Production of Aerated Foamed Concrete with Industrial Waste from the Gems and Jewels Sector of Rio Grande do Sul-Brazil
Abstract
:1. Introduction
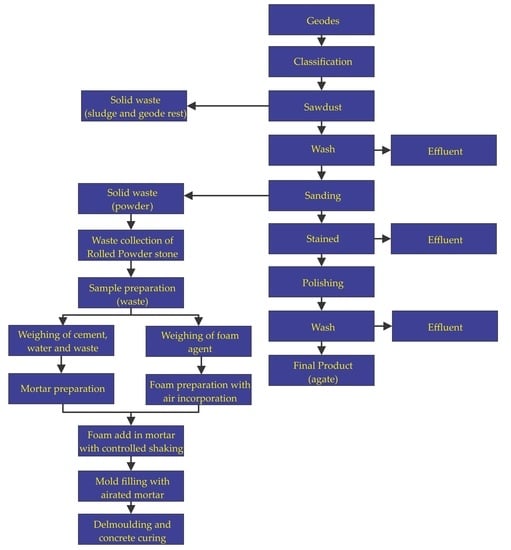
2. Materials and Methods
3. Results and Discussion
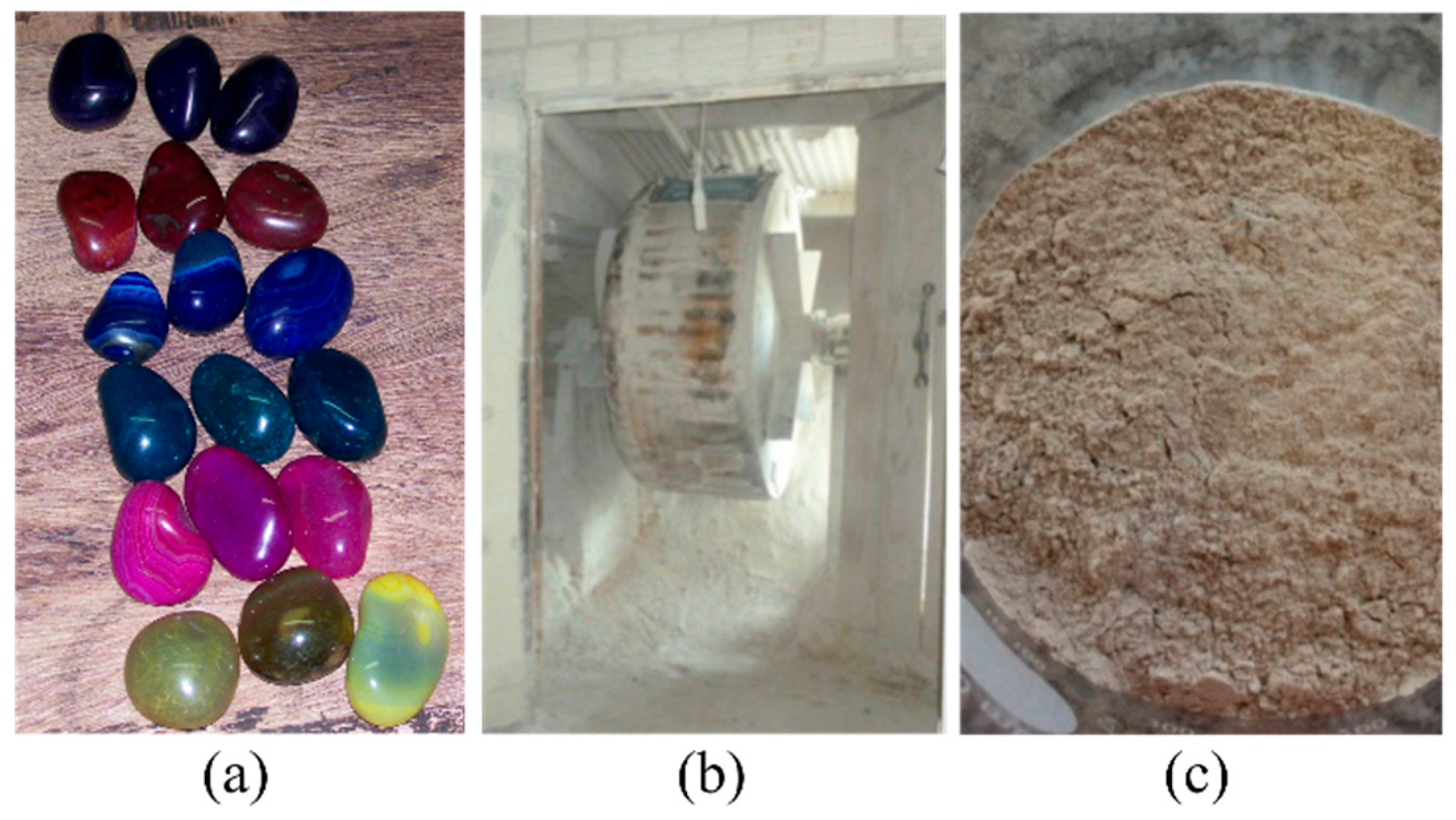
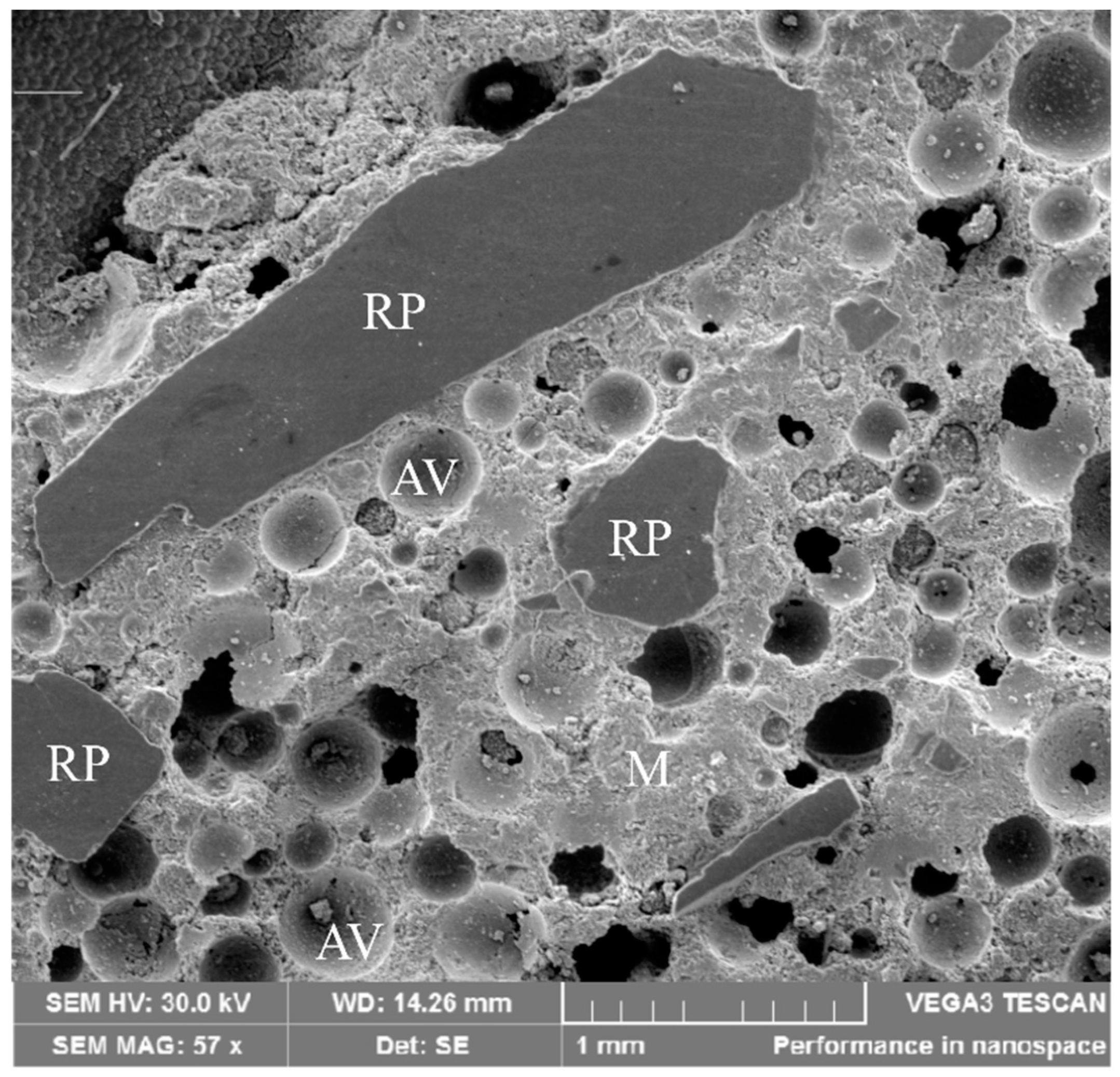
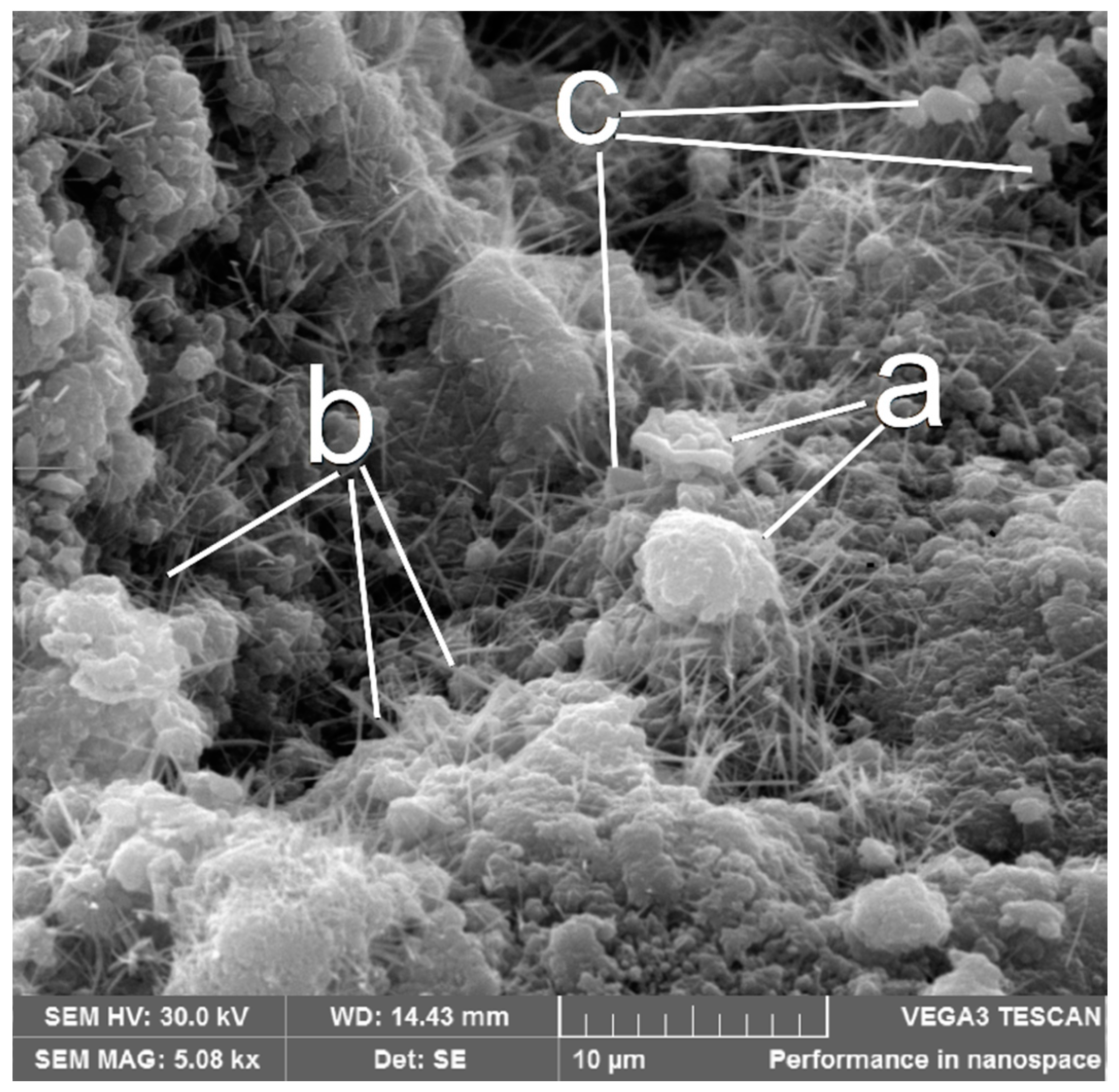
3.1. Characterization of the Rolled Powder (RP)
3.2. Characterization of AFCB
3.3. Comparison between Commercial Blocks (AFCB and ACCB) and AFCB Developed with Agate Waste
4. Conclusions
- Rolled powder can be used as an aggregate in the manufacture of AFCB, reducing the liabilities of agate manufacturing companies and reducing environmental impacts due to the irregular disposal of this waste. Also, with the use of this residue, it is possible to reduce the consumption of natural sand, which is a finite aggregate and its extraction causes environmental damages, mainly in riverbeds and lakes.
