Modelling and numerical simulation of Supercritical CO2 debinding of Inconel 718 components elaborated by Metal Injection Molding
Abstract
:Featured Application
Abstract
1. Introduction
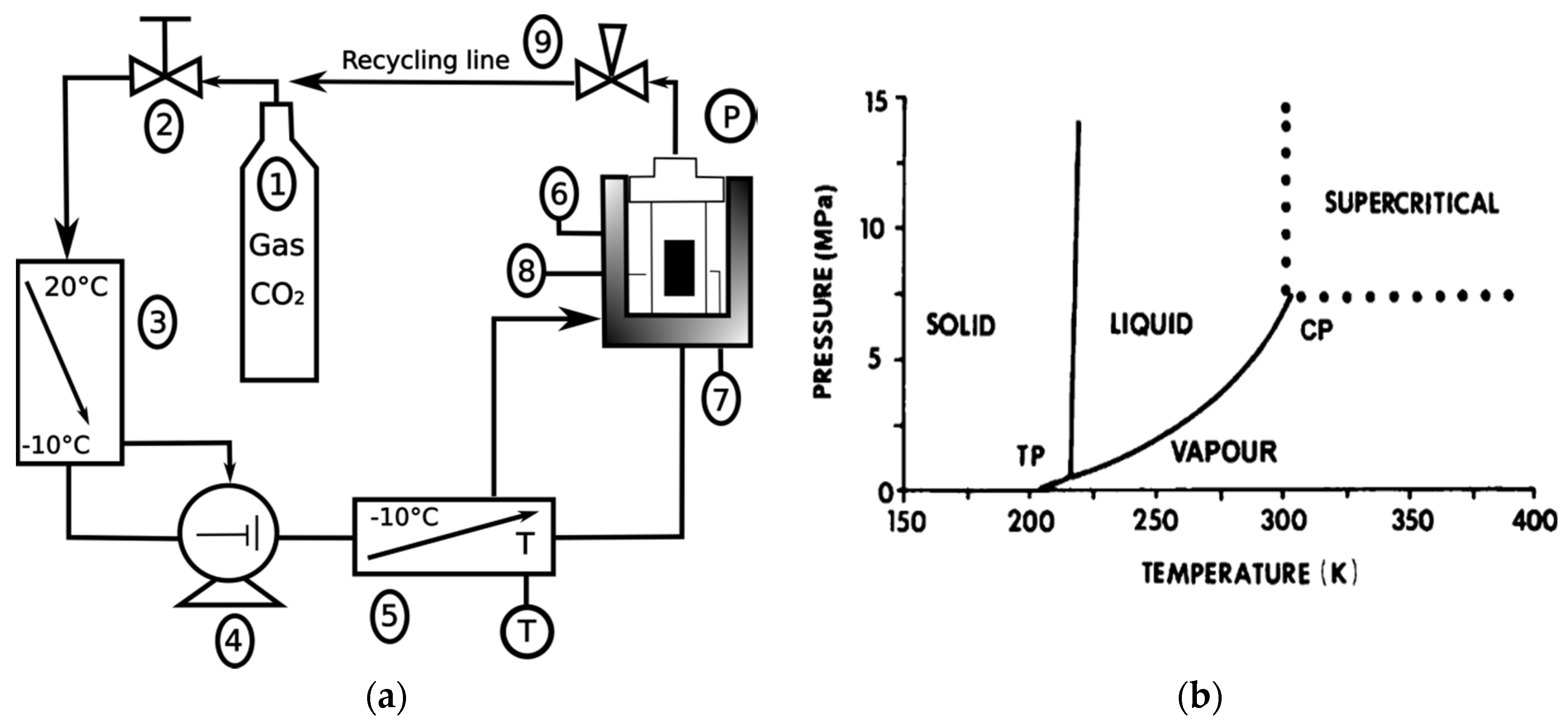
2. Materials and Methods
3. Results and Discussion
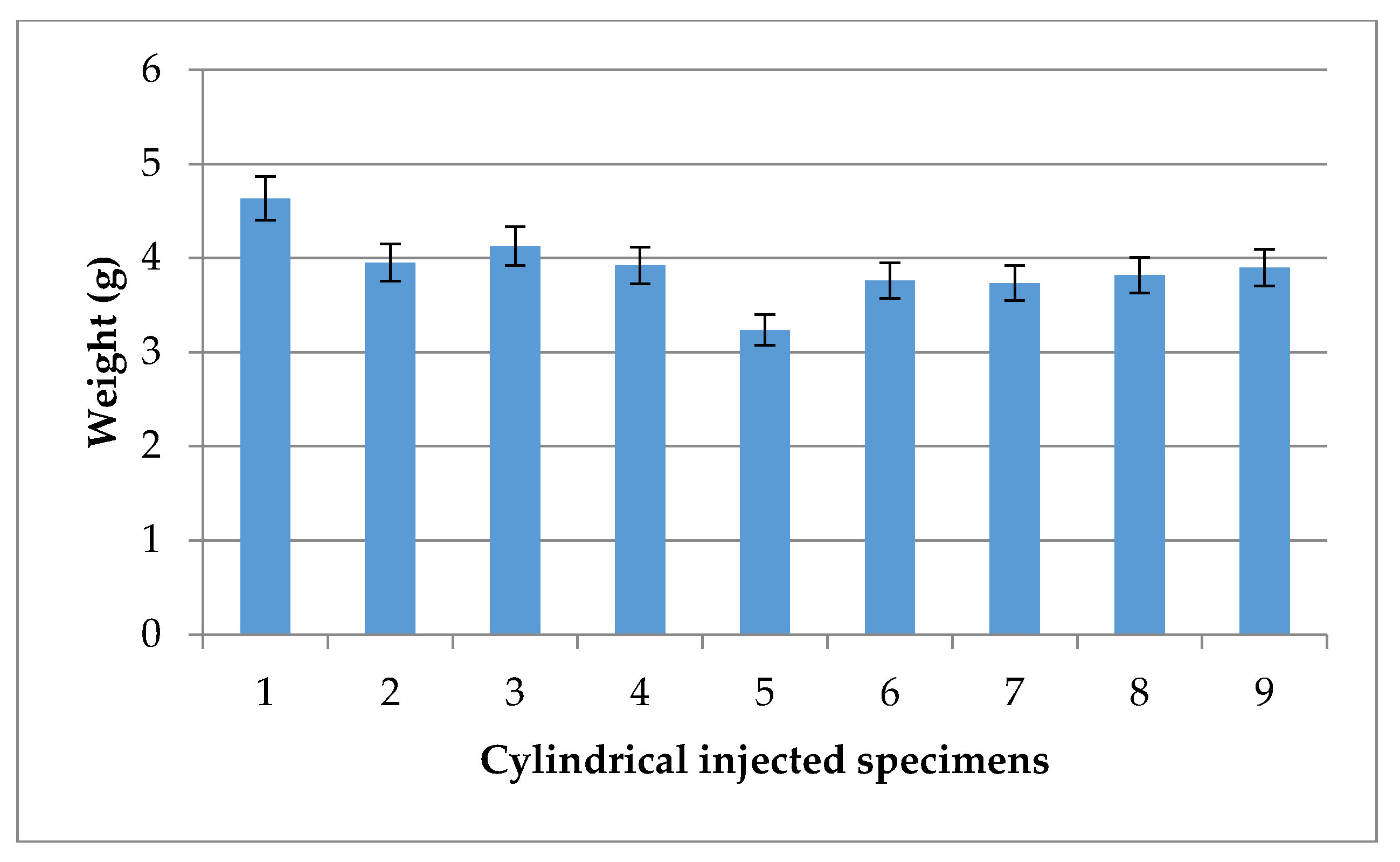
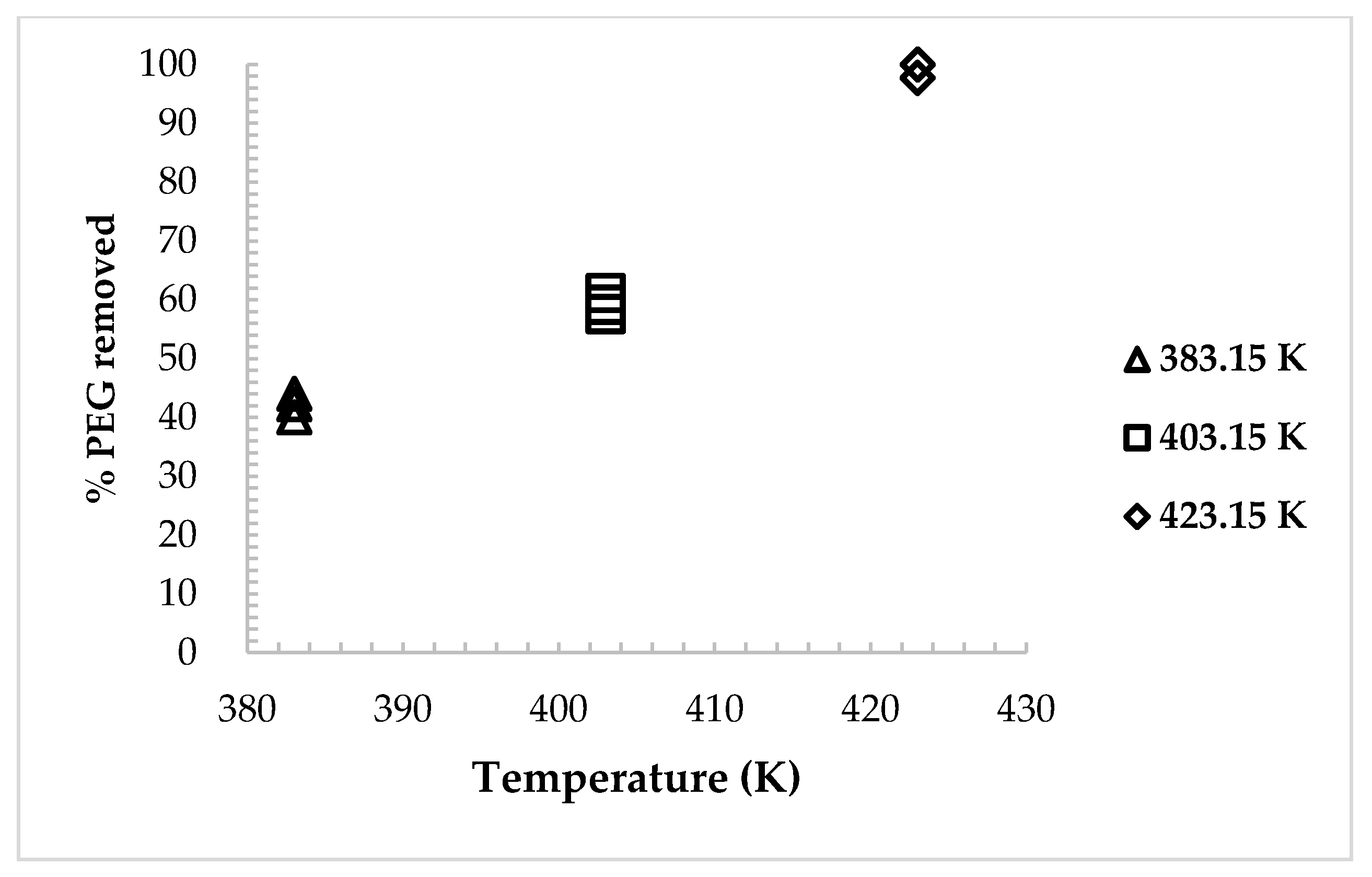
3.1. Supercritical Debinding
