Improving Corrosion Resistance and Biocompatibility of Magnesium Alloy by Sodium Hydroxide and Hydrofluoric Acid Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formation of the Chemical Conversion Layers
2.2. Surface Characterization
2.3. Corrosion Behaviors
2.3.1. Potentiodynamic Polarization Curve
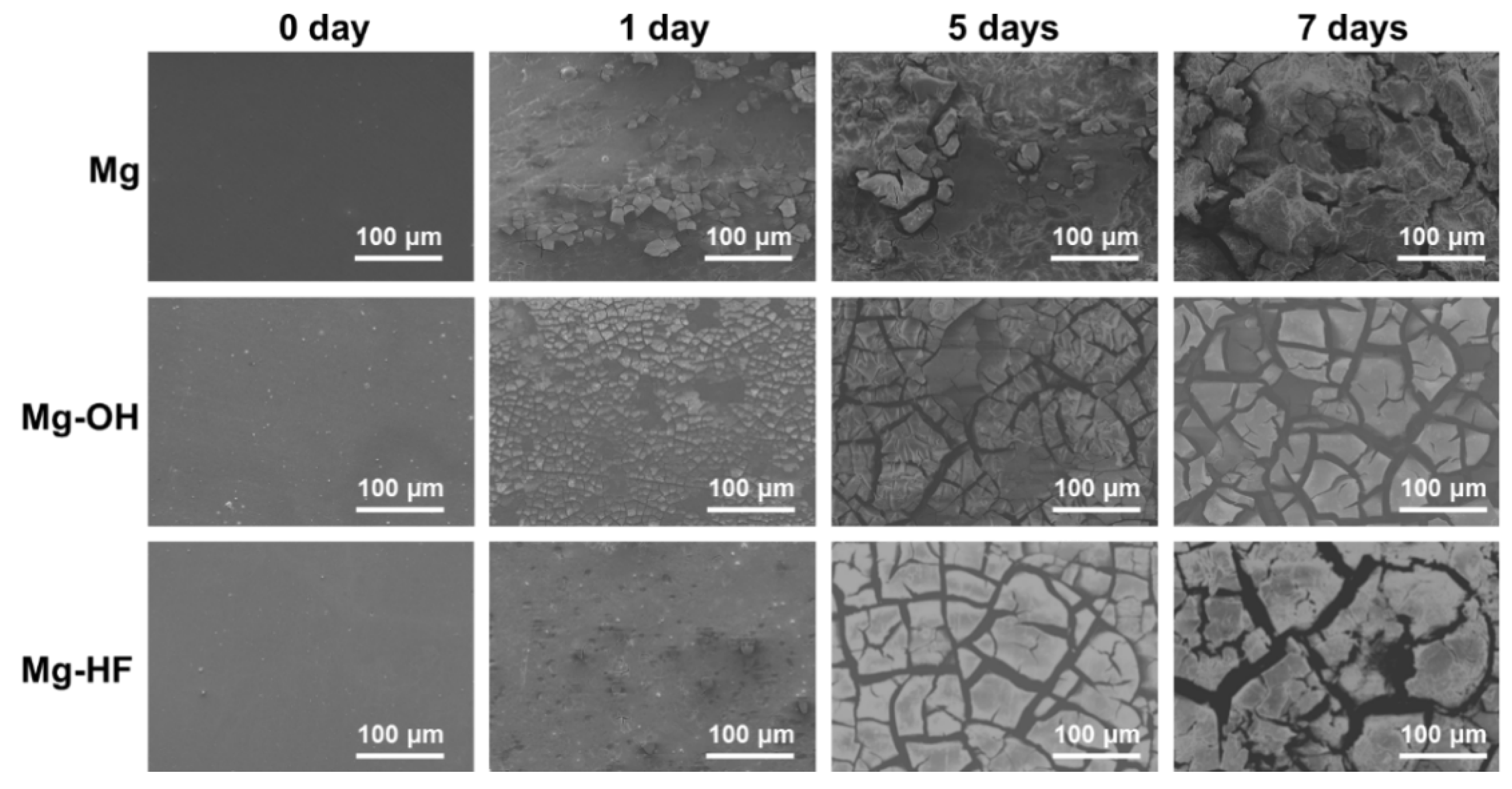
2.3.2. Immersion Test
2.4. Anticoagulation
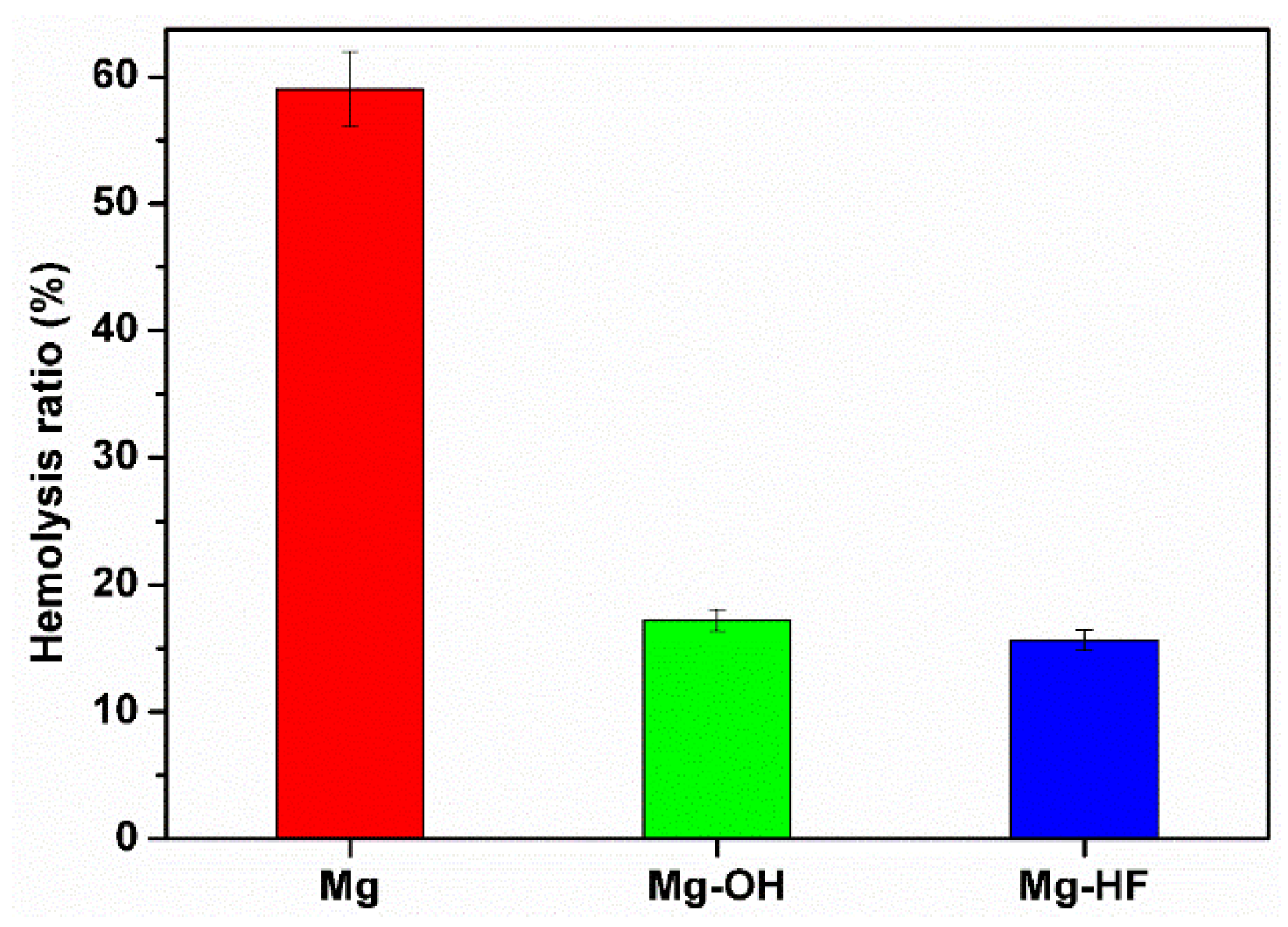
2.4.1. Hemolysis Rate
2.4.2. Platelet Adhesion
2.5. Endothelial Cell Behaviors
2.5.1. Cell Adhesion
2.5.2. CCK-8
2.6. Statistical Analysis
3. Results and Discussion
3.1. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR) and X-ray Photoelectron Spectroscopy (XPS)
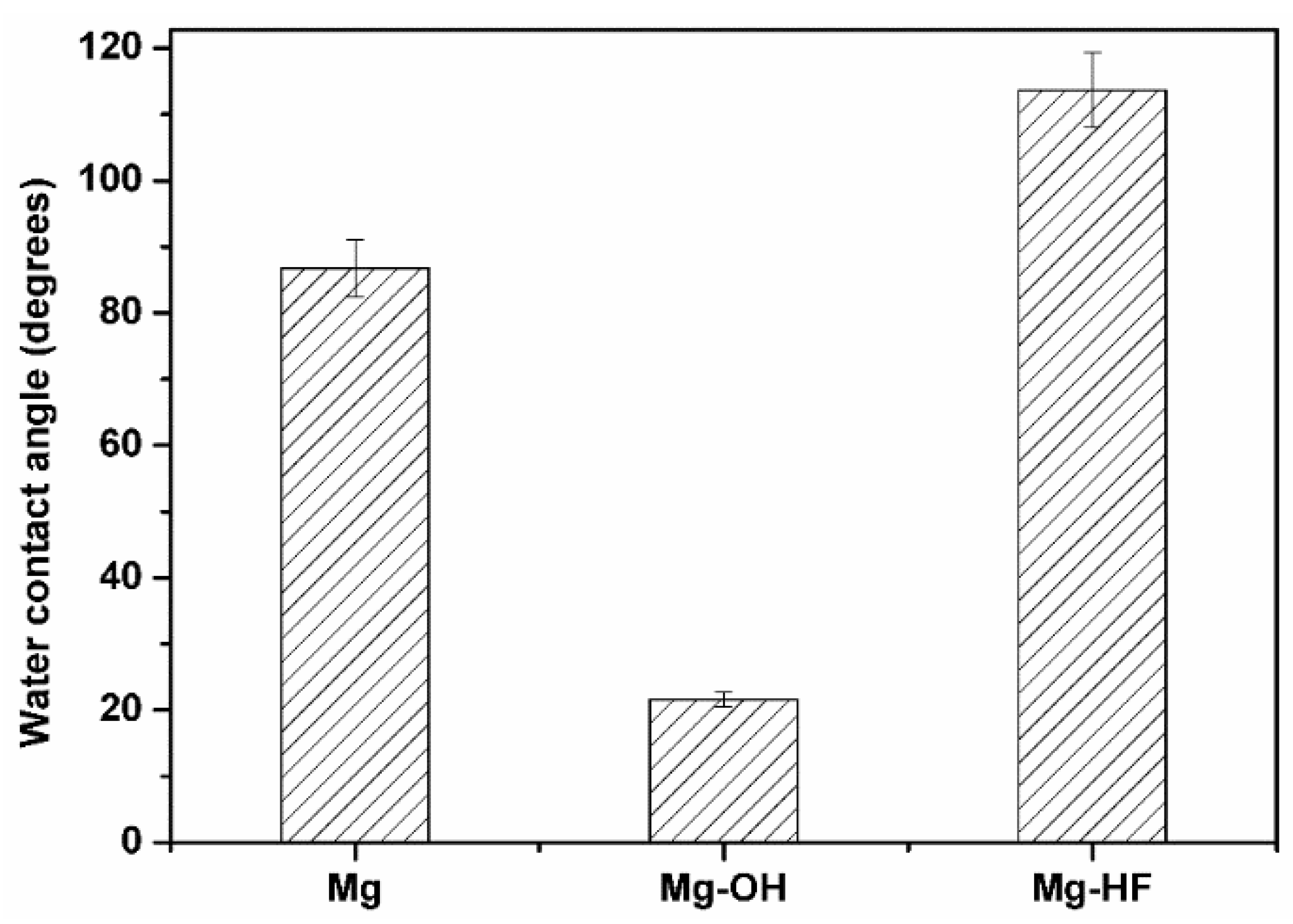
3.2. Surface Hydrophilicity
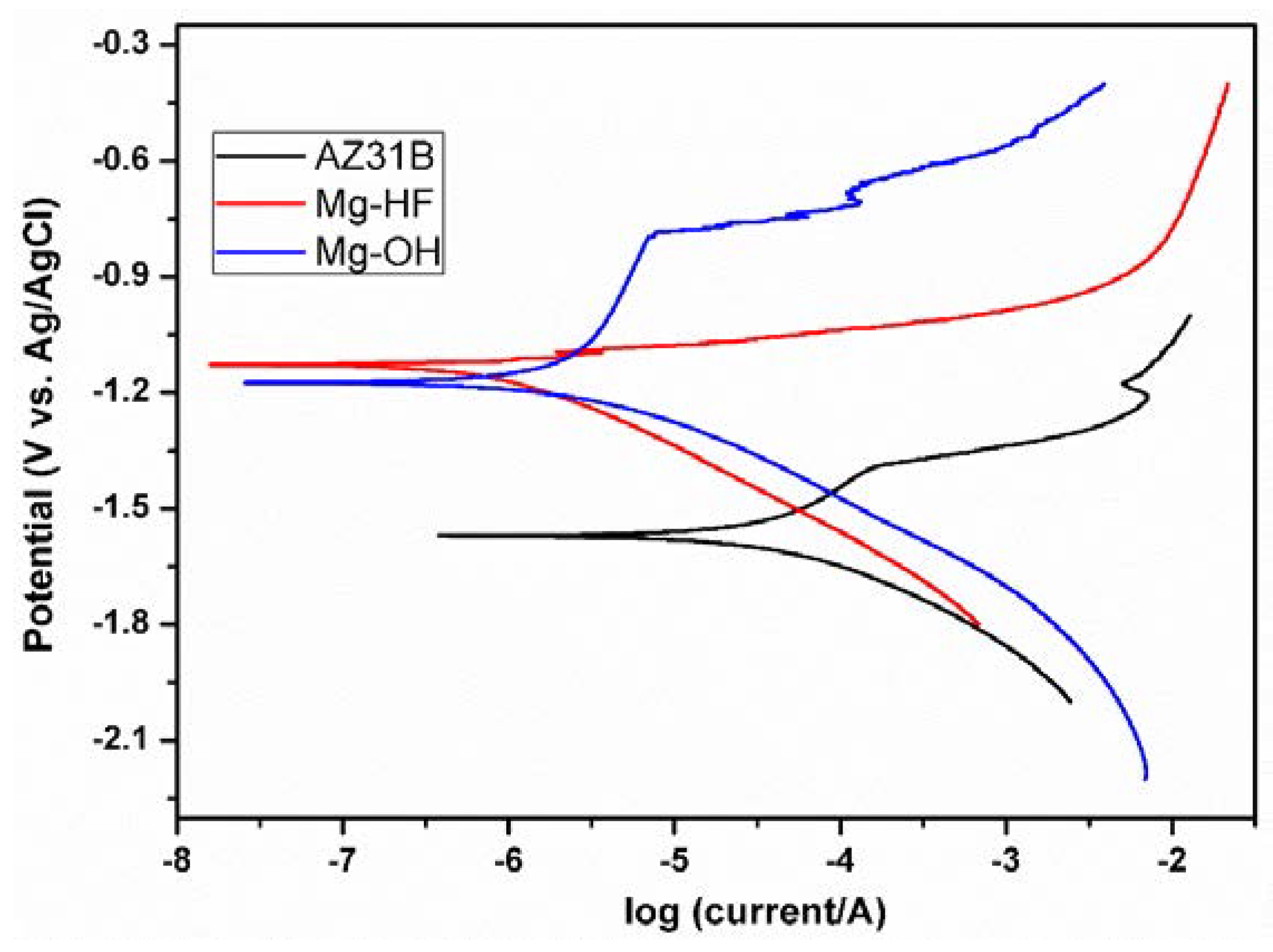
3.3. Corrosion Behaviors
3.4. Anticoagulation
3.5. Interactions with Endothelial Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.F.; Staiger, M.P.; Dias, G.J. Magnesium biomaterials for orthopedic application: A review from biological perspective. J. Biomed. Mater. Res. Part B Appl. Biometer. 2014, 102B, 1316–1331. [Google Scholar] [CrossRef] [PubMed]
- Manivasagam, G.; Suwas, S. Biodegradable Mg and Mg based alloys for biomedical implants. Mater. Sci. Technol. 2014, 30, 515–520. [Google Scholar] [CrossRef]
- Gu, X.N.; Li, S.S.; Li, X.M.; Fan, Y.B. Magnesium based degradable biomaterials: A review. Front. Mater. Sci. 2014, 8, 200–218. [Google Scholar] [CrossRef]