- The results of the RP characterization show that the granulometry and the chemical composition were suitable for the development of AFCB. It is relevant to note that PR was used without any process of beneficiation.
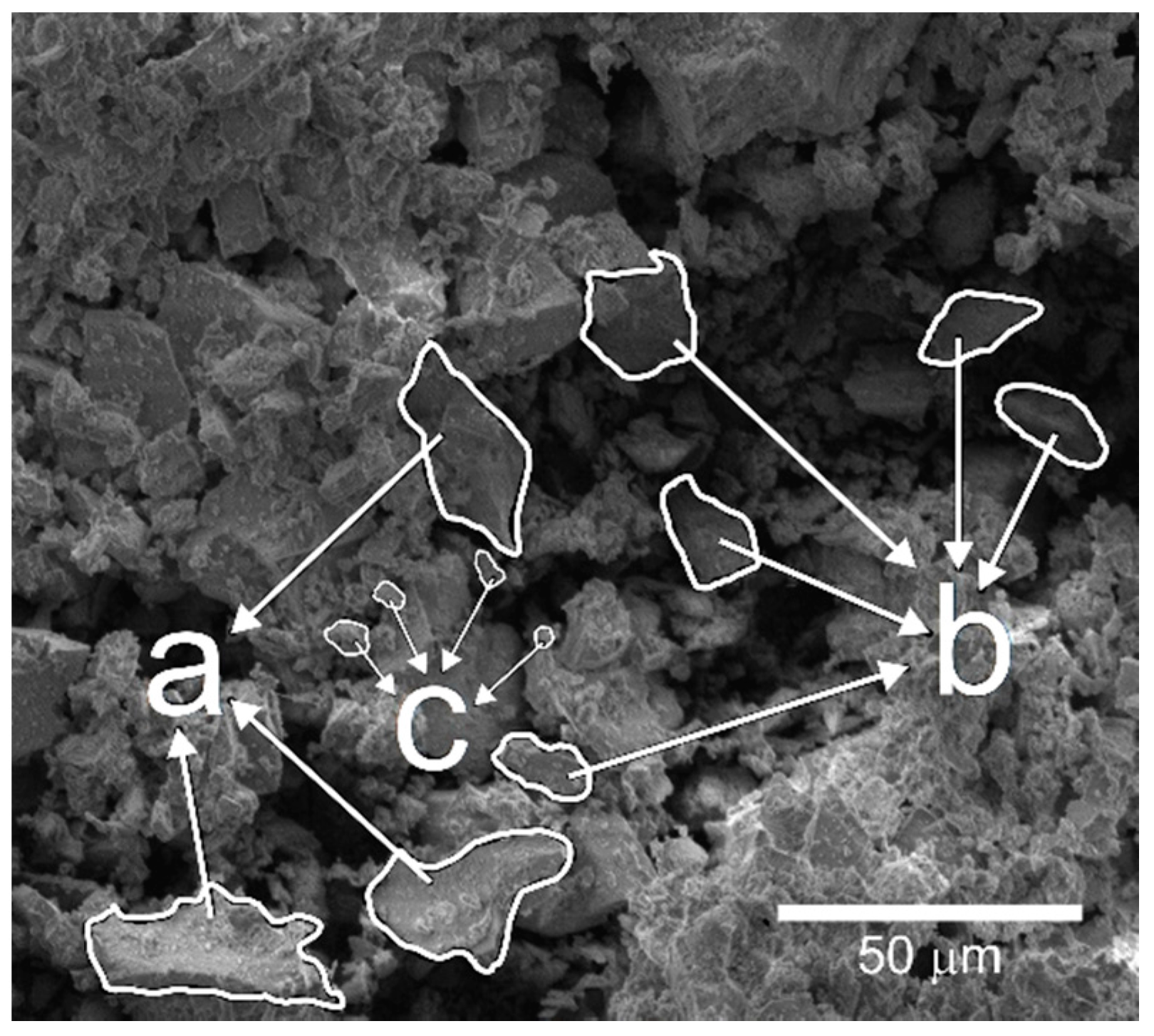
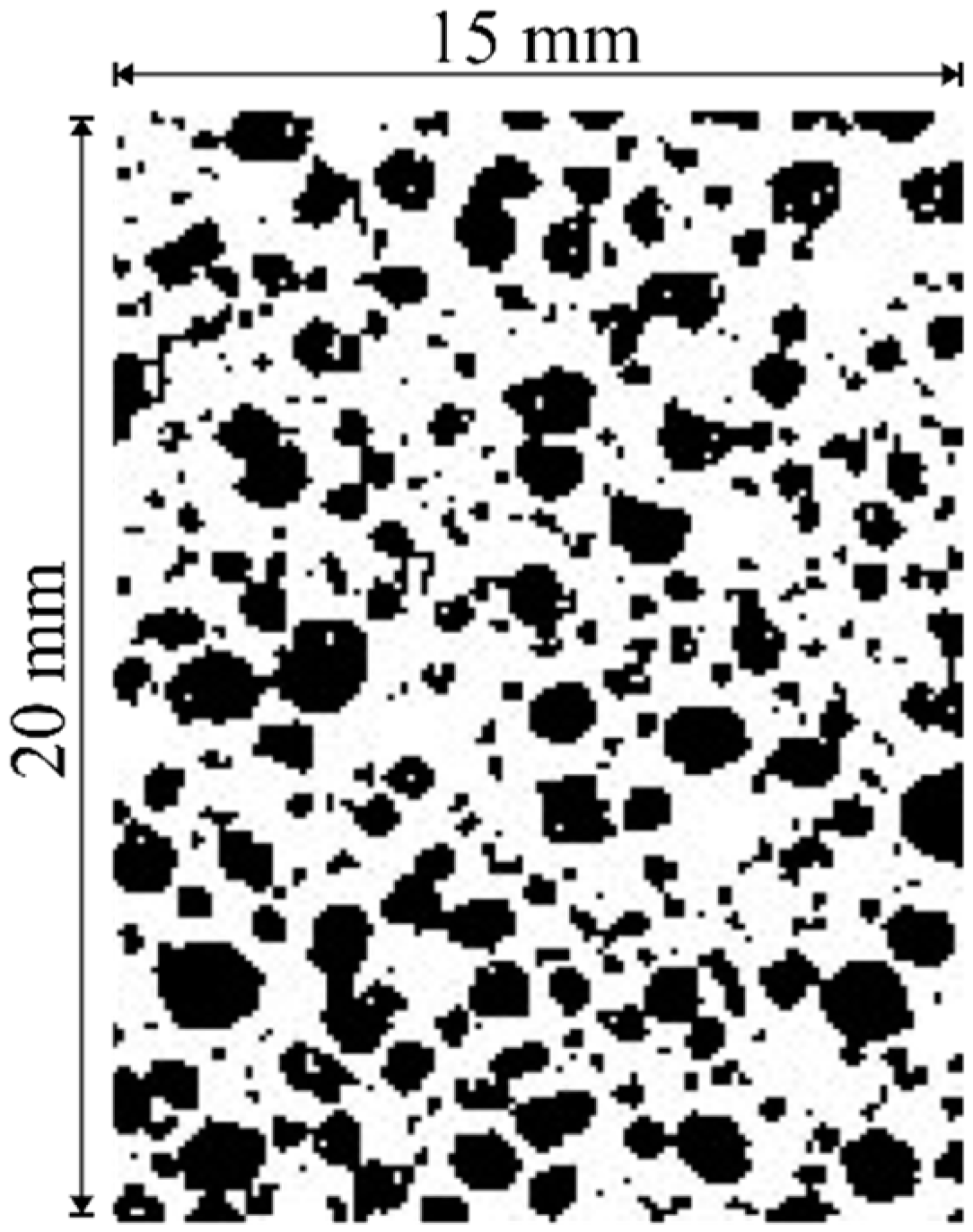
- The concrete blocks showed a microstructure with a mean pore size of 264.0 μm, which is characteristic of this type of material. Also, calcite formation in macropores was found due rate of hydration reactions.
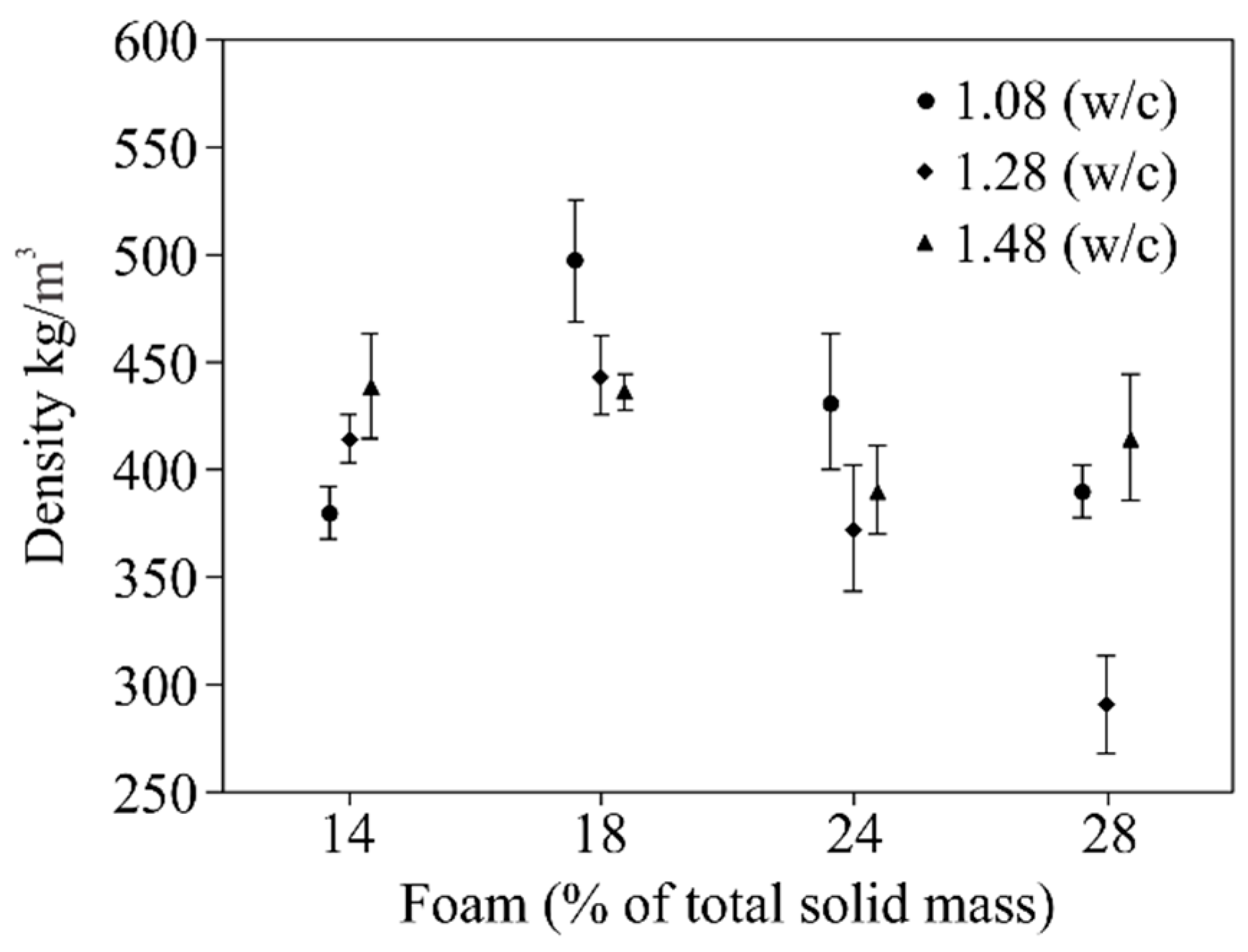
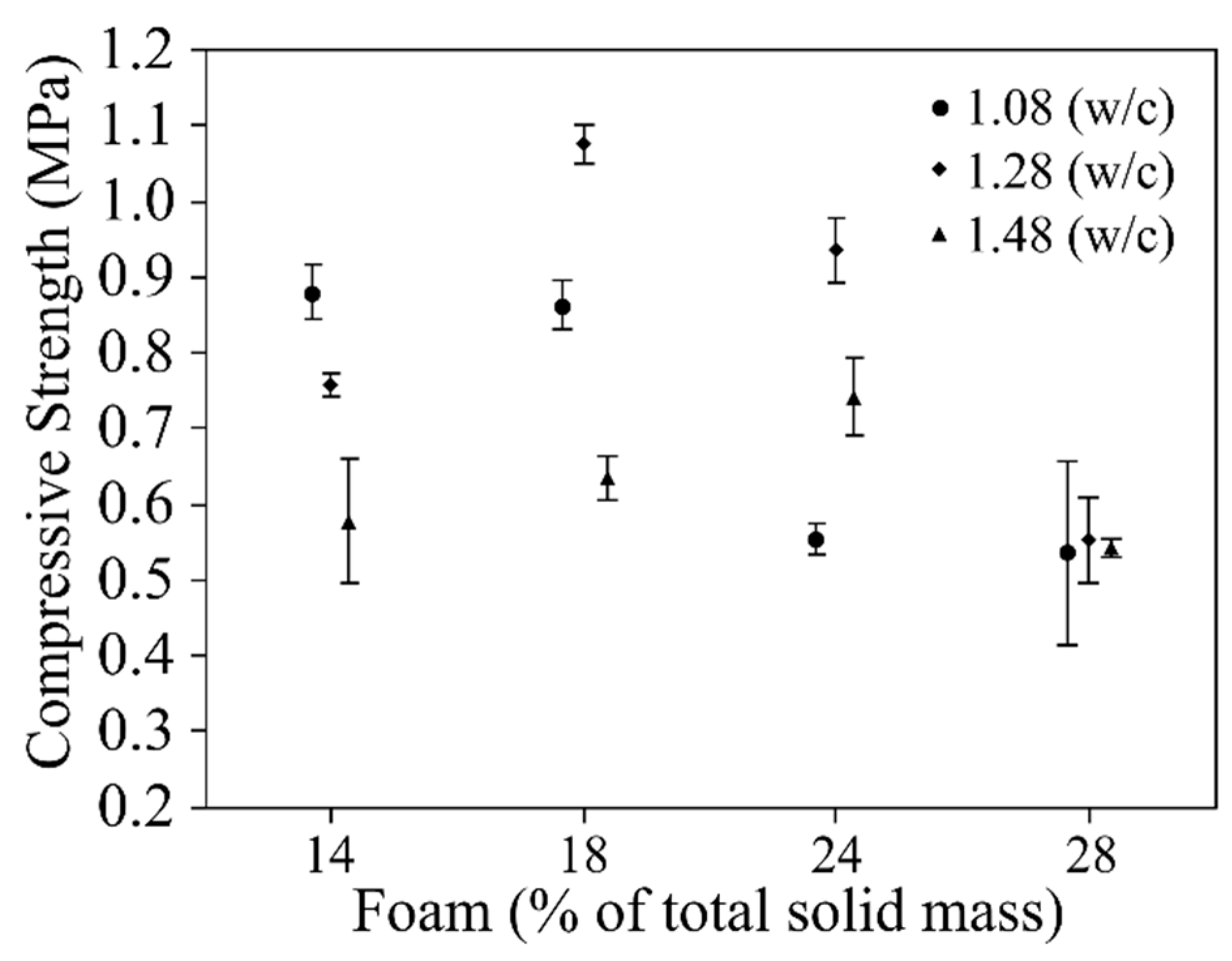
- The w/c ratio and the amount of foam had a significant influence on density and compressive strength. The results for the compressive strength and density of AFCB