1. Introduction
In the search of green lubricant, vegetable oil and glycerol were considered as the replacement for traditional mineral oil. Vegetable oil and glycerol are both environmental friendly lubricants, however, the thermal stability and flow properties of glycerol are better than vegetable oil. Glycerol based oil has been used as industrial lubricant, for example, the lubricant branded biolube GLY produced by Molylub of Germany is used in food processing machinery. The glycerol product branded Glycerin of P&G Co. (Cincinnati, OH, USA) is also used as a lubricant in the manufacturing of paper.
A glycerol based solution for use as a lubricant for plug valves was reported in March’s [
1] patent. In their invention, 78 wt %–89 wt % of glycerol was used as the base oil and has the effect of preventing the corrosion of underlying metal. Hefner’s invention [
2] of glycerol triether compounds were excellent lubricants for dissolution in internal combustion engine fuels, especially spark ignition engine fuels. Kyllönen [
3] formulated a lubricant that consists of glycerol, water, carboxymethyl cellulose, and sodium polyphosphate. The lubricant is used as a transport chain lubricant for the food industry, steel industry, and car industry.
In recent years, researchers have pointed out the numerous advantages of adding nanoparticles to lubricant oil. With its miniscule size, nanoparticles can easily mix with the lubricant oil to enhance its lubricating properties. The nanoparticles added to the lubricant oil help in preventing corrosion, reducing friction, and modifying the viscosity of the oil. Numerous articles have shown the effectiveness of adding nanoparticles to lubricants. Peña-Parás et al. [
4] compared the influence of two kinds of nanoparticles as additives on GL-4 fully-formulated oil and poly-alpha olefin 8 base oil. Their experimental results showed that CuO additives had better effects on tribological behavior than Al
2O
3 nanoparticles in two types of synthetic oils. With 2 wt % CuO concentration dispersed in PAO 8, the wear scar diameter reduced by 14% and the coefficient of friction reduced by 18%. Research studies [
5,
6] tested the tribological behavior of ZnO/Al
2O
3 composite nanoparticles as additives to the lubricant. Their results showed that when added to lubricant the composite nanoparticles reduced friction and wear better than pure ZnO and Al
2O
3. Gu et al. [
7] synthesized and modified the surface of TiO
2 nanoparticles with a double-coated agent. The concentration of nanoparticles ranged from 0.1 vt % to 1.6 vt % in a water-based medium. Their experimental results showed that the TiO
2-modified nanoparticles could increase the applicable load, reduce the friction force, and increase the anti-wear capacity of pure water.
Chang et al. [
8] added 25 nm TiO
2 nanoparticles into a base oil. Their experiments using lubricant with 0.01 wt % TiO
2 nanoparticle additives showed significant reduction in coefficient of friction (COF) and wear rate in comparison to using a pure base oil lubricant. Zin et al. [
9] investigated the tribology behavior of Pegasus 1005 oil with carbon nano-horns, TiO
2, and Cu nanoparticle additives. Their results showed that the friction reduction of engine oil containing Cu nanoparticles of 130 nm was more effective than the engine oil containing 50 nm nanoparticles. The COF of carbon nano-horns additives decreased by 12% when compared with raw oil, while the effect on the coefficient of friction with TiO
2 nanoparticles was negligible.
Zhang et al. [
10] synthesized copper nanoparticles and added them into paraffin oil to investigate their tribological performance. Their results showed that Cu nanoparticles of size 2–5 nm, when modified with oleic acid and oleylamine, reduced the COF of steel pair and displayed excellent thermal stability. The mechanisms of the decreased friction and inhibition of wear due to the nanoparticles in lubricant through colloidal effect, rolling effect, protective film effect, and the third body were presented in several previous studies [
11,
12,
13,
14].
The ANOVA technique and Taguchi robust design method were used to investigate the effect of parameters, such as the nanoparticle additive concentration, the applied load, temperature, and speed to the tribology behavior of lubricant. Peng et al. [
15] employed the Taguchi technique to determine the optimal aluminum nanoparticles size in oleic acid and ethanol. Their results revealed that the optimal nanoparticles size is 65 nm. Thakre et al. [
16,
17] added three kinds of Al
2O
3 nanoparticle sizes with different concentrations to regular engine oil to evaluate their tribological properties. L18 orthogonal array and ANOVA analysis were used to determine the optimum COF. The analysis results showed that the minimum coefficient of friction was obtained with 0.8 wt % of medium nanoparticles size (60 nm) and 160 N apply force at 1200 rpm rotational speed.
Koshy et al. [
18] enhanced the stabilization properties of MoS
2 nanoparticles in oil by mixing in a sodium dodecyl sulfate surfactant. The ANOVA analysis method was adopted by the authors to investigate the effect of surfactant-modified MoS
2 nanoparticles as additives on the thermal-physical properties as well as the tribological behavior.
