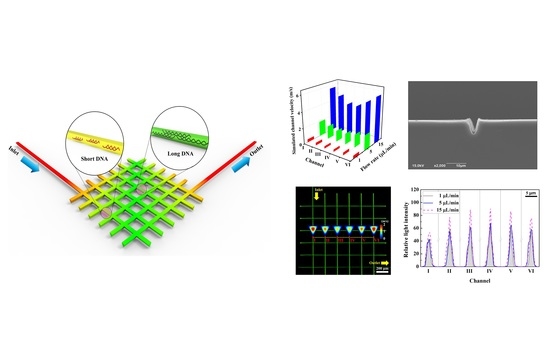
Femtosecond Laser-Inscripted Direct Ultrafast Fabrication of a DNA Distributor Using Microfluidics
Abstract
:1. Introduction
2. Methods
2.1. PDMS Sample Preparation
2.2. Micro-Scribing with a Femtosecond Laser
2.3. DNA Preparation
2.4. Computer-Aided Simulation for Microfluidic Channel Optimization
2.5. Microfluidic Experiment
3. Results and Discussions
3.1. Analysis of Cross-Sectional Images of the Micro-channel
3.2. Computational Analysis of a DNA Distributor
3.3. Microfluidic Distribution of the DNA
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Von der Linde, D.; Sokolowski-Tinten, K. The physical mechanisms of short-pulse laser ablation. Appl. Surf. Sci. 2000, 154, 1–10. [Google Scholar] [CrossRef]
- Hsieh, Y.K.; Chen, S.C.; Huang, W.L.; Hsu, K.P.; Gorday, K.A.V.; Wang, T.; Wang, J. Direct Micromachining of Microfluidic Channels on Biodegradable Materials Using Laser Ablation. Poymers 2017, 9, 242. [Google Scholar] [CrossRef]
- Sun, Y.L.; Dong, W.F.; Niu, L.G.; Jiang, T.; Liu, D.X.; Zhang, L.; Wang, Y.S.; Kim, D.P.; Sun, H.B. Protein-based soft micro-optics fabricated by femtosecond laser direct writing. Light Sci. Appl. 2014, 3, e129. [Google Scholar] [CrossRef]
- Malek, C.G.K. Laser processing for bio-microfluidics applications (part I). Anal. Bioanal. Chem. 2006, 385, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Malek, C.G.K. Laser processing for bio-microfluidics applications (part II). Anal. Bioanal. Chem. 2006, 385, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kawano, H.; Liu, W.; Hanada, Y.; Lu, P.; Miyawaki, A.; Midorikawa, K.; Sugioka, K. Controllable alignment of elongated microorganisms in 3D microspace using electrofluidic devices manufactured by hybrid femtosecond laser microfabrication. Microsyst. Nanoeng. 2017, 3, 16078. [Google Scholar] [CrossRef]
- Tavangar, A.; Tan, B.; Venkatakrishnan, K. Synthesis of bio-functionalized three-dimensional titania nanofibrous structures using femtosecond laser ablation. Acta Biomater. 2011, 7, 2726–2732. [Google Scholar] [CrossRef] [PubMed]
- Oraevsky, A.A.; Silva, L.B.D.; Rubenchik, A.M.; Feit, M.D.; Glinsky, M.E.; Perry, M.D.; Mammini, B.M.; Small, W.; Stuart, B.C. Plasma mediated ablation of biological tissues with nanosecond-to-femtosecond laser pulses: Relative role of linear and nonlinear absorption. IEEE J. Sel. Top. Quant. 1996, 2, 801–809. [Google Scholar] [CrossRef]
- Shah, R.; Shah, S.; Sengupta, S. Results of small incision lenticule extraction: All-in-one femtosecond laser refractive surgery. J. Cataract Refract. Surg. 2011, 37, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Serbin, J.; Bauer, T.; Fallnich, C.; Kasenbacher, A.; Arnold, W.H. Femtosecond lasers as novel tool in dental surgery. Appl. Surf. Sci. 2002, 197, 737–740. [Google Scholar] [CrossRef]
- Gomez, C.; Costela, A.; García-Moreno, I.; Llanes, F.; Teijón, J.M.; Blanco, D. Laser treatments on skin enhancing and controlling transdermal delivery of 5-fluorouracil. Lasers Surg. Med. 2008, 40, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal, V.; Colombo, L.; Gellert, P.; Schwab, M.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Murakami, M.; Ichikawa, Y.; Che, Y. Highly efficient and controllable PEGylation of gold nanoparticles prepared by femtosecond laser ablation in water. J. Phys. Chem. C 2011, 115, 23293–23298. [Google Scholar] [CrossRef]
- Knowles, M.; Rutterford, G.; Karnakis, D.; Ferguson, A. Micro-machining of metals, ceramics and polymers using nanosecond lasers. Int. J. Adv. Manuf. Technol. 2007, 33, 95–102. [Google Scholar] [CrossRef]
- Barberoglou, M.; Zorba, V.; Stratakis, E.; Spanakis, E.; Tzanetakis, P.; Anastasiadis, S.; Fotakis, C. Bio-inspired water repellent surfaces produced by ultrafast laser structuring of silicon. Appl. Surf. Sci. 2009, 255, 5425–5429. [Google Scholar] [CrossRef]
- Koch, J.; Fadeeva, E.; Engelbrecht, M.; Ruffert, C.; Gatzen, H.; Ostendorf, A.; Chichkov, B. Maskless nonlinear lithography with femtosecond laser pulses. Appl. Phys. A Mater. 2006, 82, 23–26. [Google Scholar] [CrossRef]