3.2. Process Modelling
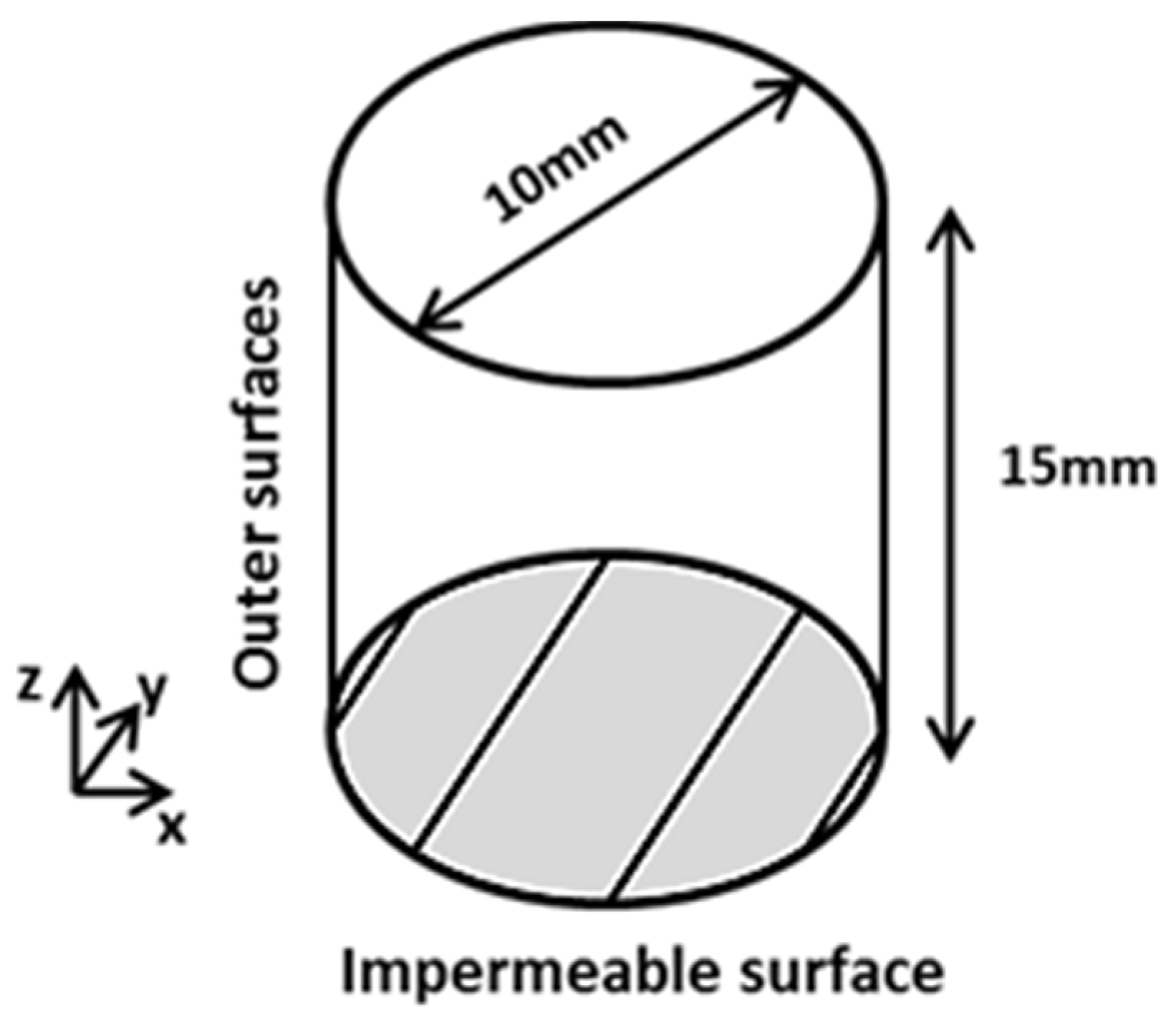
3.3. Boundary and Initial Conditions
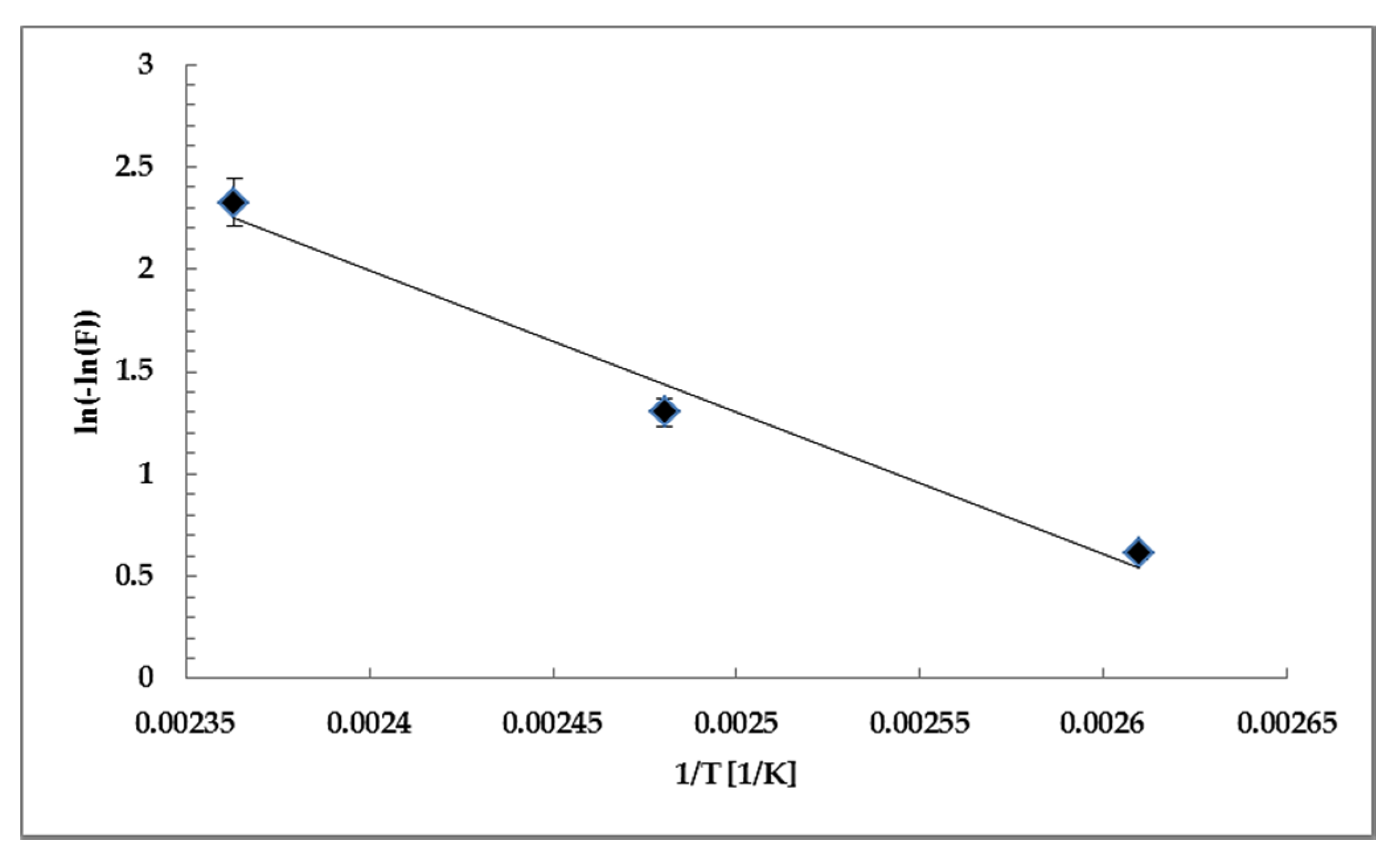
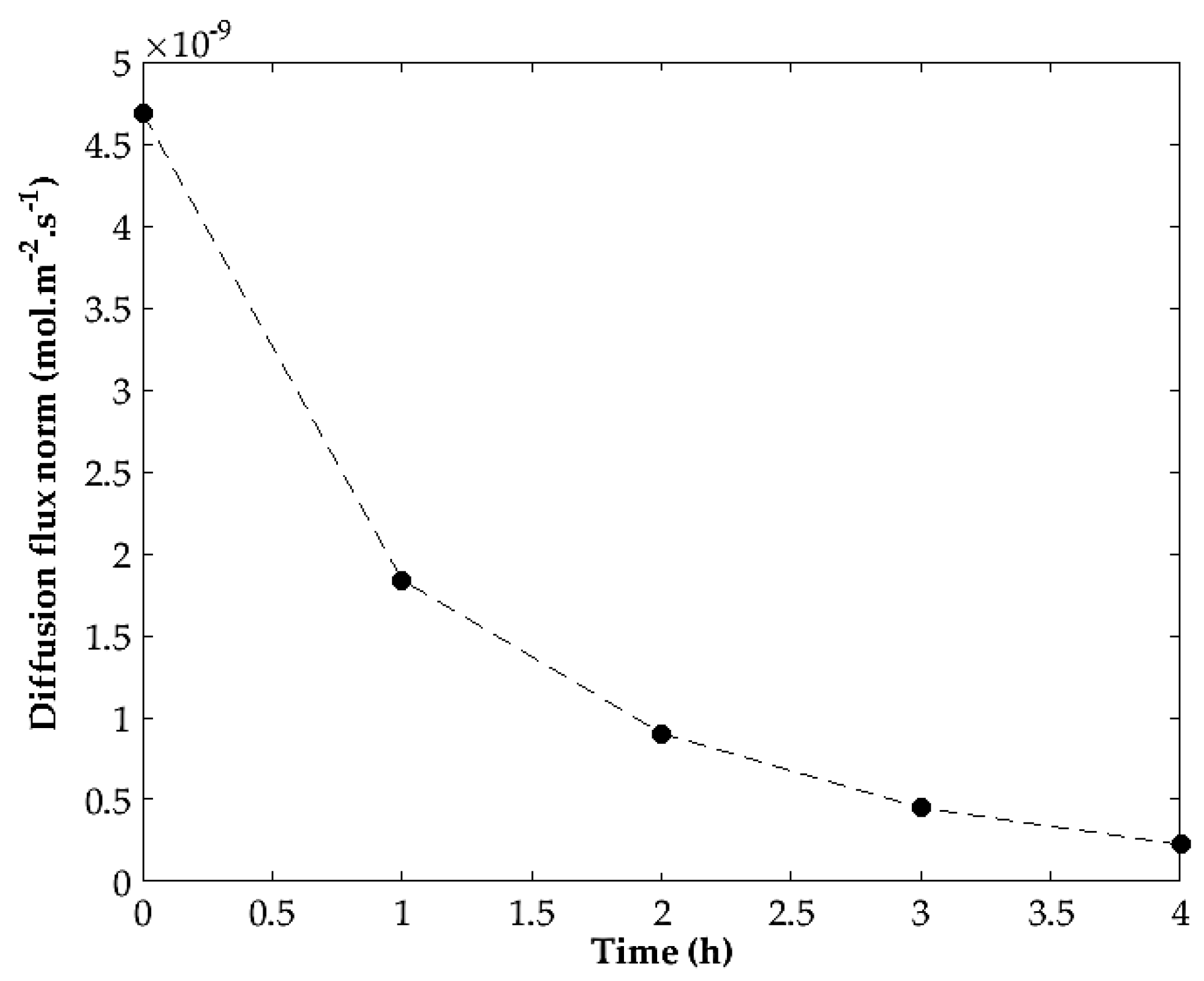