were slightly lower than those required by ABNT 13438. However, it is important to emphasize that this standard complies with the ACCB, which are autoclaved.
- Overall, the tests enabled the best ratio between PC, RP, water, and foam to be established, which was identified for AFCB with 1.28 of w/c, thus providing the greatest restriction with the lowest density. The w/c ratio set at 1.28 with an addition of 18% foam generated a sample with a density of 430 ± 18 kg/m3, and a compressive strength about of 1.07 ± 0.02 MPa. This result is close to meeting the requirements of the standard for density classes <450 kg/m3 NBR 13438 [32] for ACCB.
- Economic and financial aspects must be emphasized, since the use of agate waste can significantly reduce the production cost of the companies that generate these wastes. This is because the transportation and environmentally correct costs, today approximately of 55 USD per m3, would reduce to zero. Moreover, the AFCB manufacturing cost of using the RP can be 18% less than conventional AFCB cost.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tubino, L.; Sampaio, C. Industrial treatment of raw agate: Spectrocolorimetry and scanning electron microscopy (SEM) analyses. Dev. Miner. Process. 2000, 13, C1-9–C1-16. [Google Scholar]
- Agostini, I.M.; Fiorentini, J.A.; Brum, T.M.M.; Juchem, P.L. Ágata do Rio Grande do Sul; Série Difusão Tecnológica; Ministério de Minas e Energia, Departamento Nacional de Produção Mineral: Brasília, Brazil, 1998; No. 5; p. 272.
