Synthesis and Electroluminescence Properties of 3-(Trifluoromethyl)phenyl-Substituted 9,10-Diarylanthracene Derivatives for Blue Organic Light-Emitting Diodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Considerations
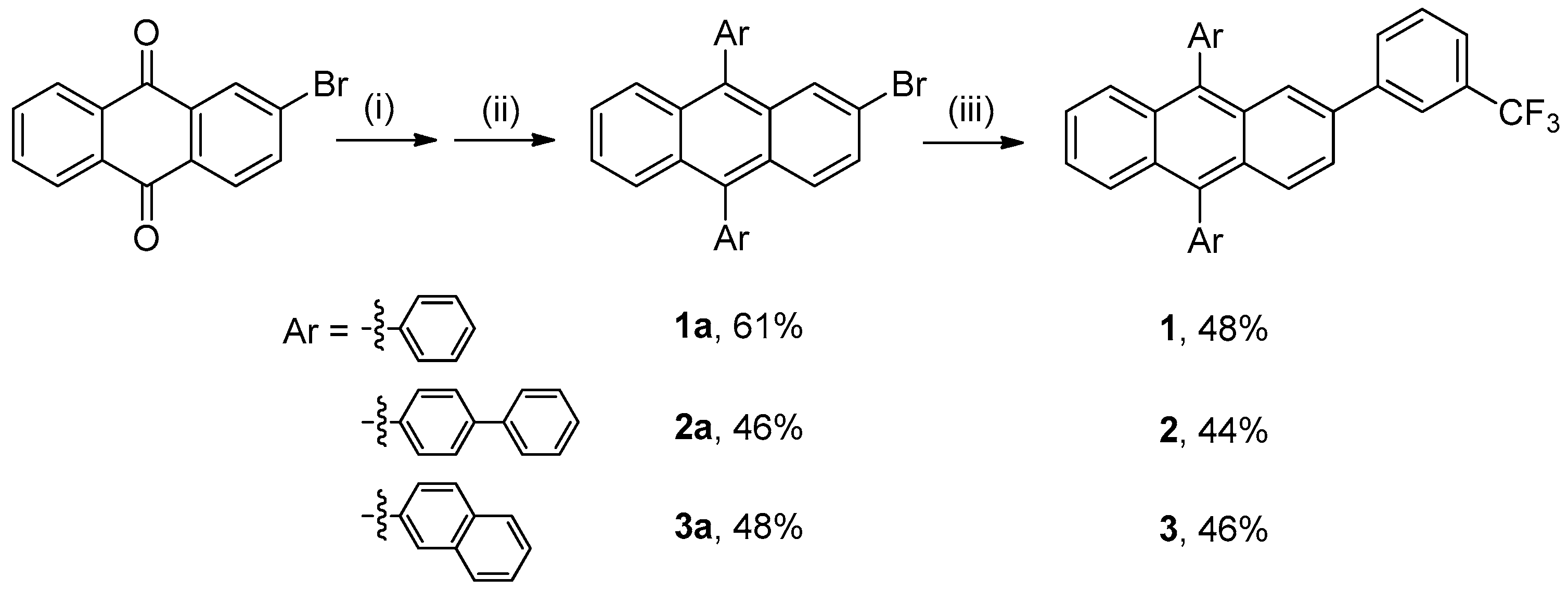
2.2. General Synthesis of 9,10-aryl-2-(3-(trifluoromethyl)phenyl)anthracene (1–3)
2.2.1. Data for 9,10-diphenyl-2-(3-(trifluoromethyl)phenyl)anthracene (1)
2.2.2. Data for 9,10-di([1,1′-biphenyl]-4-yl)-2-(3-(trifluoromethyl)phenyl)anthracene (2)
2.2.3. Data for 9,10-di(naphthalen-2-yl)-2-(3-(trifluoromethyl)phenyl)anthracene (3)
2.3. Cyclic Voltammetry
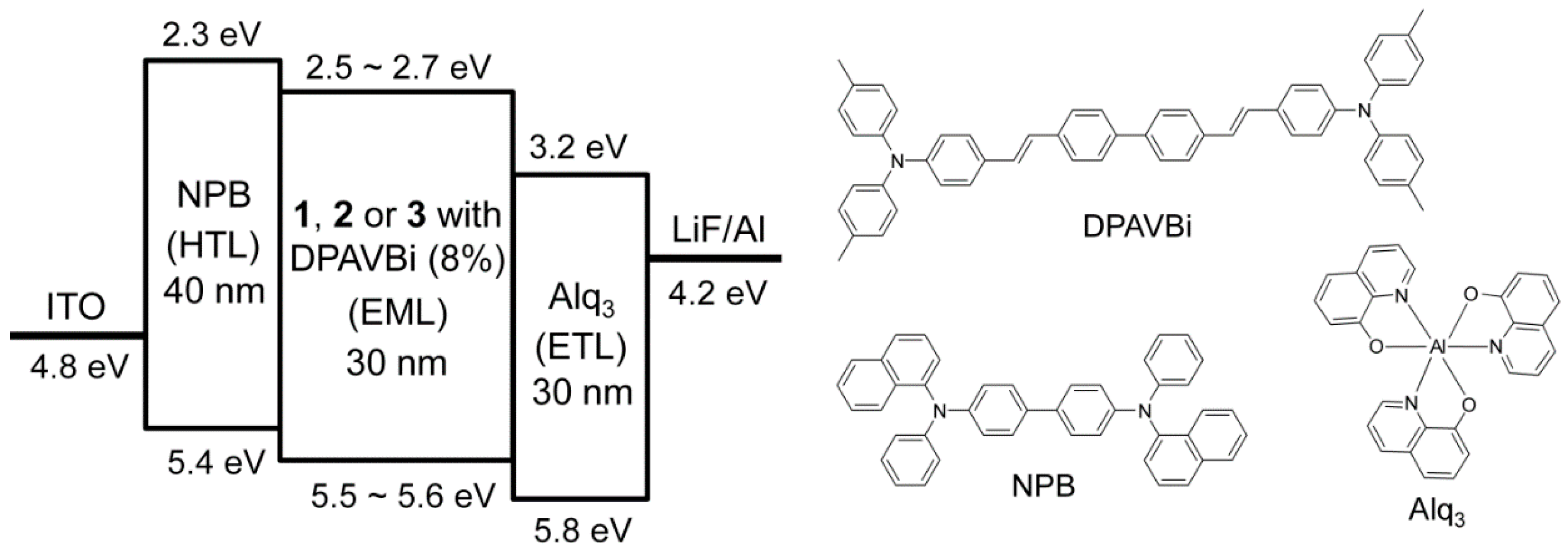
2.4. Device Fabrication and Characterization
3. Results and Discussion
3.1. Synthesis and Characterization
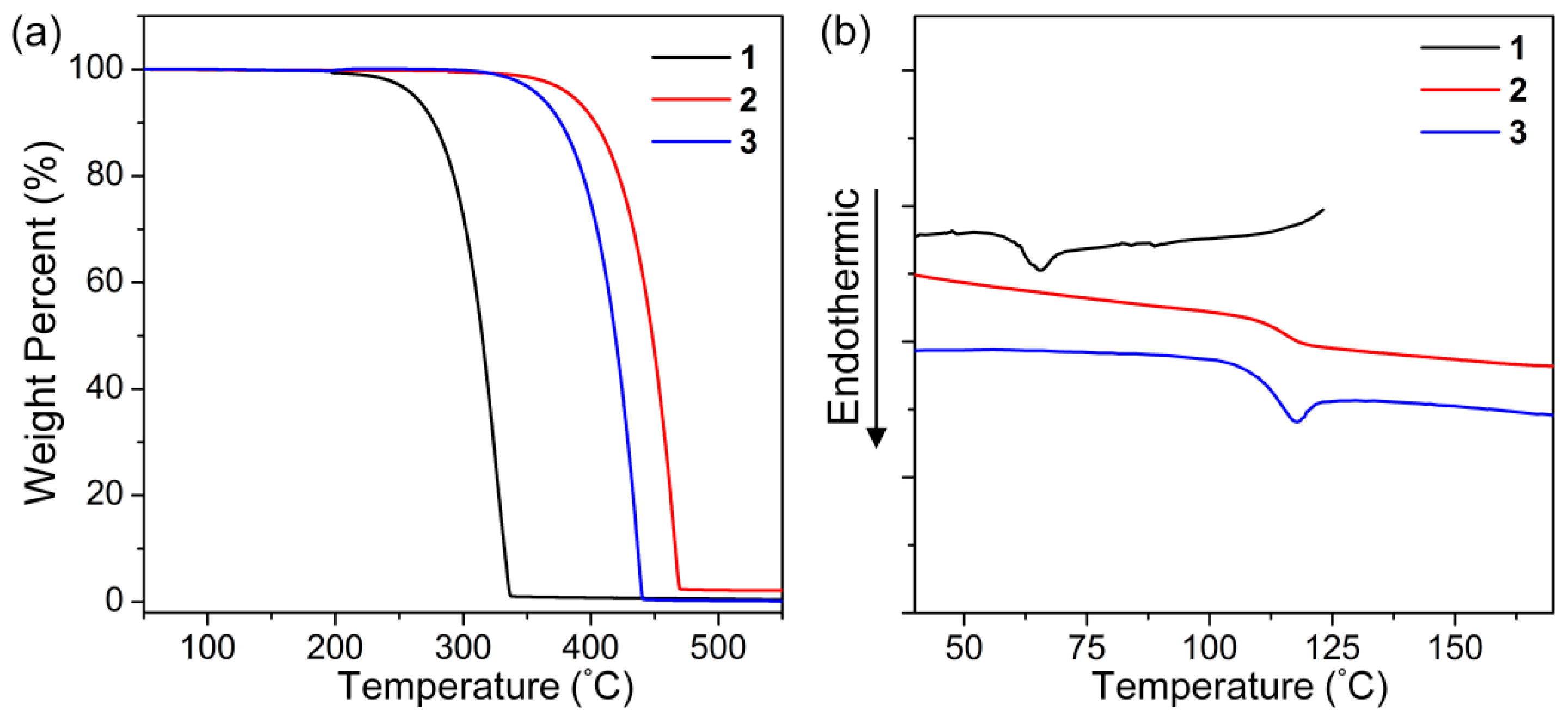
3.2. Thermal Properties
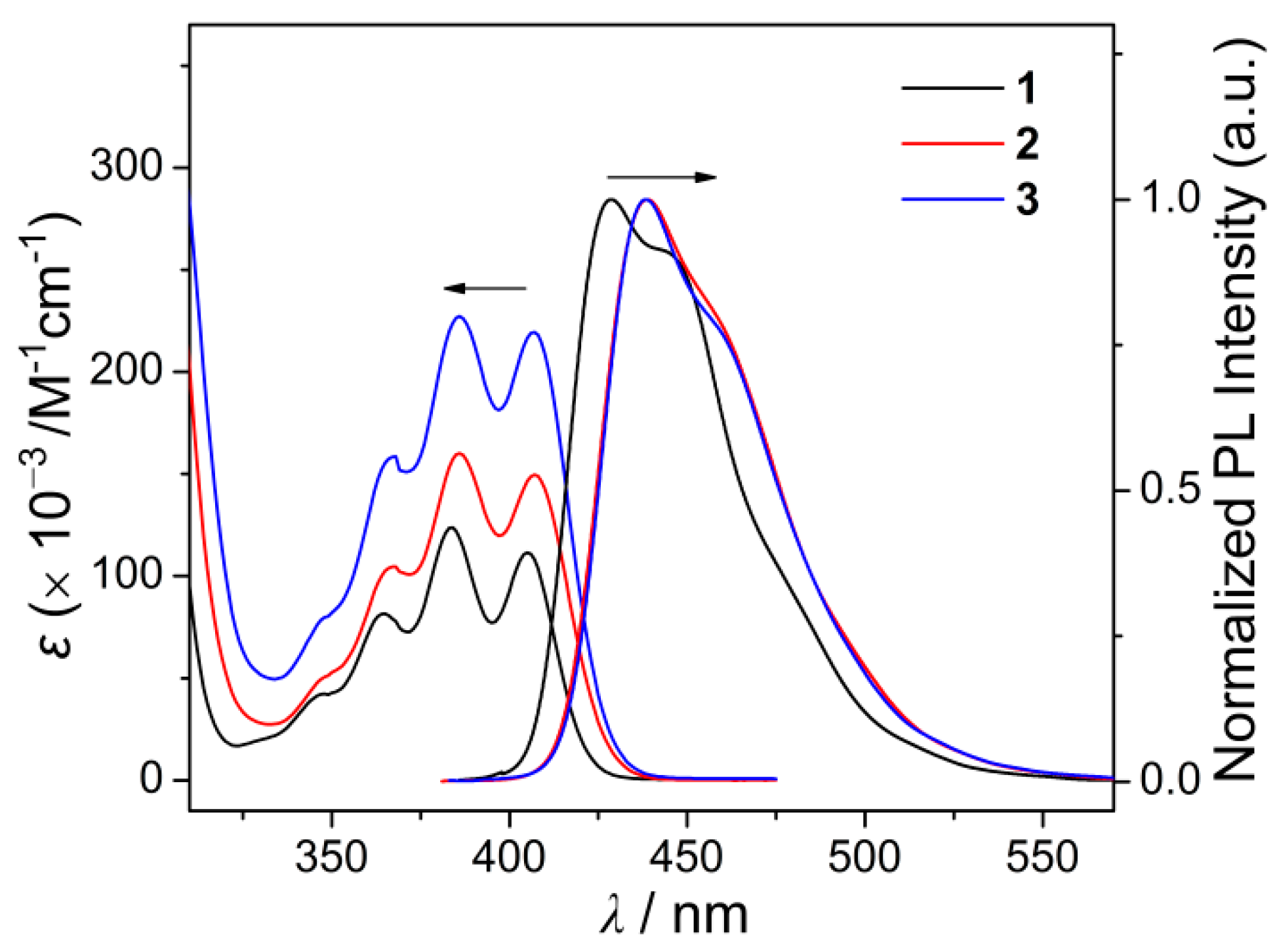
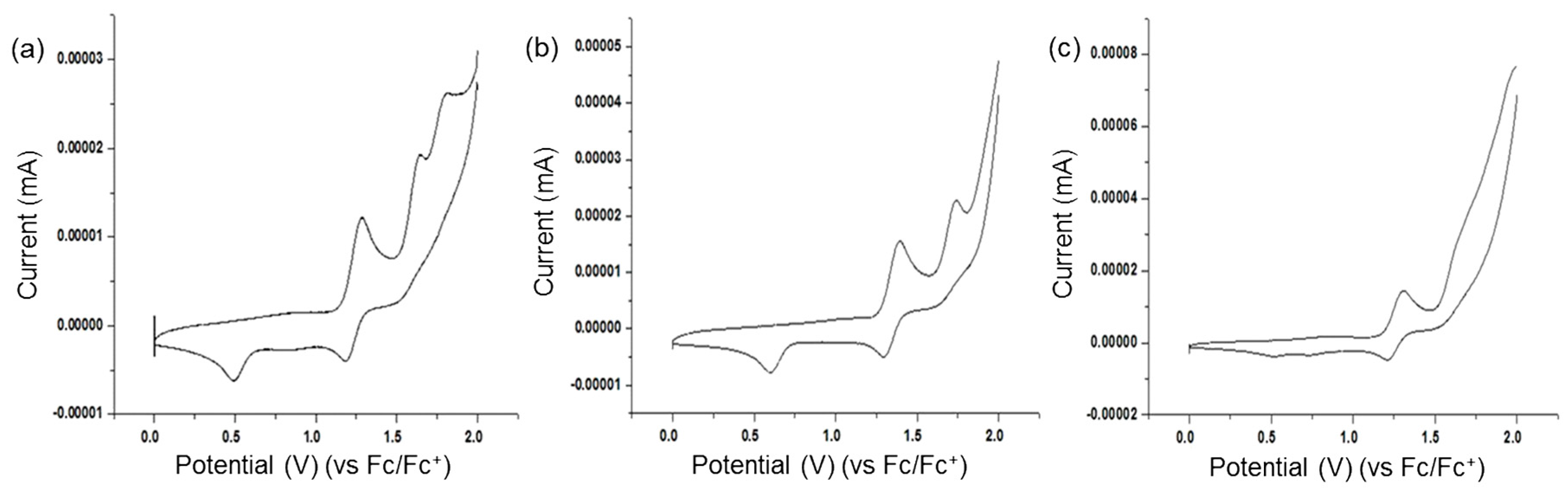
3.3. Photophysical and Electrochemical Properties
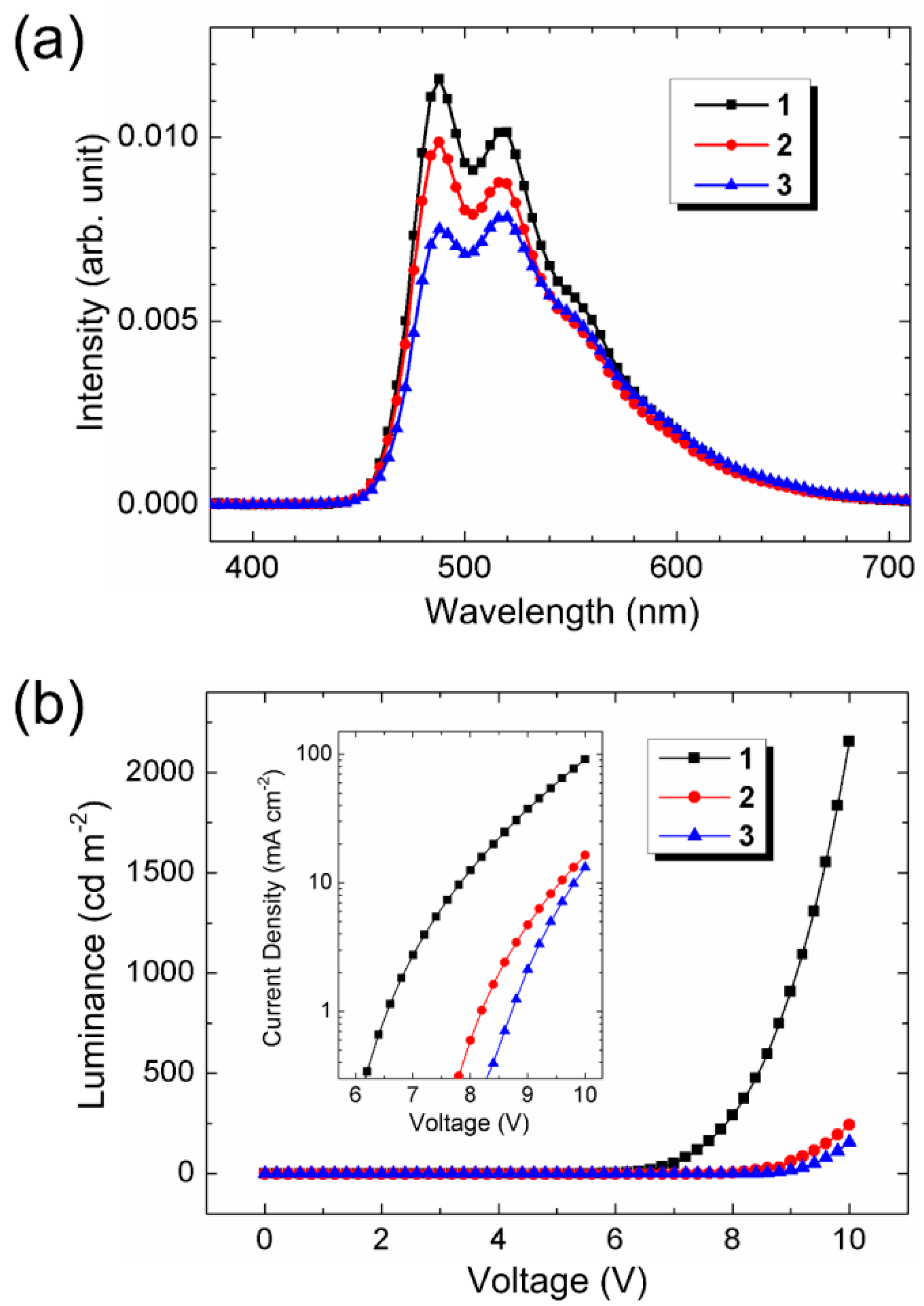
3.4. Electroluminescent Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burns, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Kido, J.; Kimura, M.; Nagai, K. Multilayer white light-emitting organic electroluminescent device. Science 1995, 267, 1332–1334. [Google Scholar] [CrossRef] [PubMed]