3.4. Parameter identification
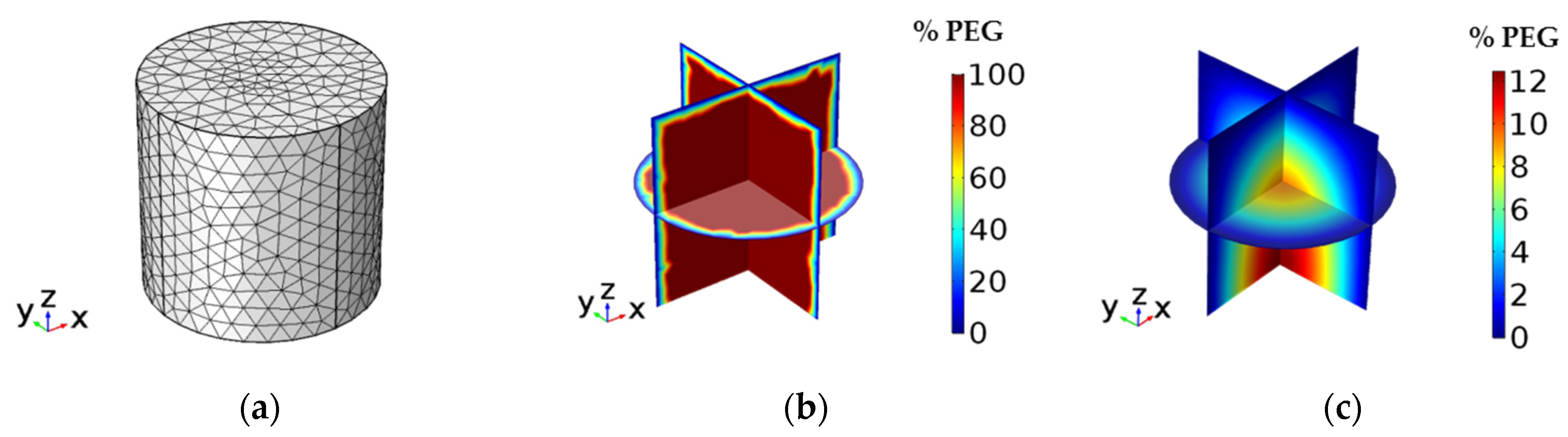
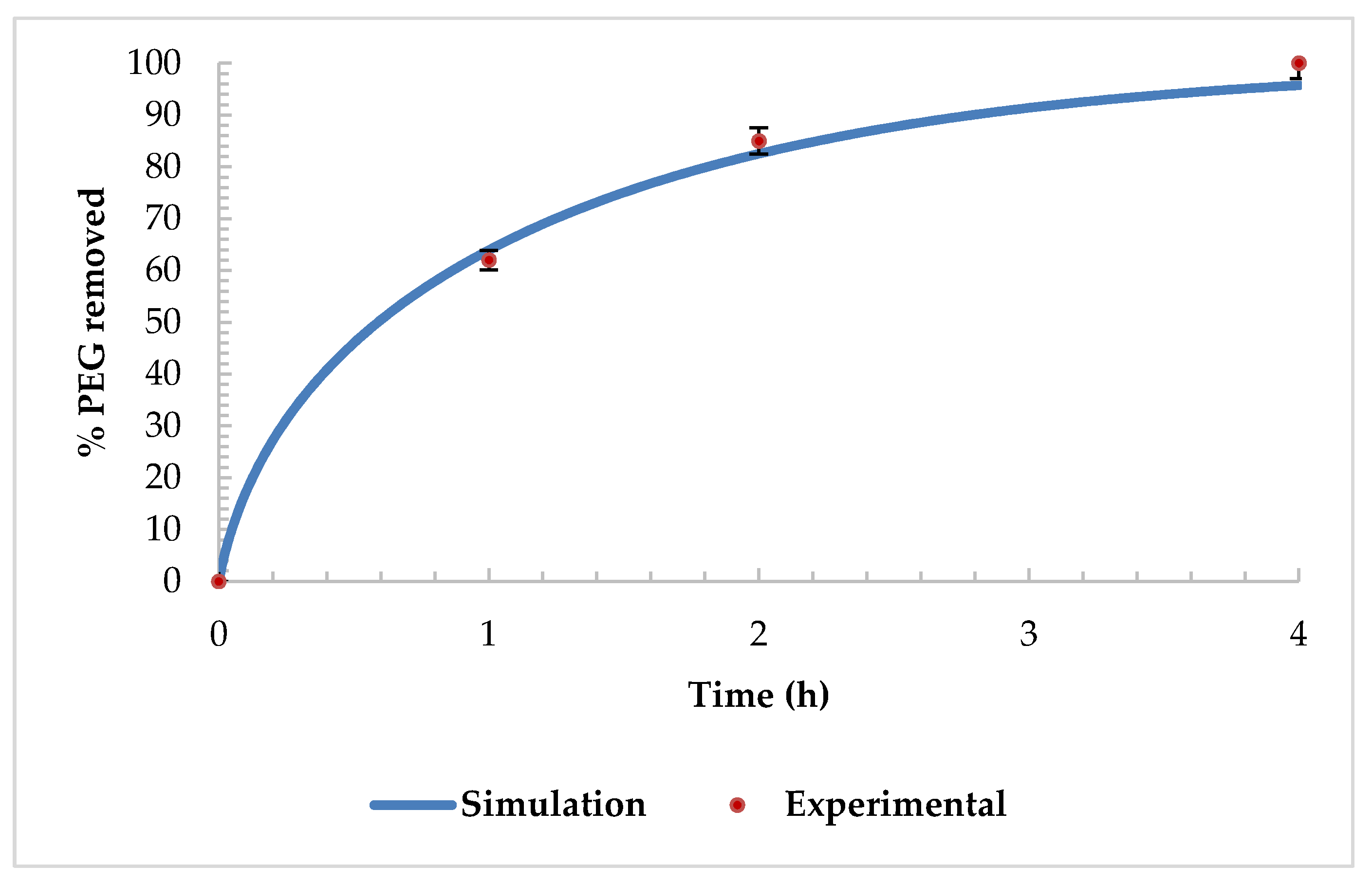
3.5. Numerical Simulations of Supercritical Debinding
- the component is continuous;
- the soluble polymer (PEG) is distributed homogeneously in the component;
- PEG is the only soluble binder;
- the weight loss is only due to the dissolution and elimination of the soluble binder;
- there is no interaction between the other binder constituents (PP, SA) contained in the component; and,
- there is no temperature variation during the supercritical debinding.
