette smoke on the lung and systemic immunity. J. Physiol. Pharmacol. 2008, 59, 19–34. [Google Scholar] [PubMed]
| Fluorophores | Excitation (nm) | Emission Peak (nm) | FWHM (nm) |
|---|---|---|---|
| Free NADH [39] | 405 | 487 | 84 |
| Protein-bound NADH [39] | 405 | 501 | 64 |
| Free FAD [39] | 405 | 544 | 75 |
| Basic form of porphyrins [40] | 405 | 590 | 25 |
| Neutral form of porphyrins [40] | 405 | 630 | 25 |
| Tryptophan [38] | 287 | 342 | 65 |
| Collagen [38] | 339 | 380 | 40 |
| Elastin [38] | 351 | 410 | 70 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Long, F.; Tang, F.; Jing, Y.; Wang, X.; Yao, L.; Ma, J.; Fei, Y.; Chen, L.; Wang, G.; et al. Autofluorescence Imaging and Spectroscopy of Human Lung Cancer. Appl. Sci. 2017, 7, 32. https://doi.org/10.3390/app7010032
Wang M, Long F, Tang F, Jing Y, Wang X, Yao L, Ma J, Fei Y, Chen L, Wang G, et al. Autofluorescence Imaging and Spectroscopy of Human Lung Cancer. Applied Sciences. 2017; 7(1):32. https://doi.org/10.3390/app7010032
Chicago/Turabian StyleWang, Mengyan, Feng Long, Feng Tang, Yueyue Jing, Xinyi Wang, Longfang Yao, Jiong Ma, Yiyan Fei, Li Chen, Guifang Wang, and et al. 2017. "Autofluorescence Imaging and Spectroscopy of Human Lung Cancer" Applied Sciences 7, no. 1: 32. https://doi.org/10.3390/app7010032






