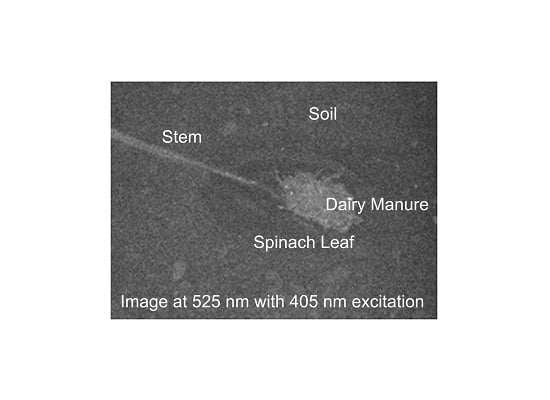
Interactions of Insolation and Shading on Ability to Use Fluorescence Imaging to Detect Fecal Contaminated Spinach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hyperspectral Imaging System
2.2. Image Acquisition
2.3. Image Analysis
2.4. Light Shroud
2.5. Experimental Protocol
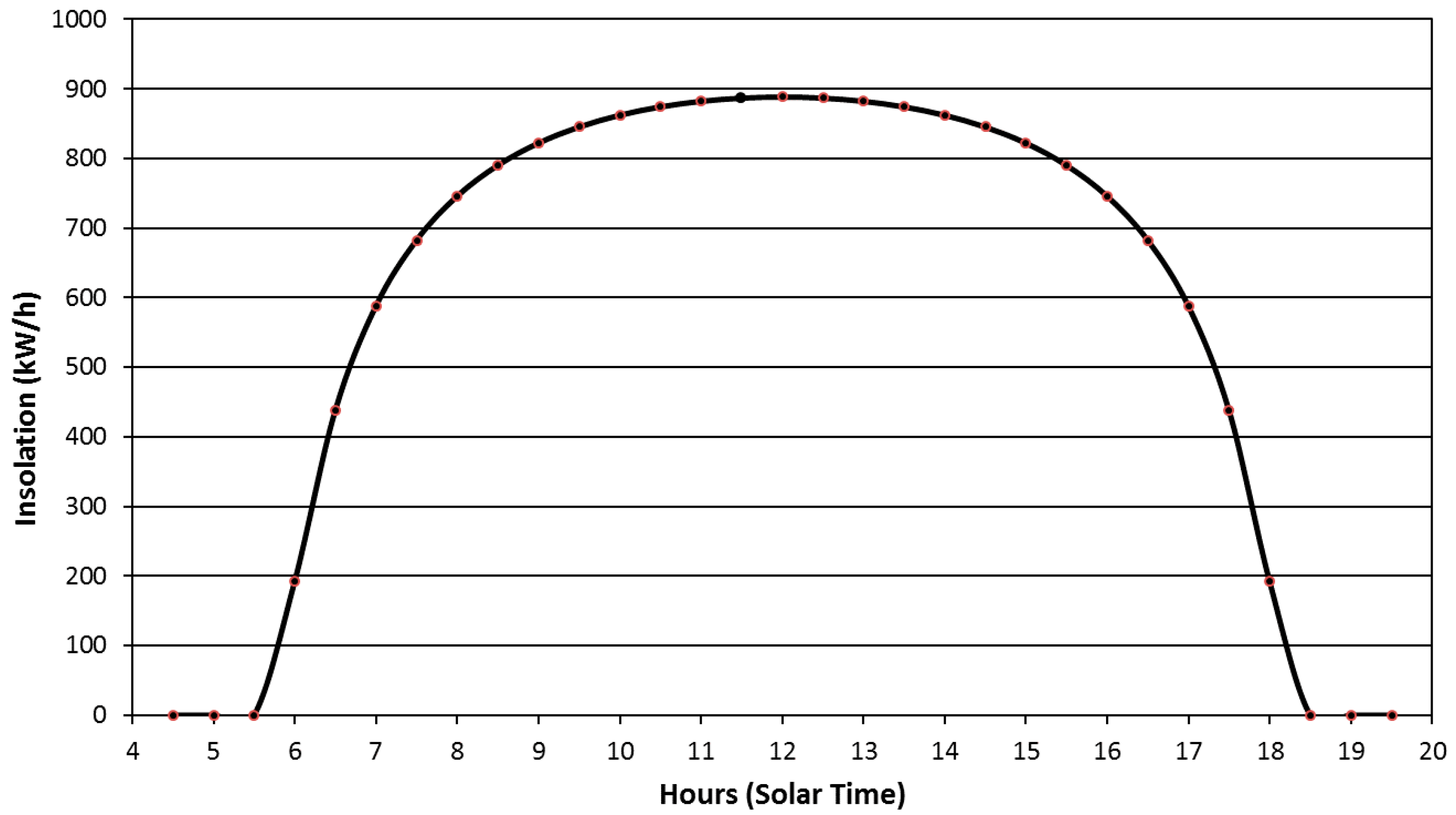
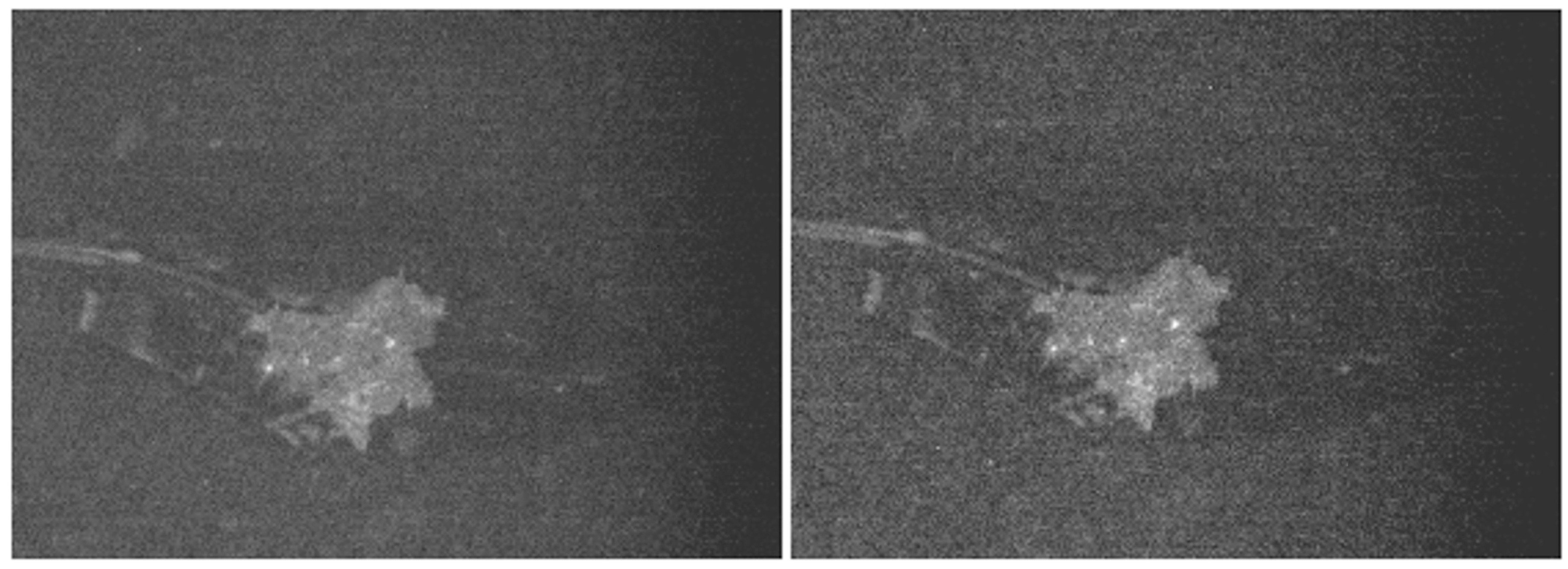
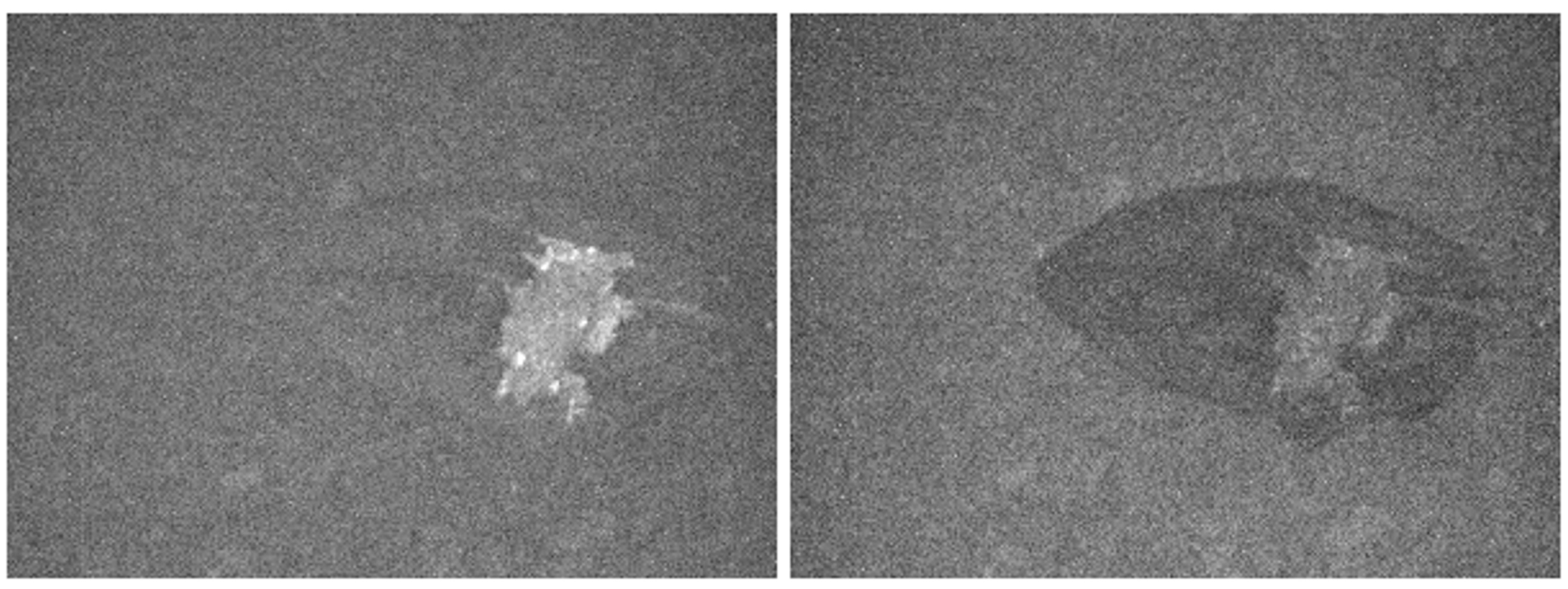
3. Results and Discussion
3.1. Goals
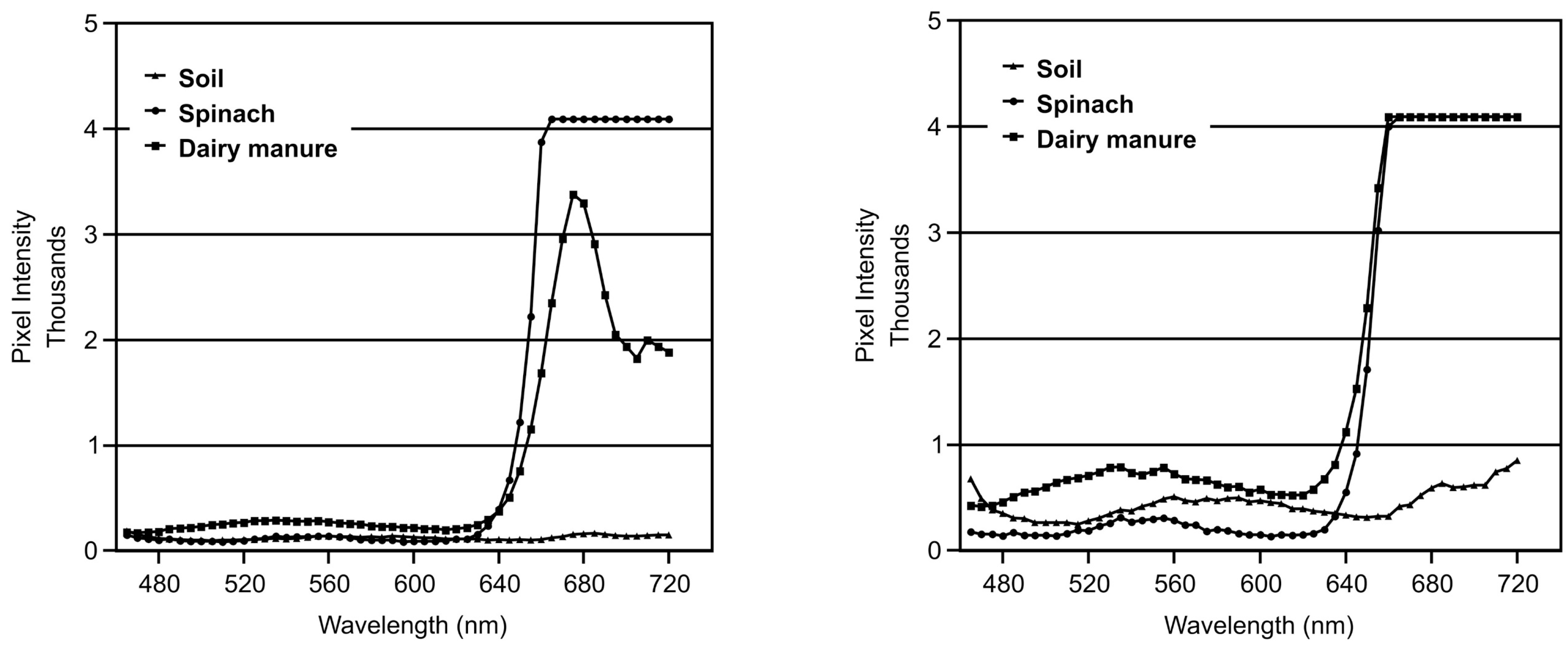
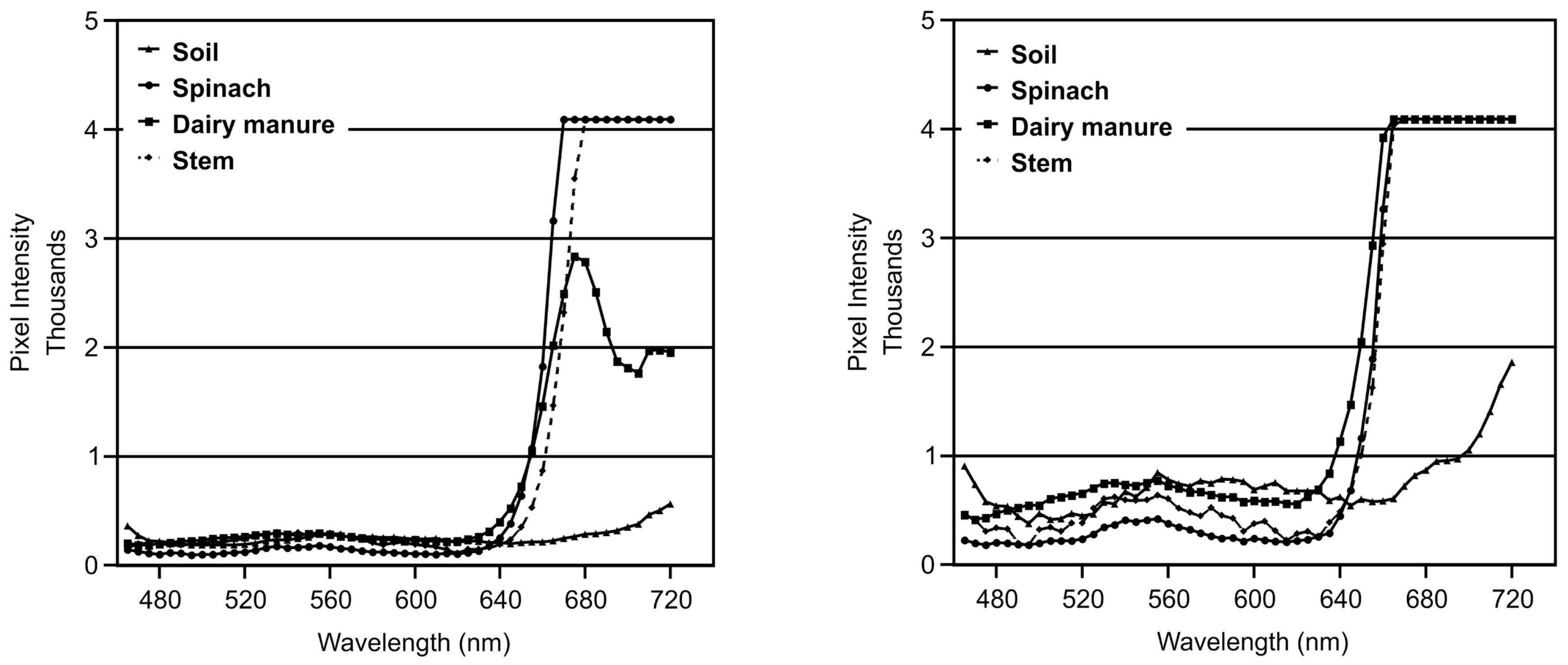
3.2. Spectra
3.3. Implications
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). CDC Estimates of Foodborne Illness in the United States: Findings. 2011. Available online: https://www.cdc.gov/foodborneburden/pdfs/factsheet_a_findings_updated4-13.pdf (accessed on 5 September 2017).
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreaks data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Local Government Management Agency (LGMA). Commodity Specific Food Safety Guidelines for the Production and Harvest of Lettuce and Leafy Greens. 2013. Available online: http://www.lgma.ca.gov/wp-content/uploads/2014/09/California-LGMA-metrics-08-26-13-Final.pdf (accessed on 31 August 2017).