- Stainger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Shen, F. Blood compatibility of a ferulic acid (FA)-eluting PHBHHx system for biodegradable magnesium stent application. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 37–45. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Wu, Y.; Zhang, Y.; Zhu, Y.; Liu, Y.; Li, M.; Zheng, G.; He, B.; Yin, Q.; Zheng, Y.; et al. Addition of Zn to the ternary Mg-Ca-Sr alloys significantly improves their antibacterial properties. J. Mater. Chem. B 2015, 3, 6676–6689. [Google Scholar] [CrossRef] [PubMed]
- Williams, D. New interests in magnesium. Med. Device Technol. 2006, 17, 9–10. [Google Scholar] [PubMed]
- Song, G. Control of biodegradation of biocompatible magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Yang, J.; Cui, F.; Lee, I.S. Surface modifications of magnesium alloys for biomedical applications. Ann. Biomed. Eng. 2011, 39, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Savalani, M.M.; Pizarro, J.M. Effect of preheat and layer thickness on selective laser melting (SLM) of magnesium. Rapid Prototyp. J. 2016, 22, 115–122. [Google Scholar] [CrossRef]
- Hu, L.; Meng, Q.; Chen, S.; Wang, H. Effect of Zn content on the chemical conversion treatments of AZ91D magnesium alloy. Appl. Surf. Sci. 2012, 259, 816–823. [Google Scholar] [CrossRef]
- Kannan, M.B.; Walter, R.; Yamamoto, A. Biocompatibility and in vitro degradation behavior of magnesium-calcium alloy coated with calcium phosphate using an unconventional electrolyte. ACS Biomater. Sci. Eng. 2016, 2, 56–64. [Google Scholar] [CrossRef]