- Thomé, A.; Abreu, A.G.D.; Brandli, L.L.; Fernandes, V.M.C.; Prietto, P.D.M. Diagnóstico dos resíduos gerados pelo setor de pedras precisas do município de Soledade/RS. In Tecnologias Para o Setor de Gemas, Joias e Mineração; Hartmann, L.A., Silva, J.T.D., Eds.; Universidade Federal do Rio Grande do Sul/UFRGS: Soledade, Brazil, 2010; Volume 1. [Google Scholar]
- Duarte, L.C.; Hartmann, L.A. Geodos preenchidos por ametistas em basaltos da Formação Arapey, Artigas, República Oriental do Uruguai: Modelo Epigenético. In 44 Congresso Brasileiro de Geologia; Sociedade Brasileira de Geologia: Curitiba, Brazil, 2008. [Google Scholar]
- Bossi, J.; Cagiano, W. Contribuición a la geologia de losya cimientos de ametista del Departamento de Artigas (Uruguai). In Congresso Brasileiro de Geologia; Sociedade Brasileira de Geologia: Porto Alegre, Brazil, 1974; Volume 5, pp. 301–318. [Google Scholar]
- Heaney, P.J. A proposed mechanism for the growth of chalcedony. Contrib. Miner. Petrol. 1993, 115, 66–74. [Google Scholar] [CrossRef]
- Brum, T.M.M.; Juchem, P.L.; Fischer, A.C.; Heemann, R.; Castro, J.H.W.; Gouvea, J.C.R., Jr. Características microscópicas da ágata do Salto do Jacuí, RS. In I Simpósio de Tratamento e Caracterizacao de Gemas; Coordenadoria de Imprensa e Editora—UFOP: Ouro Preto, Brazil, 2000. [Google Scholar]
- Thomé, A.; Schneider, I.A.; Rosa, F.D.; Consoli, N. Caracterização geotécnica de um resíduo da indústria de pedras semipreciosas e viabilidade de seu uso em estabilização dos solos. In Congresso Brasileiro de Mecânica dos Solos e Engenharia Geotécnica; ABMS: São Paulo, Brazil, 2002; Volume 1, pp. 229–240. [Google Scholar]
- Vilasbôas, F.; Santos, C.R.D.; Menezes, J.C.S.D.S.; Silva, R.D.A.; Schneider, I.A.H. Environment issues on industrial processing of raw agate. In Sustainability 16; Minerals Engineering: Falmouth, UK, 2016. [Google Scholar]
- Barros, A.L.; Pizzolato, T.M.; Carissimi, E.; Schneider, I.A.H. Decolorizing dye wastewater from the agate industry with Fenton oxidation process. Miner. Eng. 2006, 19, 87–90. [Google Scholar] [CrossRef]
- Petry, N.; Masuero, Â.B.; Kirchhein, A.P. Alkali silica reaction in aggregates derived from the valorization of waste from the agate mining. In Proceedings of the 15th International Conference on Alkali-Aggregated Reactions, São Paulo, Brazil, 3–7 July 2016. [Google Scholar]
- Hartmann, L.A.; Silva, J.; Tonezer, D. A Porto Alegre: IGEO/UFRGS. In Tecnologias Para o Setor de Gemas, Jóias e Mineração; Universidade Federal do Rio Grande do Sul—UFRGS: Porto Alegre, Brazil, 2010. [Google Scholar]
- Petry, N.D.S. Uso de Resíduos de Ágata Como Agregado em Argamassas de Cimento Portland Branco; Programa de Pós-Graduação em Engenharia Civil; Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 2015. [Google Scholar]
- Abreu, A.G.D.; Silveira, A.A.; Santos, T.M. Pó de resíduos de ágata como adição mineral. In Proceedings of the V Congresso Internacional 19a Reunión Técnica “Ing. Oscar R. Batic”, Buenos Aires, Argentina, 7–9 November 2012; Volume 1, pp. 55–62. [Google Scholar]
- Venquiaruto, S.; Ossorio, A.; I, C.Z.; Passuelo, A.; Kirchhem, A.P.; Dalmolin, D.C.C.; Massuero, A. Aproveitamento de resíduos de ágata reciclada em materiais cimentícios sustentáveis. In Tecnologia e Inovação em Gemas, Joias e Mineração; Hartmann, L., Silva, J.T.D., Eds.; Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 2014; Volume 1. [Google Scholar]
- Betat, E.F.; Pereira, F.M.; Verney, J.C.K. Concretos produzidos com resíduos do beneficiamento de ágatas: Avaliação da resistência a compressão e do consumo de cimento. Rev. Matér. 2009, 14, 10. [Google Scholar] [CrossRef]
- Chiaro, S.M.X. Reação Alcali-Agregado em Concretos Brancos com Agregados de Miúdos de Reciclados de Ágata; Universidade Federal do Rio Grande do Sul—UFRGS: Porto Alegre, Brazil, 2012. [Google Scholar]
- Correia, S.L.; Dienstmann, G.; Folgueras, M.V.; Segadaes, A.M. Effect of quartz sand replacement by agate rejects in triaxial porcelain. J. Hazard. Mater. 2009, 163, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.V.; Basegio, T.; Vilanova, D.L.; Bergmann, C.P. Beneficiamento de ágata para a preparação de Materiais Cerâmicos. In Seminário de Iniciação Científica; Universidade Federal do Rio Grande do Sul—UFRGS: Porto Alegre, Brazil, 2014. [Google Scholar]
- DallaRosa, F.; Thomé, A.; Donato, M. Análise da viabilidade técnica da aplicação do resíduo da rolagem de pedras preciosas em estruturas de pavimentos urbanos. In Tecnologia e Inovação em Gemas, Joias e Mineração; Hartmann, L.A., Silva, J.T.D., Donato, M., Eds.; Instituto de Geociências UFRGS: Porto Alegre, Brazil, 2014; Volume 1, pp. 83–94. [Google Scholar]
- Donato, M.; Bortoluzzi, E.C.; Abreu, C.T.; DallaRosa, F. Transformação de resíduos da mineração para uso como artefato de concreto e remineralizador de solo. In Inovação, Design e Pesquisas Aplicadas em Gemas, Joias e Mineração; Donato, M., Duarte, L.d.C., Hartmann, L.A., Eds.; Instituto de Geociências UFRGS: Porto Alegre, Brazil, 2015; Volume 1, pp. 90–96. [Google Scholar]
- Folle, D.; Silva, R.D.A.; Boita, J.; Carissimo, D.; Schneider, I.A.H. Waste generation in Agate processing: Use of SiO2 as a support matterial for Fe3O4. Int. J. Civ. Struct. Eng. 2015, 2, 327–331. [Google Scholar]
- Topçu, İ.B.; Bilir, T.; Uygunoğlu, T. Effect of waste marble dust content as filler on properties of self-compacting concrete. Constr. Build. Mater. 2009, 23, 1947–1953. [Google Scholar] [CrossRef]
