New Class of Wide Energy Gap Benzotriimidazole Optical Materials
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis and Structural Characterization of Benztriimidazole Materials


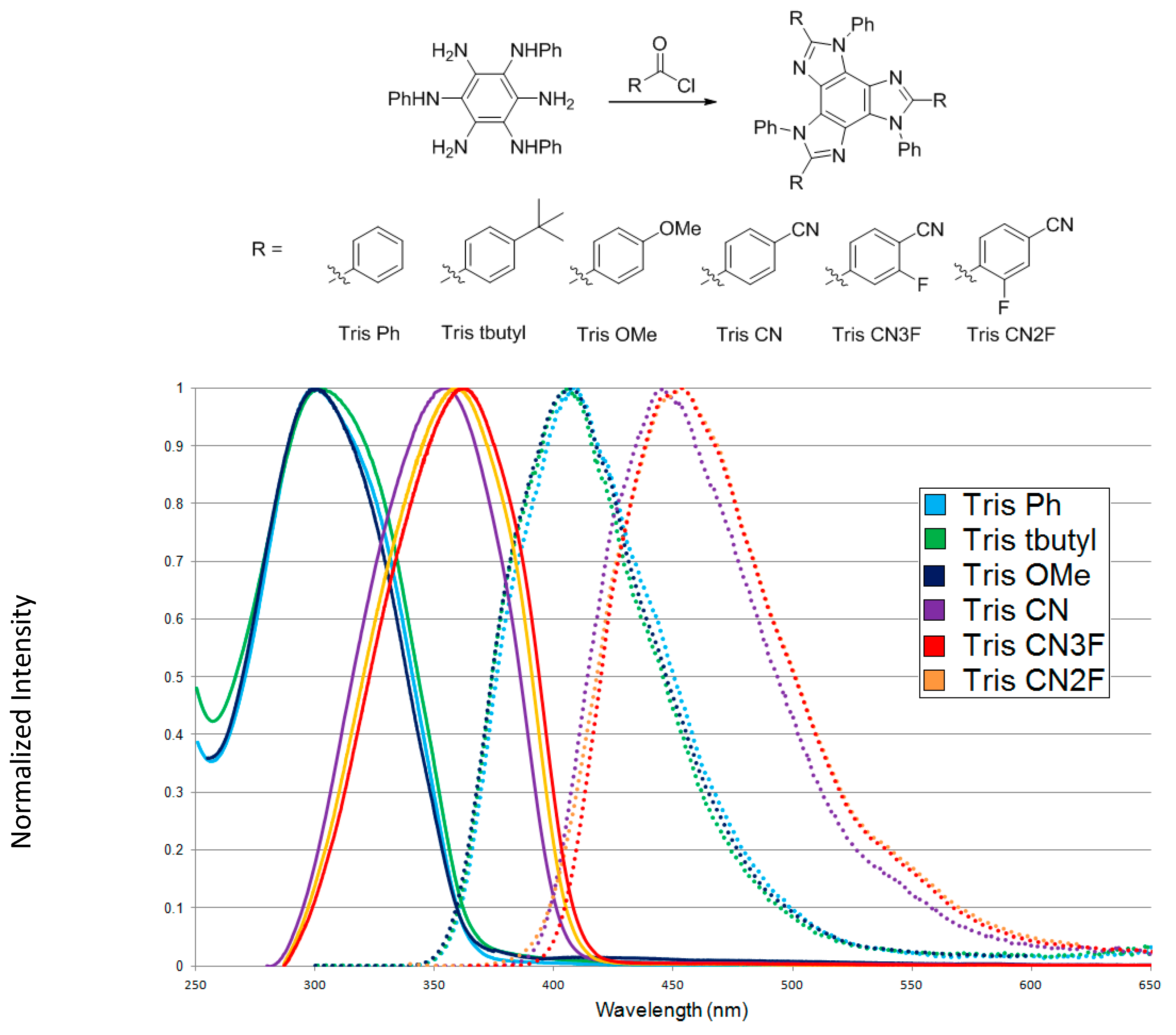
3.2. Optical Properties of Benztriimidazole Materials
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Burroughes, J.H.; Bradley, D.D.C.; Burroughes, J.H.; Friend, R.H.; Greenham, N.C.; Burn, P.L.; Holmes, A.B.; Kraft, A.M. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151. [Google Scholar]
- Yu, G.; Heeger, A.J. High efficiency photonic devices made with semiconducting polymers. Synth. Met. 1997, 85, 1183–1186. [Google Scholar] [CrossRef]
- O’Brien, B.; Lee, Y.K.; Marrs, M.; Smith, J.; Strnad, M.; Forsythe, E.; Morton, D. 70.2 L: Late-News Paper: 14.7” Active Matrix PHOLED Displays on Temporary Bonded PEN Substrates with Low Temperature IGZO TFTs. SID Symp. Digest Tech. Pap. 2013, 44, 447–450. [Google Scholar] [CrossRef]
- O’Rourke, S.M.; Loy, D.E.; Moyer, C.; Bawolek, E.J.; Ageno, S.K.; O’Brien, B.P.; Marrs, M.; Bottesch, D.; Dailey, J.; Naujokaitis, R.; et al. Direct Fabrication of A-Si:H Thin Film Transistor Arrays on Plastic and Metal Foils for Flexible Displays; Arizona State University Tempe: Tempe, AZ, USA, 2008. [Google Scholar]
- Swensen, J.S.; Wang, L.; Koech, P.K.; Polikarpov, E.; Padmaperuma, A.B.; Gaspar, D.J. Blue phosphorescent organic light-emitting devices utilizing cesium–carbonate-doped 2,4,6-tris(2′,4′-difluoro-[1,1′-biphenyl]-4-yl)-1,3,5-triazine. J. Photonics Energy 2011, 1, 011008. [Google Scholar] [CrossRef]
- Fellowes, D.A.; Wood, M.V. AMOLED (active matrix OLED) functionality and usable lifetime at temperature. In Proceedings of the SPIE 5800, Helmet- and Head-Mounted Displays X: Technologies and Applications, Orlando, FL, USA, 19 May 2005; SPIE: Bellingham, WA, USA, 2005. [Google Scholar]
- Shen, Z.; Burrows, P.E.; Bulovic, V.; Forrest, S.R.; Thompson, M.E. Three-color, tunable, organic light-emitting devices. Science 1997, 276, 2009–2011. [Google Scholar] [CrossRef]
- Hameed, T.A.S.; Predeep, P.; Baiju, M.R. Organic Light Emitting Diodes: Device Physics and Effects of Ambience on Performance Parameters, Optoelectronics-Devices and Applications; Predeep, P., Ed.; InTech: Rijeka, Croatia, 2011; ISBN 978-953-307-576-1. [Google Scholar]