- D’Andrade, B.W.; Thompson, M.E.; Forrest, S.R. Controlling exciton diffusion in multilayer white phosphorescent organic light emitting devices. Adv. Mater. 2002, 14, 147–151. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.; Liang, Y.; Yang, R. Solution-processible carbazole dendrimers as host materials for highly efficient phosphorescent organic light-emitting diodes. Adv. Funct. Mater. 2013, 23, 619–628. [Google Scholar] [CrossRef]
- Lee, S.-J.; Park, J.-S.; Song, M.; Shin, I.A.; Kim, Y.-I.; Lee, J.W.; Kang, J.-W.; Gal, Y.-S.; Kang, S.; Lee, J.Y.; et al. Synthesis and characterization of red-emitting iridium(III) complexes for solution-processable phosphorescent organic light-emitting diodes. Adv. Funct. Mater. 2009, 19, 2205–2212. [Google Scholar] [CrossRef]
- Thiery, S.; Tondelier, D.; Geffroy, B.; Jacques, E.; Robin, M.; Métivier, R.; Jeannin, O.; Rault-Berthelot, J.; Poriel, C. Spirobifluorene-2,7-dicarbazole-4′-phosphine oxide as host for high-performance single-layer green phosphorescent OLED devices. Org. Lett. 2015, 17, 4682–4685. [Google Scholar] [CrossRef] [PubMed]
- Thiery, S.; Tondelier, D.; Declairieux, C.; Geffroy, B.; Jeannin, O.; Métivier, R.; Rault-Berthelot, J.; Poriel, C. 4-Pyridyl-9,9′-spirobifluorenes as host materials for green and sky-blue phosphorescent OLEDs. J. Phys. Chem. C 2015, 119, 5790–5805. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Sun, Q.; Wu, S.-F.; Yuan, Y.; Li, Q.; Jiang, Z.-Q.; Fung, M.-K.; Liao, L.