- Sugioka, K.; Cheng, Y. Ultrafast lasers—Reliable tools for advanced materials processing. Light Sci. Appl. 2014, 3, e149. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Direct femtosecond laser surface nano/microstructuring and its applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Wolfe, D.B.; Ashcom, J.B.; Hwang, J.C.; Schaffer, C.B.; Mazur, E.; Whitesides, G.M. Customization of Poly (dimethylsiloxane) Stamps by Micromachining Using a Femtosecond-Pulsed Laser. Adv. Mater. 2003, 15, 62–65. [Google Scholar] [CrossRef]
- Darvishi, S.; Cubaud, T.; Longtin, J.P. Ultrafast laser machining of tapered microchannels in glass and PDMS. Opt. Lasers Eng. 2012, 50, 210–214. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Du, G.; Bian, H.; Zhang, D.; Si, J.; Yun, F.; Hou, X. Rapid fabrication of large-area concave microlens arrays on PDMS by a femtosecond laser. ACS Appl. Mater. Interfaces 2013, 5, 9382–9385. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.-X.; Zhang, Y.-L.; Han, D.-D.; Wang, H.; Xia, H.; Chen, Q.-D.; Ding, H.; Sun, H.-B. Solvent-tunable PDMS microlens fabricated by femtosecond laser direct writing. J. Mater. Chem. C 2015, 3, 1751–1756. [Google Scholar] [CrossRef]
- Stankova, N.; Atanasov, P.; Nikov, R.G.; Nikov, R.; Nedyalkov, N.; Stoyanchov, T.; Fukata, N.; Kolev, K.; Valova, E.; Georgieva, J. Optical properties of polydimethylsiloxane (PDMS) during nanosecond laser processing. Appl. Surf. Sci. 2016, 374, 96–103. [Google Scholar] [CrossRef]
- Surdo, S.; Piazza, S.; Ceseracciu, L.; Diaspro, A.; Duocastella, M. Towards nanopatterning by femtosecond laser ablation of pre-stretched elastomers. Appl. Surf. Sci. 2016, 374, 151–156. [Google Scholar] [CrossRef]
- Farshchian, B.; Gatabi, J.R.; Bernick, S.M.; Park, S.; Lee, G.-H.; Droopad, R.; Kim, N. Laser-induced superhydrophobic grid patterns on PDMS for droplet arrays formation. Appl. Surf. Sci. 2017, 396, 359–365. [Google Scholar] [CrossRef]
- Selimović, Š.; Piraino, F.; Bae, H.; Rasponi, M.; Redaelli, A.; Khademhosseini, A. Microfabricated polyester conical microwells for cell culture applications. Lab Chip 2011, 11, 2325–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piraino, F.; Selimović, Š.; Adamo, M.; Pero, A.; Manoucheri, S.; Bok Kim, S.; Demarchi, D.; Khademhosseini, A. Polyester μ-assay chip for stem cell studies. Biomicrofluidics 2012, 6, 044109. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.M.; Groisman, A.; Kleinfeld, D.; Schaffer, C.B. Customization of microfluidic devices using femtosecond laser micromachining. In Ultrashort Laser Microprocessing of Materials III, Proceedings of Frontiers in Optics 2003, Tucson, AZ, USA, 5 October 2003; Optical Society of America: Washington, DC, USA, 2003. [Google Scholar]
- Lopa, S.; Piraino, F.; Kemp, R.J.; Di Caro, C.; Lovati, A.B.; Di Giancamillo, A.; Moroni, L.; Peretti, G.M.; Rasponi, M.; Moretti, M. Fabrication of multi-well chips for spheroid cultures and implantable constructs through rapid prototyping techniques. Biotechnol. Bioeng. 2015, 112, 1457–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laib, S.; Rankl, M.; Ruckstuhl, T.; Seeger, S. Sizing of single fluorescently stained DNA fragments by scanning microscopy. Nucleic Acids Res. 2003, 31, e138. [Google Scholar] [CrossRef] [PubMed]







| Energy Dose (%) | Pulse Energy (μJ) | Laser Fluence (J/cm2) |
|---|---|---|
| 1 | 3.75 | 19.11 |
| 5 | 18.75 | 95.54 |
| 10 | 37.50 | 191.08 |
| 15 | 56.25 | 286.62 |
| 20 | 75.00 | 382.16 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.; Kim, H.; Jang, Y.; Jung, J.; Oh, J. Femtosecond Laser-Inscripted Direct Ultrafast Fabrication of a DNA Distributor Using Microfluidics. Appl. Sci. 2017, 7, 1083. https://doi.org/10.3390/app7101083
Shin H, Kim H, Jang Y, Jung J, Oh J. Femtosecond Laser-Inscripted Direct Ultrafast Fabrication of a DNA Distributor Using Microfluidics. Applied Sciences. 2017; 7(10):1083. https://doi.org/10.3390/app7101083
Chicago/Turabian StyleShin, Hojun, Hyojae Kim, Yeongseok Jang, Jinmu Jung, and Jonghyun Oh. 2017. "Femtosecond Laser-Inscripted Direct Ultrafast Fabrication of a DNA Distributor Using Microfluidics" Applied Sciences 7, no. 10: 1083. https://doi.org/10.3390/app7101083