- Gao, Z.; Lee, C.S.; Bello, I.; Lee, S.T.; Chen, R.-M.; Luh, T.-Y.; Shi, J.; Tang, C.W. Bright-blue electroluminescence from a silyi-substituted ter-(phenylene-vinylene) derivative. Appl. Phys. Lett. 1999, 74, 865. [Google Scholar] [CrossRef]
- Hung, L.S.; Tang, C.W.; Mason, M.G. Enhanced electron injection in organic electroluminescence devices using an Al/LiF electrode. Appl. Phys. Lett. 1997, 70, 152–154. [Google Scholar] [CrossRef]
- Wakimoto, T.; Fukuda, Y.; Nagayama, K.; Yokoi, A.; Nakada, H.; Tsuchida, M. Organic EL cells using alkaline metal compounds as electron injection materials. IEEE Trans. Electron. Dev. 1997, 44, 1245–1248. [Google Scholar] [CrossRef]
- Jabbour, G.E.; Kippelen, B.; Armstrong, N.R.; Peyghambarian, N. Aluminum based cathode structure for enhanced electron injection in electroluminescent organic devices. Appl. Phys. Lett. 1998, 73, 1185–1187. [Google Scholar] [CrossRef]
- Kido, J.; Matsumoto, T. Bright organic electroluminescent devices having a metal-doped electron-injecting layer. Appl. Phys. Lett. 1998, 73, 2866–2868. [Google Scholar] [CrossRef]
- Burrows, P.E.; Shen, Z.; Bulovic, V.; McCarty, D.M.; Forrest, S.R.; Cronin, J.A.; Thompson, M.E. Relationship between electroluminescence and current transport in organic heterojunction light-emitting devices. J. Appl. Phys. 1996, 79, 7991–8006. [Google Scholar] [CrossRef]
- Tsang, S.W.; So, S.K.; Xu, J.B. Application of admittance spectroscopy to evaluate carrier mobility in organic charge transport materials. J. Appl. Phys. 2006, 99, 013706. [Google Scholar] [CrossRef]
- Wu, C.-C.; Huang, W.-Y.; Liu, T.-L. Hole-transport properties of a furan-containing oligoaryl. J. Appl. Phys. 2003, 93, 5465–5471. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.-Q.; Duan, L.; Zhang, R.; Wang, L.-D.; Qiu, Y. Elucidation of the electron injection mechanism of evaporated cesium carbonate cathode interlayer for organic light-emitting diodes. Appl. Phys. Lett. 2007, 90, 012119. [Google Scholar] [CrossRef]
- Pfieffer, M.; Leo, K.; Zhou, X.; Huang, J.S.; Hofmann, M.; Werner, A.; Blochwitz-Nimoth, J. Doped organic semiconductors: Physics and application in light emitting diodes. Org. Electron. 2003, 4, 89–103. [Google Scholar] [CrossRef]
- Lee, T.W.; Park, O.O. The effect of different heat treatments on the luminescence efficiency of polymer light-emitting diodes. Adv. Mater. 2000, 12, 801–804. [Google Scholar] [CrossRef]