-S. Thermally activated delayed fluorescence material as host with novel spiro-based skeleton for high power efficiency and low roll-off blue and white phosphorescent devices. Adv. Fucnt. Mater. 2016, 26, 7929–7936. [Google Scholar] [CrossRef]
- Xue, M.-M.; Huang, C.-C.; Yuan, Y.; Cui, L.-S.; Li, Y.-X.; Wang, B.; Jiang, Z.-Q.; Fung, M.-K.; Liao, L.-S. De novo design of boron-based host materials for highly efficient blue and white phosphorescent OLEDs with low efficiency roll-off. ACS Appl. Mater. Interfaces 2016, 8, 20230–20236. [Google Scholar] [CrossRef] [PubMed]
- Poriel, C.; Rault-Berthelota, J. Structure–property relationship of 4-substituted-spirobifluorenes as hosts for phosphorescent organic light emitting diodes: An overview. J. Mater. Chem. C 2017, 5, 3869–3897. [Google Scholar] [CrossRef]
- Yang, X.; Xu, X.; Zhou, G. Recent advances of the emitters for high performance deep-blue organic light-emitting diodes. J. Mater. Chem. C 2015, 3, 913–944. [Google Scholar] [CrossRef]
- Seifert, R.; Rabelo de Moraes, I.; Scholz, S.; Gather, M.C.; Lüssem, B.; Leo, K. Chemical degradation mechanisms of highly efficient blue phosphorescent emitters used for organic light emitting diodes. Org. Electron. 2013, 14, 115–123. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, Y.S.; Kim, S.H.; Jeong, S.; Lee, S.E.; Kim, Y.K.; Yoon, S.S. Synthesis and electroluminescent properties of tert-butylated arylamine substituted 9,10-diphenylanthracene derivatives for organic light-emitting diodes. Synth. Met. 2016, 220, 628–634. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, W.; Xu, C.; Ji, B.; Zheng, C.; Zhang, X. Excellent deep-blue emitting materials based on anthracene derivatives for non-doped organic light-emitting diodes. Opt. Mater. 2016, 58, 260–267. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, K.H.; Kang, S.; Lee, J.Y.; Park, J.S.; Seo, J.H.; Kim, Y.K.; Yoon, S.S. Highly efficient blue-emitting materials based on 10-naphthylanthracene derivatives for OLEDs. Org. Electron. 2010, 11, 905–915. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.; Kang, I.; Chu, H.Y.; Lee, J.-I.; Kwon, S.-K.; Kim, Y.-H. Highly rigid and twisted anthracene derivatives: A strategy for deep blue OLED materials with theoretical limit efficiency. J. Mater. Chem. 2012, 22, 2695–2700. [Google Scholar] [CrossRef]
- Cho, I.; Kim, S.H.; Kim, J.H.; Park, S.; Park, S.Y. Highly efficient and stable deep-blue emitting anthracene-derived molecular glass for versatile types of non-doped OLED applications. J. Mater. Chem. 2012, 22, 123–129. [Google Scholar] [CrossRef]
- Bin, J.-K.; Hong, J.-I. Efficient blue organic light-emitting diode using anthracene-derived emitters based on polycyclic aromatic hydrocarbons. Org. Electron. 2011, 12, 802–808. [Google Scholar] [CrossRef]