- Yang, J.; Cui, F.Z.; Lee, I.S.; Wang, X. Plasma surface modification of magnesium alloy for biomedical application. Surf. Coat. Technol. 2010, 205, 5182–5187. [Google Scholar] [CrossRef]
- Ye, S.H.; Jang, Y.S.; Yun, Y.H.; Shankarraman, V.; Woolley, J.R.; Hong, Y.; Gamble, L.J.; Ishihara, K.; Wagner, W.R. Surface modification of a biodegradable magnesium alloy with phosphorylcholine (PC) and sulfobetaine (SB) functional macromolecules for reduced thrombogenicity and acute corrosion resistance. Langmuir 2013, 29, 8320–8327. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, F.; Li, H.L.; Zhu, W.J.; Pan, H.B.; Tan, G.X.; Lao, Y.H.; Ning, C.Y.; Ni, G.X. Corrosion mechanism of micro-arc oxidation treated biocompatible AZ31 magnesium alloy in simulated body fluid. Prog. Nat. Sci. Mater. Int. 2014, 24, 516–522. [Google Scholar] [CrossRef]
- Xue, D.; Yun, Y.; Schulz, M.J.; Shanov, V. Corrosion protection of biodegradable magnesium implants using anodization. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 215–223. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sharkeev, Y.P.; Sinebryukhov, S.L.; Khrisanfova, O.A.; Legostaeva, E.V.; Zavidnaya, A.G.; Puz, A.V.; Khlusov, I.A.; Opra, D.P. Functional coatings formed on the titanium and magnesium alloys as implant materials by plasma electrolytic oxidation technology: Fundamental principles and synthesis conditions. Corros. Rev. 2016, 34, 65–83. [Google Scholar] [CrossRef]