- for x = 0 at t ≥ 0;
- C = Co = 0 for outer surfaces at t = 0;
- C = Ci = 100% at t = 0.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- German, R.M. Injection Molding of Metals and Ceramics. Met. Powder Ind. Fed. 1997, 3. [Google Scholar] [CrossRef]
- Wolff, M.; Schaper, J.G.; Suckert, M.R.; Dahms, M.; Feyerabend, F.; Ebel, T.; Willumeit-Römer, R.; Klassen, T. Metal Injection Molding (MIM) of Magnesium and Its Alloys. Metals 2016, 6, 118. [Google Scholar] [CrossRef]
- Fang, W.; He, X.; Zhang, R.; Yang, S.; Qu, X. Evolution of stresses in metal injection molding parts during sintering. Trans. Nonferrous Met. Soc. China 2015, 25, 552–558. [Google Scholar] [CrossRef]
- Romero, A.; Herranz, G. Development of feedstocks based on steel matrix composites for metal injection moulding. Powder Technol. 2017, 308, 472–478. [Google Scholar] [CrossRef]
- Lin, D.; Sanetrnik, D.; Cho, H.; Chung, S.T.; Kwon, Y.S.; Kate, K.H.; Hausnerova, B.; Atre, S.V.; Park, S.J. Rheological and thermal debinding properties of blended elemental Ti-6Al-4V powder injection molding feedstock. Powder Technol. 2017, 311, 357–363. [Google Scholar] [CrossRef]
- Royer, A.; Barrière, T.; Gelin, J.-C. Development and Characterization of a Metal Injection Molding Bio Sourced Inconel 718 Feedstock Based on Polyhydroxyalkanoates. Metals 2016, 6, 89. [Google Scholar] [CrossRef]
- Valencia, J.; Spirko, J.; Schmees, R. Sintering Effect on the Microstructure and Mechanical Properties of Alloy 718 Processed by Powder Injection Molding - Superalloys. Superalloys 718 625 706 Var. Deriv. Ed. EA Loria Miner. Met. Mater. Soc. 1997, 1997, 753–762. [Google Scholar]
- Chen, G.; Cao, P.; Wen, G.; Edmonds, N. Debinding behaviour of a water soluble PEG/PMMA binder for Ti metal injection moulding. Mater. Chem. Phys. 2013, 139, 557–565. [Google Scholar] [CrossRef]
- Belgacem, M.; Thierry, B.; Jean-Claude, G. Investigations on thermal debinding process for fine 316L stainless steel feedstocks and identification of kinetic parameters from coupling experiments and finite element simulations. Powder Technol. 2013, 235, 192–202. [Google Scholar] [CrossRef]
- Chartier, T.; Ferrato, M.; Baumard, J.F. Supercritical debinding of injection molded ceramics. J. Am. Ceram. Soc. 1995, 78, 1787–1792. [Google Scholar] [CrossRef]
- Hwang, K.S.; Tsou, T.H. Thermal debinding of powder injection molded parts: Observations and mechanisms. Metall. Trans. A 1992, 23, 2775–2782. [Google Scholar] [CrossRef]
- Bloemacher, M.; Weinand, D. CatamoldTM-a new direction for powder injection molding. J. Mater. Process. Technol. 1997, 63, 918–922. [Google Scholar] [CrossRef]
- Kim, S.W.; Lee, H.-W.; Song, H.; Kim, B.H. Pore structure evolution during solvent extraction and wicking. Ceram. Int. 1996, 22, 7–14. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Stringari, G.; Emri, I. Powder Injection Molding of Metal and Ceramic Parts. In Some Critical Issues for Injection Molding; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Masia, S.; Calvert, P.D.; Rhine, W.E.; Bowen, H.K. Effect of oxides on binder burnout during ceramics processing. J. Mater. Sci. 1989, 24, 1907–1912. [Google Scholar] [CrossRef]
- Yang, W.-W.; Yang, K.-Y.; Wang, M.-C.; Hon, M.-H. Solvent debinding mechanism for alumina injection molded compacts with water-soluble binders. Ceram. Int. 2003, 29, 745–756. [Google Scholar] [CrossRef]
- Hwang, K.S.; Hsieh, Y.M. Comparative study of pore structure evolution during solvent and thermal debinding of powder injection molded parts. Metall. Mater. Trans. A 1996, 27, 245–253. [Google Scholar] [CrossRef]
- Enneti, R.K.; Shivashankar, T.S.; Park, S.-J.; German, R.M.; Atre, S.V. Master debinding curves for solvent extraction of binders in powder injection molding. Powder Technol. 2012, 228, 14–17. [Google Scholar] [CrossRef]
- Nalawade, S.P.; Picchioni, F.; Janssen, L.P.B.M. Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications. Prog. Polym. Sci. 2006, 31, 19–43. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Lee, Y.-W.; Park, J.-K.; Lee, C.-H.; Lim, J. S. Supercritical Carbon Dioxide debinding in metal injection molding (MIM) process. Korean J. Chem. Eng. 2002, 19, 986–991. [Google Scholar] [CrossRef]