- Lee, K.H.; You, J.N.; Kang, S.; Lee, J.Y.; Kwon, H.J.; Kim, Y.K.; Yoon, S.S. Synthesis and electroluminescent properties of blue-emitting t-butylated bis(diarylaminoaryl) anthracenes for OLEDs. Thin Solid Films 2010, 518, 6253–6258. [Google Scholar] [CrossRef]
- Zheng, C.-J.; Zhao, W.-M.; Wang, Z.-Q.; Huang, D.; Ye, J.; Ou, X.-M.; Zhang, X.-H.; Lee, C.-S.; Lee, S.-T. Highly efficient non-doped deep-blue organic light-emitting diodes based on anthracene derivatives. J. Mater. Chem. 2010, 20, 1560–1566. [Google Scholar] [CrossRef]
- Kim, S.-K.; Yang, B.; Ma, Y.; Lee, J.-H.; Park, J.-W. Exceedingly efficient deep-blue electroluminescence from new anthracenes obtained using rational molecular design. J. Mater. Chem. 2008, 18, 3376–3384. [Google Scholar] [CrossRef]
- Park, J.-Y.; Jung, S.Y.; Lee, J.Y.; Baek, Y.G. High efficiency in blue organic light-emitting diodes using an anthracene-based emitting material. Thin Solid Films 2008, 516, 2917–2921. [Google Scholar] [CrossRef]
- Shih, P.-I.; Chuang, C.-Y.; Chien, C.-H.; Diau, E.W.-G.; Shu, C.-F. Highly efficient non-doped blue-light-emitting diodes based on an anthrancene derivative end-capped with tetraphenylethylene groups. Adv. Funct. Mater. 2007, 17, 3141–3146. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, S.-L.; Tong, Q.-X.; Lo, M.-F.; Ng, T.-W.; Chan, M.-Y.; Wen, Z.-C.; He, J.; Jeff, K.-S.; Tang, X.-L.; et al. High efficiency nondoped deep-blue organic light emitting devices based on imidazole-π-triphenylamine derivatives. Chem. Mater. 2012, 24, 61–70. [Google Scholar] [CrossRef]