- Kang, M.H.; Jang, T.S.; Jung, H.D.; Kim, S.M.; Kim, H.E.; Koh, Y.H.; Song, J. Poly (ether imide)-silica hybrid coatings for tunable corrosion behavior and improved biocompatibility of magnesium implants. Biomed. Mater. 2016, 11, 035003. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Yoo, Y. Synthesis and characterization of thin films on magnesium alloy using a hydrothermal method. Surf. Coat. Technol. 2015, 284, 26–30. [Google Scholar] [CrossRef]
- Mao, L.; Shen, L.; Chen, J.H.; Wu, Y.; Kwak, M.; Lu, Y.; Xue, Q.; Pei, J.; Zhang, L.; Yuan, G.Y.; et al. Enhanced bioactivity of Mg-Nd-Zn-Zr alloy achieved with nanoscale MgF2 surface for vascular stent application. ACS Appl. Mater. Interfaces 2015, 7, 5320–5330. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.P.; Zhang, E.L.; Yang, K. Phosphating treatment and corrosion properties of Mg-Mn-Zn alloy for biomedical application. J. Maters Sci. Mater. Med. 2009, 20, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.M.; Hussein, K.H.; Woo, H.M.; Park, C.H.; Kim, C.S. One-Step anodization deposition of anticorrosive bioceramic compounds on AZ31B magnesium alloy for biomedical application. Ceram. Int. 2015, 41, 10861–10870. [Google Scholar] [CrossRef]
- Xu, R.; Wu, G.; Yang, X.; Hu, T.; Lu, Q.; Chu, P.K. Controllable degradation of biomedical magnesium by chromium and oxygen dual ion implantation. Mater. Lett. 2011, 65, 2171–2173. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Zhang, P.; Li, Y.; Lai, Y.; Qin, L. Surface modifications of magnesium alloys developed for bioabsorbable orthopedic implants: A general review. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100B, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wan, G.; Wang, J.; Zhao, S.; Zhao, Y.; Huang, N. Covalent immobilization of phytic acid on Mg by alkaline pre-treatment: Corrosion and degradation behavior in phosphate buffered saline. Corros. Sci. 2013, 75, 280–286. [Google Scholar] [CrossRef]
- Pan, C.J.; Hou, Y.; Wang, Y.N.; Gao, F.; Liu, T.; Hou, Y.H.; Zhu, Y.F.; Ye, W.; Wang, L.R. Effects of self-assembly of 3-phosphonopropionic acid, 3-aminopropyltrimethoxysilane and dopamine on the corrosion behaviors and biocompatibility of a magnesium alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.J.; Hou, Y.H.; Zhang, B.B.; Dong, Y.X.; Ding, H.Y. Blood compatibility and interaction with endothelial cells of titanium modified by sequential immobilization of poly (ethylene glycol) and heparin. J. Mater. Chem. B 2014, 2, 892–902. [Google Scholar] [CrossRef]
- Zhen, Z.; Liu, X.; Huang, T.; Xi, T.; Zheng, Y. Hemolysis and cytotoxicity mechanisms of biodegradable magnesium and its alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.K.; Kwon, O.H.; Lee, Y.M.; Yong, K.S. Preparation and surface characterization of functional group-grafted and heparin-immobilized polyurethanes by plasma glow discharge. Biomaterials 1996, 17, 841–847. [Google Scholar] [CrossRef]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Role of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.B.; Rodriguez, I.; Venkatraman, S.S. The effect of topography of polymer surfaces on platelet adhesion. Biomaterials 2010, 31, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Sask, K.N.; McClung, W.G.; Berry, L.R.; Chan, A.K.C.; Brash, J.L. Immobilization of an antithrombin-heparin complex on gold: Anticoagulation properties and platelet interactions. Acta Biomater. 