- Binici, H.; Shah, T.; Aksogan, O.; Kaplan, H. Durability of concrete made with granite and marble as recycle aggregates. J. Mater. Process. Technol. 2008, 208, 299–308. [Google Scholar] [CrossRef]
- Sua-iam, G.; Makul, N. Utilization of high volumes of unprocessed lignite-coal fly ash and rice husk ash in self-consolidating concrete. J. Clean. Prod. 2014, 78, 184–194. [Google Scholar] [CrossRef]
- Kunchariyakun, K.; Asavapisit, S.; Sombatsompop, K. Properties of autoclaved aerated concrete incorporating rice husk ash as partial replacement for fine aggregate. Cem. Concr. Compos. 2015, 55, 11–16. [Google Scholar] [CrossRef]
- Kurama, H.; Topçu, İ.B.; Karakurt, C. Properties of the autoclaved aerated concrete produced from coal bottom ash. J. Mater. Process. Technol. 2009, 209, 767–773. [Google Scholar] [CrossRef]
- Naceri, A.; Hamina, M.C. Use of waste brick as a partial replacement of cement in mortar. Waste Manag. 2009, 29, 2378–2384. [Google Scholar] [CrossRef] [PubMed]
- Esmaily, H.; Nuranian, H. Non-autoclaved high strength cellular concrete from alkali activated slag. Constr. Build. Mater. 2012, 26, 200–206. [Google Scholar] [CrossRef]
- Walczaka, P.; Małolepszyb, J.; Rebenb, M.; Szymański, P.; Rzepa, K. Utilization of waste glass in autoclaved aerated concrete. Procedia Eng. 2015, 122, 302–309. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials, 4th ed.; McGraw Hill Professiona’s Access Engineering: New York, NY, USA, 2014. [Google Scholar]
- Associação Brasileira Normas Tecnicas (ABNT). NBR13438—Blocos de Concreto Celular Autoclavado-Requisitos; Associação Brasileira Normas Tecnicas: Rio de Janeiro, Brazil, 2013; p. 5. [Google Scholar]
- Fouad, F.H. Significance of Tests and Properties of Concrete and Concrete-Making Materials, STP 169D; Lamond, J.F., Pielert, J.H., Eds.; American Society for Testing and Materials International: Bridgeport, NJ, USA, 2006; pp. 561–569. [Google Scholar]
- American Society for Testing and Materials (ASTM) International. C495-99a—Standard Test Method for Compressive Strength of Lightweight Insulating Concrete; American Society for Testing and Materials International: West Conshohocken, PA, USA, 1999. [Google Scholar]
- Narayanan, N.; Ramamurthy, K. Structure and properties of aerated concrete: A review. Cem. Concr. Compos. 2000, 22, 321–329. [Google Scholar] [CrossRef]
- Ferreira, O.A.R. Concreto Celulares Espumosos; Boletin Técnico do Departamento de Engenharia Construção Civil; Escola Politécnica da Universidade de São Paulo: São Paulo, Brazil, 1987. [Google Scholar]
- Wongkeo, W.; Chaipanich, A. Compressive strength, microstructure and thermal analysis of autoclaved and air cured structural lightweight concrete made with coal bottom ash and silica fume. Mater. Sci. Eng. A 2010, 527, 3676–3684. [Google Scholar] [CrossRef]
- Krämer, C.; Schauerte, M.; Kowald, T.L.; Trettin, R.H.F. Three-phase-foams for foam concrete application. Mater. Charact. 2015, 102, 173–179. [Google Scholar] [CrossRef]
- Associação Brasileira Normas Tecnicas (ABNT). NBR 5733/91 (EB 2)—Cimento Portland de Alta Resistência Inicial; Associação Brasileira Normas Tecnicas: Rio de Janeiro, Brazil, 1991. [Google Scholar]
- Associação Brasileira Normas Tecnicas (ABNT). NBR 5737—Cimentos Portland Resistentes a Sulfatos; Associação Brasileira Normas Tecnicas: Rio de Janeiro, Brazil, 1992. [Google Scholar]
- Paiva, M.P.D. Caracterização e Flotação de Minérios de Fosfato da Mina de Sofia-Chile; Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 2012. [Google Scholar]
- Oliveira, C.H.; Binotti, R.S.; Barrientos-Astigarraga, R.E.; Graudenz, G.S.; Neto, A.C. Surfactantes derivados do fruto de coco (Cocos nucifera L.) e sensibilidade cutânea. Rev. Bras. Alerg. Imunopatol. 2005, 28, 155–160. [Google Scholar]
- American Society for Testing and Materials (ASTM) International. ASTM C796/C796M-12—Standard Test Method for Foaming Agents for Use in Producing Cellular Concrete Using Preformed Foam; American Society for Testing and Materials International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- American Society for Testing and Materials (ASTM) International. C869/C869M-11—Standard Specification for Foaming Agents Used in Making Preformed Foam for Cellular Concrete; American Society for Testing and Materials International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- American Society for Testing and Materials (ASTM) International. C138/C138M-16a—Standard Test Method for Density (Unit Weight), Yield, and Air Content (Gravimetric) of Concrete; American Society for Testing and Materials International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- American Society for Testing and Materials (ASTM) International. C457/C457M-12—Standard Test Method for Microscopical Determination of Parameters of the Air-Void System in Hardened Concrete; American Society for Testing and Materials International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Will, G.; Bellotto, M.; Parrish, W.; Hart, M. Crystal structures of quartz and magnesium germanate by profile analysis of synchrotron-radiation high-resolution powder data. J. Appl. Crystallogr. 1988, 21, 182–191. [Google Scholar] [CrossRef]
- Barth, T.F.W. The cristobalite structures. Am. J. Sci. 1932, 136, 350–356. [Google Scholar] [CrossRef]
- Graetsch, H.A. Modulated crystal structure of incommensurate low tridymite. Acta Crystallogr. Sect. B Struct. Sci. 2009, 65, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Dollase, W. Reinvestigation of the structure of low cristobalite. Z. Kristallogr. Cryst. Mater. 1965, 121, 369–377. [Google Scholar] [CrossRef]