- Thangthong, A.; Meunmart, D.; Prachumrak, N.; Jungsuttiwong, S.; Keawin, T.; Sudyoadsuk, T.; Promarak, V. Bifunctional anthracene derivatives as non-doped blue emitters and hole-transporters for electroluminescent devices. Chem. Commun. 2011, 47, 7122–7124. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Duan, L.; Zhang, D.; Qiao, J.; Dong, G.; Wang, L.; Qiu, Y. A pyridine-containing anthracene derivative with high electron and hole mobilities for highly efficient and stable fluorescent organic light-emitting diodes. Adv. Funct. Mater. 2011, 21, 1881–1886. [Google Scholar] [CrossRef]
- Choi, I.-S.; Kim, S.H.; Ahn, H.-C.; Kim, Y.; Chung, Y. 2-Triphenylsilyl-9,10-di-1-naphthalenylanthracene and its application for blue organic light emitting diodes. Bull. Korean Chem. Soc. 2013, 34, 2211–2214. [Google Scholar] [CrossRef]
- Lyu, Y.-Y.; Kwak, J.; Kwon, O.; Lee, S.-H.; Kim, D.; Lee, C.; Char, K. Silicon-cored anthracene derivatives as host materials for highly efficient blue organic light-emitting devices. Adv. Mater. 2008, 20, 2720–2729. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Park, J.K.; Seo, J.H.; Park, S.W.; Kim, Y.S.; Kim, Y.K.; Yoon, S.S. Efficient deep-blue and white organic light-emitting diodes based on triphenylsilane-substituted anthracene derivatives. J. Mater. Chem. 2011, 21, 13640–13648. [Google Scholar] [CrossRef]