- Shi, J.; Tang, C.W.; Chen, C.H. Blue Organic Electroluminescent Devices. U.S. Patent 5,645,948, 8 July 1997. [Google Scholar]
- Shi, J.; Tang, C.W.; Chen, C.H. Electron Transporting Materials for Organic Electroluminescent Devices. U.S. Patent 5,766,779, 16 June 1998. [Google Scholar]
- Fu, R.; Shi, J.; Forsythe, E.; Blomquist, S.; Srour, M.; Morton, D.C. Improvement of device efficiency for blue organic light emitting diodes by controlling the Cs2CO3-doped electron transport layer. J. Photonics Energy 2014, 4, 043595. [Google Scholar] [CrossRef]
- Fu, R.; Forsythe, E.; Shi, J.; Srour, M.; Blomquis, S.; Morton, D. Temperature dependence of cesium carbonate-doped electron transporting layers on organic light-emitting diodes. Synth. Met. 2015, 209, 128–134. [Google Scholar] [CrossRef]
- Hanifi, D.; Cao, D.; Klivansky, L.M.; Liu, Y. Novel C3-symmetric n-type tris(aroyleneimidazole) and its analogs: Synthesis, physical properties and self-assembly. Chem. Commun. 2011, 47, 3454–3456. [Google Scholar] [CrossRef] [PubMed]
- Debeaux, M.; Thesen, M.W.; Schneidenbach, D.; Hopf, H.; Janietz, S.; Krueger, H.; Wedel, A.; Kowalsky, W.; Johannes, H.-H. Charge-Transporting Polymers based on Phenylbenzoimidazole Moieties. Adv. Funct. Mater. 2010, 20, 399–408. [Google Scholar] [CrossRef]


| Compound | λabs max (nm) a | λem max (nm) a,b | Δλstokes (nm) c | Φ (%) a,d | Eg (eV) e |
|---|---|---|---|---|---|
| Tris Ph | 301 | 409 | 108 | 38.9 | 3.41 |
| Tris tbutyl | 302 | 405 | 103 | 39.4 | 3.40 |
| Tris OMe | 300 | 407 | 107 | 36.2 | 3.37 |
| Tris CN | 355 | 446 | 91 | 75.0 | 3.06 |
| Tris CN2F | 359 | 455 | 96 | 77.2 | 3.02 |
| Tris CN3F | 362 | 454 | 92 | 76.5 | 3.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Chudomel, J.M.; Fu, R. New Class of Wide Energy Gap Benzotriimidazole Optical Materials. Appl. Sci. 2017, 7, 1078. https://doi.org/10.3390/app7101078
Shi J, Chudomel JM, Fu R. New Class of Wide Energy Gap Benzotriimidazole Optical Materials. Applied Sciences. 2017; 7(10):1078. https://doi.org/10.3390/app7101078
Chicago/Turabian StyleShi, Jianmin, J. Matthew Chudomel, and Richard Fu. 2017. "New Class of Wide Energy Gap Benzotriimidazole Optical Materials" Applied Sciences 7, no. 10: 1078. https://doi.org/10.3390/app7101078