2011, 7, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K. Platelet activation mechanisms and markers in arterial thrombosis. J. Intern. Med. 1996, 239, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Sitia, S.; Tomasoni, L.; Atzeni, F.; Ambrosio, G.; Cordiano, C.; Catapano, A.; Tramontana, S.; Perticone, F.; Naccarato, P.; Camici, P.; et al. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev. 2010, 9, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.C.; Jiang, J.; Huo, K.F.; Tang, G.Y.; Chu, P.K. Corrosion resistance and cytocompatibility of biodegradable surgical magnesium alloy coated with hydrogenated amorphous silicon. J. Biomed. Mater. Res. A 2009, 89A, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, G.; Jiang, J.; Wong, H.M.; Yeung, K.W.K.; Chu, P.K. Improved corrosion resistance and cytocompatibility of magnesium alloy by two-stage cooling in thermal treatment. Corros. Sci. 2012, 59, 360–365. [Google Scholar] [CrossRef]
- Lorenz, C.; Brunner, J.G.; Kollmannsberger, P.; Jaafar, L.; Fabry, B.; Virtanen, S. Effect of surface pre-treatments on biocompatibility of magnesium. Acta Biomater. 2009, 5, 2783–2789. [Google Scholar] [CrossRef] [PubMed]
- Benhabbour, S.R.; Sheardown, H.; Adronov, A. Cell adhesion and proliferation on hydrophilic dendritically modified surfaces. Biomaterials 2008, 29, 4177–4186. [Google Scholar] [CrossRef] [PubMed]