- Popov, M.; Zakrevskaya, L.; Vaganov, V.; Hempel, S.; Mechtcherine, V. Performance of lightweight concrete based on Granulated Foamglass. In 2nd International Conference on Innovative Materials, Structures and Technologies, Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2015. [Google Scholar]
- Alexanderson, J. Relations between structure and mechanical properties of autoclaved aerated concrete. Cem. Concr. Res. 1979, 9, 507–514. [Google Scholar] [CrossRef]
- Ramamurthy, K.; Kunhanandan Nambiar, E.K.; Indu Siva Ranjani, G. A classification of studies on properties of foam concrete. Cem. Concr. Compos. 2009, 31, 388–396. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer foam concrete: An emerging material for sustainable construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Laukaitis, A.; Fiks, B. Acoustical properties of aerated autoclaved concrete. Appl. Acoust. 2006, 67, 284–296. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Farzadnia, N.; Abang Ali, A.A. Properties and applications of foamed concrete: A review. Constr. Build. Mater. 2015, 101, 990–1005. [Google Scholar] [CrossRef]
- Secco, M.P.; Tiecher, C.; Ribeiro, B.P.; Santos, T.J.P.D.; Meira, C.; Silva, M.D.; Silva, R.D.A. Obtenção de bloco de concreto celular como tema gerador para ensino médio. Rev. Eng. Civ. IMED 2015, 2, 35–43. [Google Scholar] [CrossRef]
- Hilal, A.A.; Thom, N.H.; Dawson, A.R. Failure mechanism of foamed concrete made with/without additives and lightweight aggregate. J. Adv. Concr. Technol. 2016, 14, 511–520. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.H.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
| Physical Properties | |||||||||
| Materials | Specific Gravity g/cm3 | Blaine Fineness m2/kg | Particle Size | pH Paste | |||||
| Pass in 75 μm | D10% Μm | D50% μm | D90% μm | Daverage μm | |||||
| Portland Cement (PC) | 3.1 | 430 | 0.5% | 1.0 | 9.6 | 27.6 | 12.3 | 13.0 | |
| Rolled Powder (PR) | 2.7 | 80 | 3.2% | 2.8 | 31.4 | 151.5 | 57.6 | 8.2 | |
| Chemical Composition | |||||||||
| Materials | Al2O3 | CaO | CdO | SiO2 | Fe2O3 | Na2O | All Others | SO3 | Loss on Ignition |
| Portland Cement (PC) | 3.1 | 60.6 | 4.3 | 18.9 | 0.5 | - | 13.0 | 2.0 | 2.9 |
| Rolled Powder (RP) | 1.2 | 0.4 | 0.1 | 92.2 | 1.1 | 0.1 | 0.1 | - | 3.5 |
| Order | w/c Rate | % Foam (of Total Solid Mass) |
|---|---|---|
| 1 | 1.08 | 14 |
| 2 | 1.08 | 18 |
| 3 | 1.08 | 24 |
| 4 | 1.08 | 28 |
| 5 | 1.28 | 14 |
| 6 | 1.28 | 18 |
| 7 | 1.28 | 24 |
| 8 | 1.28 | 28 |
| 9 | 1.48 | 14 |
| 10 | 1.48 | 18 |
| 11 | 1.48 | 24 |
| 12 | 1.48 | 28 |
| Fase | Crystaline System | Lattice Parameters Refined | Quantification | |
|---|---|---|---|---|
| % Mass | % Volumetric | |||
| Quartzo (SiO2) | Trigonal | a = 4.9113 ± 1.58·10−4 Å | 95.09 ± 0.09 | 89.03 ± 0.09 |
| c = 5.4016 ± 2.79·10−4 Å | ||||
| Cristobalite-low (SiO2) | Tetragonal | a = 5.026 ± 4.73·10−4 Å | 1.86 ± 0.12 | 2 ± 0.12 |
| c = 6.8446 ± 2·10−4 Å | ||||
| Tridimite-low (SiO2) | Triclinic | a = 5.030 ± 0.013 Å | 1.54 ± 0.17 | 7.25 ± 0.17 |
| b = 9.082 ± 0.06 Å | ||||
| c = 8.306 ± 0.05 Å | ||||
| α = 91.11 ± 0.92° | ||||
| β = 93.79 ± 0.48° | ||||
| γ = 91.47 ± 0.81° | ||||
| Cristobalite (SiO2) | Cubic | a = 7.1436 ± 0.0057 Å | 1.5 ± 0.26 | 1.7 ± 0.26 |
| Sum | 99.99% | 99.98% | ||
| Blocks | Compressive Strength (MPa) | Density (kg/m3) | Description of Manufacturing Process |
|---|---|---|---|
| Commercial AFCB | 2.14 | 830 | water/cement rate of 0.82; 25 kg of natural sand; 25 kg of cement Portland (V-ARI); 20 kg of water; The materials were added in a mixer and stirred for 10 min. Sequentially, 50 liters of foam were added and mixed for another 5 min. The concrete curing was of 28 days in open environment. |
| Commercial ACCB | 4.50 | 600 | Autoclave curing process. Other information not provided by the manufacturer. |
| Commercial ACCB | 1.20 | 400 | Autoclave curing process. Other information not provided by the manufacturer. |
| AFCB with RP | 1.07 | 430 | water/cement rate of 1.28; 500 g of RP; 500 g of cement Portland (V-ARI); 400 g of water; The materials were added in a mixer and stirred for 10 min. Sequentially, 230 g of foam were added and mixed for another 5 min. The concrete curing was of 28 days in open environment. |
| Materials | Commercial AFCB Cost (USD) | AFCB with RP Cost (USD) |
|---|---|---|
| Cement | 45.20 | 45.20 |
| Natural sand | 6.45 | 0.00 |
| Water | 0.40 | 0.40 |
| Agate waste | 0.00 | 0.00 |
| Commercial foam | 5.81 | 0.00 |
| Foam of study | 0.00 | 1.94 |
| Total cost | 57.86 | 47.54 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedro, R.; Tubino, R.M.C.; Anversa, J.; De Col, D.; Lermen, R.T.; Silva, R.D.A. Production of Aerated Foamed Concrete with Industrial Waste from the Gems and Jewels Sector of Rio Grande do Sul-Brazil. Appl. Sci. 2017, 7, 985. https://doi.org/10.3390/app7100985
Pedro R, Tubino RMC, Anversa J, De Col D, Lermen RT, Silva RDA. Production of Aerated Foamed Concrete with Industrial Waste from the Gems and Jewels Sector of Rio Grande do Sul-Brazil. Applied Sciences. 2017; 7(10):985. https://doi.org/10.3390/app7100985
Chicago/Turabian StylePedro, Rudimar, Rejane M. C. Tubino, Jonas Anversa, Denisar De Col, Richard Thomas Lermen, and Rodrigo De Almeida Silva. 2017. "Production of Aerated Foamed Concrete with Industrial Waste from the Gems and Jewels Sector of Rio Grande do Sul-Brazil" Applied Sciences 7, no. 10: 985. https://doi.org/10.3390/app7100985