- Jay-Russell, M.T. What is the Risk from Wild Animals in Food-Borne Pathogen Contamination of Plants? CAB Rev. 2013, 8, 1–16. [Google Scholar] [CrossRef]
- Gil, M.I.; Selma, M.V.; Suslow, T.; Jacxsens, L.; Uyttendaele, M.; Allende, A. Pre-and Postharvest Preventive Measures and Intervention Strategies to Control Microbial Food Safety Hazards of Fresh Leafy Vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Gonzalez, N.; Jay-Russell, M.T. Use of Falconry to Deter Nuisance Birds in Leafy Greens Fields in Northern California. In Proceedings of the 27th Vertebrate Pest Conference, Newport Beach, CA, USA, 7–20 March 2016; Timm, R.M., Baldwin, R.A., Eds.; University of California, Davis: Davis, CA, USA, 2016; pp. 209–216. [Google Scholar]
- Yang, C.C.; Kim, M.S.; Kang, S.; Cho, B.K.; Chao, K.; Lefcourt, A.M.; Chan, D.E. Red to Far-Red Multispectral Fluorescence Image Fusion for Detection of Fecal Contamination on Apples. J. Food Eng. 2012, 108, 312–319. [Google Scholar] [CrossRef]
- Lefcourt, A.M.; Kim, M.S.; Chen, Y.R. Detection of Fecal Contamination on Apples with Nanosecond-Scale Time-Resolved Imaging of Laser-induced Fluorescence. Appl. Opt. 2005, 44, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Lefcourt, A.M.; Kim, M.S.; Chen, Y.R. Automated Detection of Fecal Contamination of Apples by Multispectral Laser-induced Fluorescence Imaging. Appl. Opt. 2003, 42, 3935–3943. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.M.; Kim, M.S.; Tao, Y.; Lefcourt, A.M.; Chen, Y.R.; Luo, Y.; Song, Y.; Buchanan, R. Detection of Fecal Contamination on Cantaloupes using Hyperspectral Fluorescence Imagery. J. Food Sci. 2005, 70, e471–e476. [Google Scholar] [CrossRef]
- Tewey, K.J. Time-Resolved Hyperspectral Line-Scan Imaging of Pulsed-Laser Induced Fluorescence: Food Safety Inspection; University of Maryland: Baltimore, MD, USA, 2013. [Google Scholar]
- Lefcourt, A.M.; Wiederoder, M.S.; Liu, N.T.; Kim, M.S.; Lo, Y.M. Development of a Portable Hyperspectral Imaging System for Monitoring the Efficacy of Sanitation Procedures in Food Processing Facilities. J. Food Eng. 2013, 117, 59–66. [Google Scholar] [CrossRef]
- Lefcourt, A.M.; Kistler, R.; Gadsden, S.A.; Kim, M.S. Automated Cart with VIS/NIR Hyperspectral Reflectance and Fluorescence Imaging Capabilities. Appl. Sci. 2016, 7, 3. [Google Scholar] [CrossRef]
- Everard, C.D.; Kim, M.S.; Lee, H.; O’Donnell, C.P. Identifying Fecal Matter Contamination in Produce Fields using Multispectral Reflectance Imaging under Ambient Solar Illumination. In Proceedings of the SPIE Commercial + Scientific Sensing and Imaging, Baltimore, MD, USA, 17 May 2016; p. 986416. [Google Scholar]
- Jenkins, T.; Bolivar-Mendoza, G. Solar Time, Angles, and Irradiance Calculator: User Manual. 2014. Available online: http://aces.nmsu.edu/pubs/_circulars/CR674/welcome.html (accessed on 6 September 2017).
- Kim, M.S.; Lefcourt, A.M.; Chen, Y.R. Optimal Fluorescence Excitation and Emission Bands for Detection of Fecal Contamination. J. Food Prot. 2003, 66, 1198–1207. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefcourt, A.M.; Siemens, M.C. Interactions of Insolation and Shading on Ability to Use Fluorescence Imaging to Detect Fecal Contaminated Spinach. Appl. Sci. 2017, 7, 1041. https://doi.org/10.3390/app7101041
Lefcourt AM, Siemens MC. Interactions of Insolation and Shading on Ability to Use Fluorescence Imaging to Detect Fecal Contaminated Spinach. Applied Sciences. 2017; 7(10):1041. https://doi.org/10.3390/app7101041
Chicago/Turabian StyleLefcourt, Alan M., and Mark C. Siemens. 2017. "Interactions of Insolation and Shading on Ability to Use Fluorescence Imaging to Detect Fecal Contaminated Spinach" Applied Sciences 7, no. 10: 1041. https://doi.org/10.3390/app7101041