- Bacáková, L.; Mares, V.; Bottone, M.G.; Pellicciari, C.; Lisá, V.; Svorcík, V. Fluorine ion-implanted polystyrene improves growth and viability of vascular smooth muscle cells in culture. J. Biomed. Mater. Res. 2000, 49, 369–379. [Google Scholar] [CrossRef]





| Samples | Mg | C | O | F | Al | Zn |
|---|---|---|---|---|---|---|
| Mg | 42.05 | 12.72 | 43.22 | - | 1.47 | 0.54 |
| Mg–OH | 22.15 | 22.87 | 54.12 | - | 0.86 | - |
| Mg–HF | 28.19 | 25.96 | 8.94 | 35.97 | 0.94 | - |
| Samples | Ecorr (V) | icorr (A·cm−2) |
|---|---|---|
| AZ31B | −1.570 | 3.726 × 10−5 |
| Mg–OH | −1.174 | 2.052 × 10−6 |
| Mg–HF | −1.128 | 6.821 × 10−7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, C.-J.; Pang, L.-Q.; Hou, Y.; Lin, Y.-B.; Gong, T.; Liu, T.; Ye, W.; Ding, H.-Y. Improving Corrosion Resistance and Biocompatibility of Magnesium Alloy by Sodium Hydroxide and Hydrofluoric Acid Treatments. Appl. Sci. 2017, 7, 33. https://doi.org/10.3390/app7010033
Pan C-J, Pang L-Q, Hou Y, Lin Y-B, Gong T, Liu T, Ye W, Ding H-Y. Improving Corrosion Resistance and Biocompatibility of Magnesium Alloy by Sodium Hydroxide and Hydrofluoric Acid Treatments. Applied Sciences. 2017; 7(1):33. https://doi.org/10.3390/app7010033
Chicago/Turabian StylePan, Chang-Jiang, Li-Qun Pang, Yu Hou, Yue-Bin Lin, Tao Gong, Tao Liu, Wei Ye, and Hong-Yan Ding. 2017. "Improving Corrosion Resistance and Biocompatibility of Magnesium Alloy by Sodium Hydroxide and Hydrofluoric Acid Treatments" Applied Sciences 7, no. 1: 33. https://doi.org/10.3390/app7010033