Studies of glycerol used as lubricant have demonstrated that it has the potential to reduce friction and wear. Shi et al. [
19] studied the viscosity of glycerol solution and its lubrication characteristics and found they are better than rapeseed oil, particularly when the water content is less than 20 wt %. Zhou et al. [
20] investigated the effect of temperature and water content in glycerol in respect of friction and wear behavior using a Si
3N
4 ball on a disc tribo-tester. They found that the wear was intensified with an increase in temperature, and at 30 °C, the amount of wear was less than when the concentration of glycerol was higher. Yan et al. [
21] showed that when the ethylene glycol concentration is larger than 10% in lubricant solution, it exhibits good tribological properties in the sliding bearings of self-mated Si
3N
4 balls.
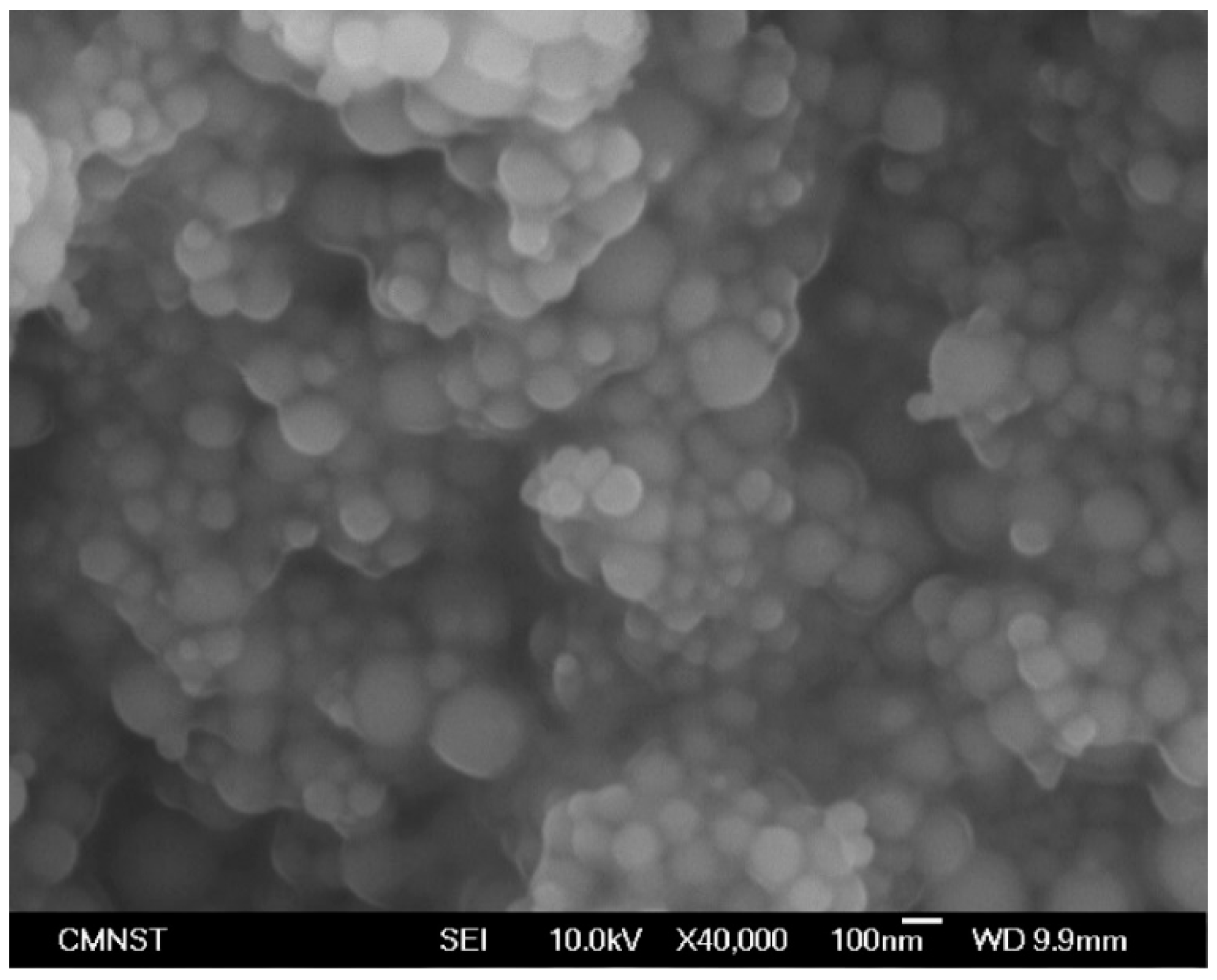
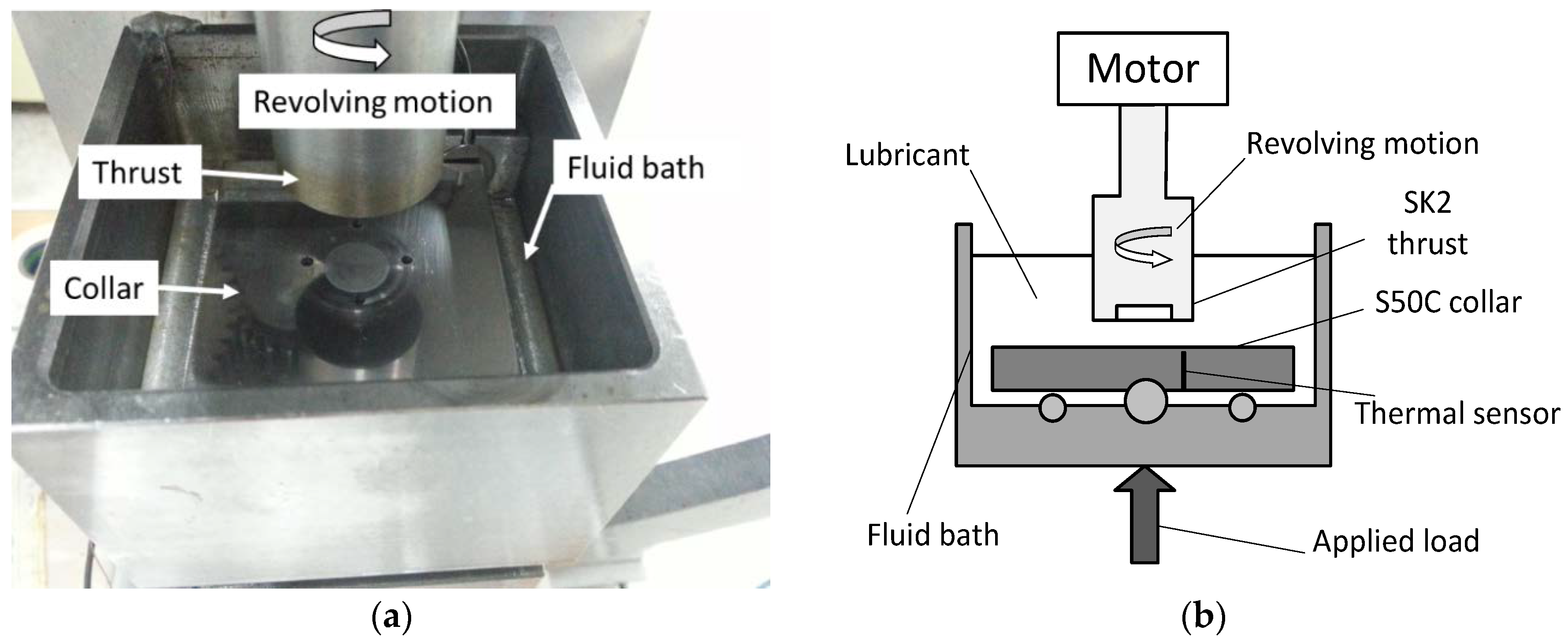
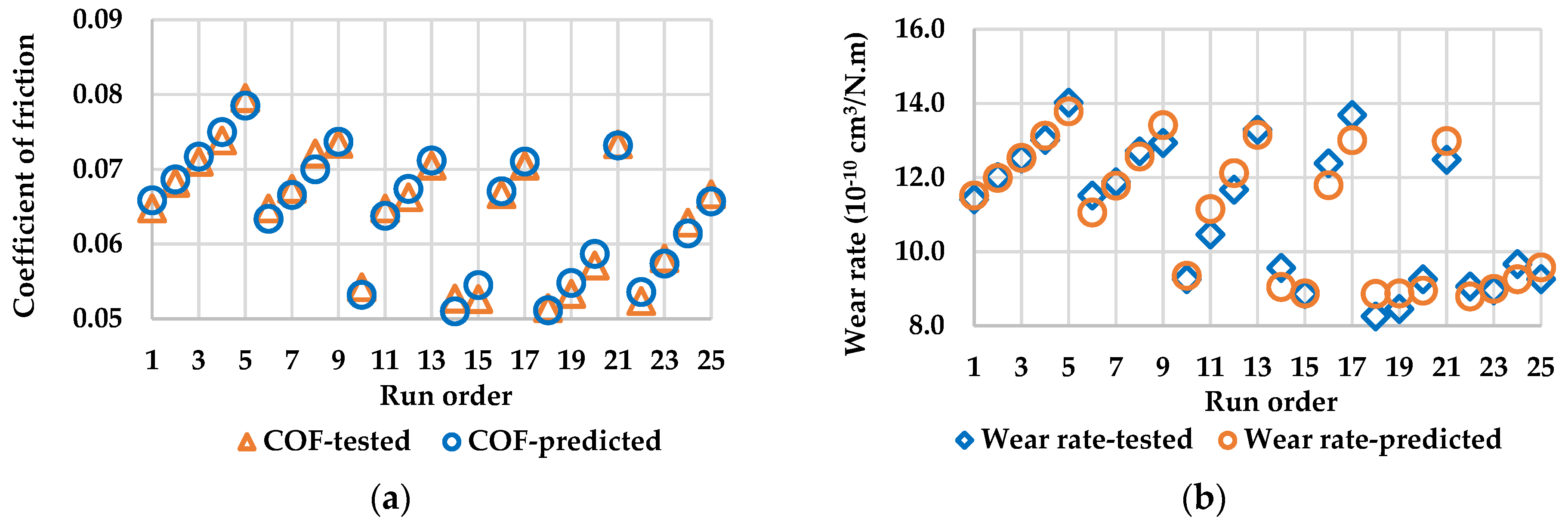
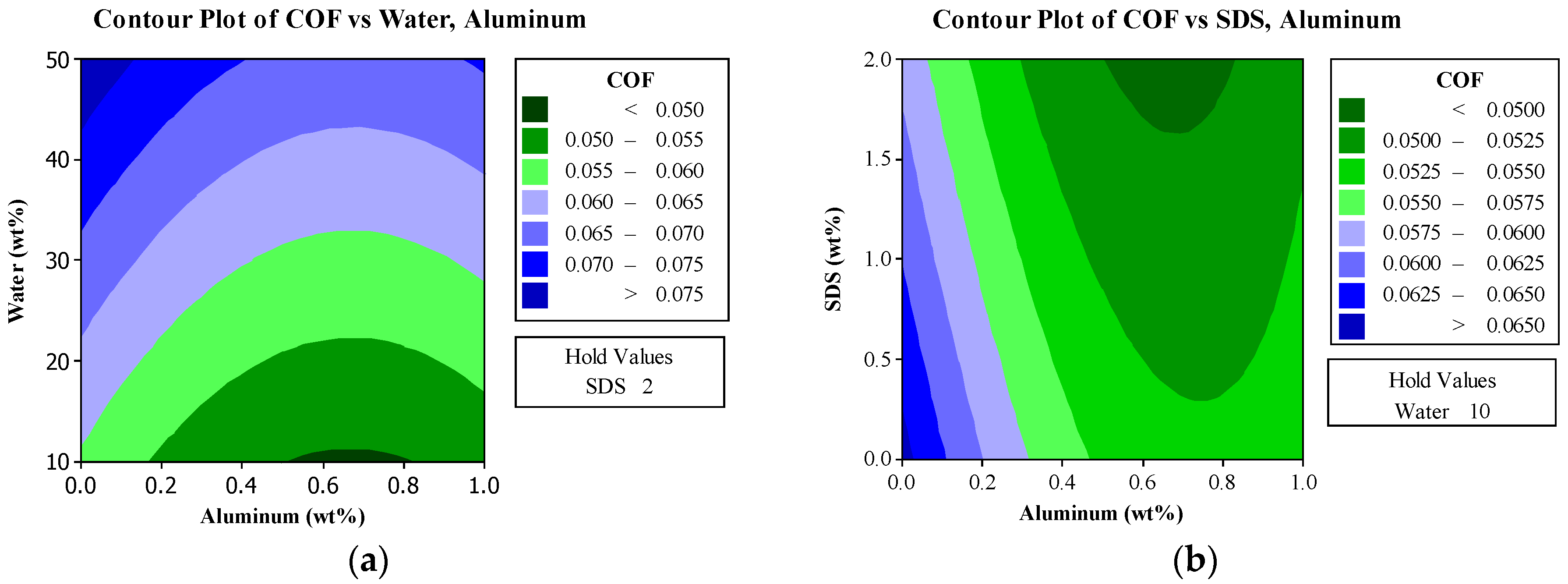
In this research, the influences of aluminum nanoparticles, sodium dodecyl sulfate as the dispersal agent, and the water content of glycerol on the characteristics of friction and wear were studied. The experimental design included methods such as Taguchi technique, ANOVA analysis, and response surface methodology in this research. Surface roughness measurement, scanning electron microscope (SEM), and energy dispersive spectrometer (EDS) are used to study the effects of aluminum nanoparticles as the additive to glycerol lubricant.