- Kim, S.W. Debinding behaviors of injection molded ceramic bodies with nano-sized pore channels during extraction using supercritical carbon dioxide and n-heptane solvent. J. Supercrit. Fluids 2010, 51, 339–344. [Google Scholar] [CrossRef]
- Chartier, T.; Bordet, F.; Delhomme, E.; François Baumard, J. Extraction of binders from green ceramic bodies by supercritical fluid: influence of the porosity. J. Eur. Ceram. Soc. 2002, 22, 1403–1409. [Google Scholar] [CrossRef]
- Chartier, T.; Ferrato, M.; Baumard, J.-F. Influence of the debinding method on the mechanical properties of plastic formed ceramics. J. Eur. Ceram. Soc. 1995, 15, 899–903. [Google Scholar] [CrossRef]
- Avelino, H.M.N.T.; Fareleira, J.M.N.A.; Gourgouillon, D.; Igreja, J. M.; Nunes da Ponte, M. Viscosity of poly(ethyleneglycol) 200 [PEG 200] saturated with supercritical carbon dioxide. J. Supercrit. Fluids 2017, 128, 300–307. [Google Scholar] [CrossRef]
- Chartier, T.; Delhomme, E.; Baumard, J.-F. Mechanisms of Binder Removal Involved in Supercritical Debinding of Injection Moulded Ceramics. J. de Physique III 1997, 7, 291–302. [Google Scholar] [CrossRef]
- Zhu, B.; Qu, X.; Tao, Y. Mathematical model for condensed-solvent debinding process of PIM. J. Mater. Process. Technol. 2003, 142, 487–492. [Google Scholar] [CrossRef]
- Krauss, V.A.; Oliveira, A.A. M.; Klein, A.N.; Al-Qureshi, H.A.; Fredel, M.C. A model for PEG removal from alumina injection moulded parts by solvent debinding. J. Mater. Process. Technol. 2007, 182, 268–273. [Google Scholar] [CrossRef]
- German, R.M.; Lin, S.T. Extraction Debinbding of Injection Molded Parts by Condensed Solvent. Powder Metall. Int. 1989, 21, 19–24. [Google Scholar]
- Shivashankar, T.S.; German, R.M. Effective Length Scale for Predicting Solvent-Debinding Times of Components Produced by Powder Injection Molding. J. Am. Ceram. Soc. 1999, 82, 1146–1152. [Google Scholar] [CrossRef]
- Özgün, Ö.; Gülsoy, H.Ö.; Yılmaz, R.; Fındık, F. Microstructural and mechanical characterization of injection molded 718 superalloy powders. J. Alloys Compd. 2013, 576, 140–153. [Google Scholar] [CrossRef]
- Özgün, Ö.; Özkan Gülsoy, H.; Yilmaz, R.; Findik, F. Injection molding of nickel based 625 superalloy: Sintering, heat treatment, microstructure and mechanical properties. J. Alloys Compd. 2013, 546, 192–207. [Google Scholar] [CrossRef]
- Mendes, R.L.; Nobre, B.P.; Cardoso, M.T.; Pereira, A.P.; Palavra, A.F. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Protag. Chem. Frausto Silva 2003, 356, 328–334. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Škerget, M.; Knez, Ž. Density and viscosity of the binary polyethylene glycol/CO2 systems. J. Supercrit. Fluids 2014, 95, 641–668. [Google Scholar] [CrossRef]
- Ben Said, A.; Guinot, C.; Ruiz, J.-C.; Grandjean, A.; Dole, P.; Joly, C.; Chalamet, Y. Modeling of supercritical CO2 extraction of contaminants from post-consumer polypropylene: Solubilities and diffusion coefficients in swollen polymer at varying pressure and temperature conditions. Chem. Eng. Res. Des. 2017, 117, 95–109. [Google Scholar] [CrossRef]
- Barrenechea, G.R.; Knobloch, P. Analysis of a group finite element formulation. Appl. Numer. Math. 2017, 118, 238–248. [Google Scholar] [CrossRef]
| Ni | Fe | Cr | Nb | Mo | Ti | Al | C |
|---|---|---|---|---|---|---|---|
| 52.5 | 19.0 | 19.0 | 5.3 | 3.0 | 0.9 | 0.5 | 0.0 |
| Polymer | Weight Fraction, % | Density, g/cm3 | Melting Point, K |
|---|---|---|---|
| PP | 2.68 | 0.90 | 418 |
| PEG | 4.95 | 1.21 | 335 |
| SA | 0.35 | 0.94 | 341 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agne, A.; Barrière, T. Modelling and numerical simulation of Supercritical CO2 debinding of Inconel 718 components elaborated by Metal Injection Molding. Appl. Sci. 2017, 7, 1024. https://doi.org/10.3390/app7101024
Agne A, Barrière T. Modelling and numerical simulation of Supercritical CO2 debinding of Inconel 718 components elaborated by Metal Injection Molding. Applied Sciences. 2017; 7(10):1024. https://doi.org/10.3390/app7101024
Chicago/Turabian StyleAgne, Aboubakry, and Thierry Barrière. 2017. "Modelling and numerical simulation of Supercritical CO2 debinding of Inconel 718 components elaborated by Metal Injection Molding" Applied Sciences 7, no. 10: 1024. https://doi.org/10.3390/app7101024