- Kim, R.; Lee, S.; Kim, K.-H.; Lee, Y.-J.; Kwon, S.-K.; Kim, J.-J.; Kim, Y.-H. Extremely deep blue and highly efficient non-doped organic light emitting diodes using an asymmetric anthracene derivative with a xylene unit. Chem. Commun. 2013, 49, 4664–4666. [Google Scholar] [CrossRef] [PubMed]
- Wee, K.-R.; Han, W.-S.; Kim, J.-E.; Kim, A.-L.; Kwon, S.; Kang, S.O. Asymmetric anthracene-based blue host materials: Synthesis and electroluminescence properties of 9-(2-naphthyl)-10-arylanthracenes. J. Mater. Chem. 2011, 21, 1115–1123. [Google Scholar] [CrossRef]
- Choi, I.-S.; Jeong, M.-H.; Lee, S.H.; Park, M.H.; Chung, Y. Synthesis of asymmetric anthracene derivatives and their application for blue organic light-emitting diodes. Bull. Korean Chem. Soc. 2016, 37, 136–141. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chien, C.-H.; Hsu, F.-M.; Shih, P.-I.; Shu, C.-F. Efficient non-doped blue-light-emitting diodes incorporating an anthracene derivative end-capped with fluorene groups. J. Mater. Chem. 2009, 19, 1464–1470. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Yeh, S.-C.; Chen, Y.-H.; Chen, C.-T.; Lee, R.-H.; Jeng, R.-J. New carbazole-substituted anthracene derivatives based non-doped blue light-emitting devices with high brightness and efficiency. Dyes Pigments 2013, 99, 577–587. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lin, S.-L.; Chang, Y.-C.; Chen, Y.-C.; Lin, J.-T.; Lee, R.-H.; Kuo, W.-J.; Jeng, R.-J. Efficient non-doped blue light emitting diodes based on novel carbazole-substituted anthracene derivatives. Org. Electron. 2012, 13, 43–52. [Google Scholar] [CrossRef]
- Wu, C.-L.; Chang, C.-H.; Chang, Y.-T.; Chen, C.-T.; Chen, C.-T.; Su, C.-J. High efficiency non-dopant blue organic light-emitting diodes based on anthracene-based fluorophores with molecular design of charge transport and red-shifted emission proof. J. Mater. Chem. C 2014, 2, 7188–7200. [Google Scholar] [CrossRef]
- Jo, W.J.; Kim, K.-H.; No, H.C.; Shin, D.-Y.; Oh, S.-J.; Son, J.-H.; Kim, Y.-H.; Cho, Y.-K.; Zhao, Q.-H.; Lee, K.-H.; et al. High efficient organic light emitting diodes using new 9,10-diphenylanthracene derivatives containing bulky substituents on 2,6-positon. Synth. Met. 2009, 159, 1359–1364. [Google Scholar] [CrossRef]
- Romain, M.; Tondelier, D.; Geffroy, B.; Jeannin, O.; Jacques, E.; Rault-Berthelot, J.; Poriel, C. Donor/acceptor dihydroindeno[1,2-a]fluorene and dihydroindeno[2,1-b]fluorene: Towards new families of organic semiconductors. Chem. Eur. J. 2015, 21, 9426–9439. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ren, J.; Li, Z.; Yuan, S.; Wu, Z.; Cui, Y.; Jia, H.; Wang, H.; Shi, F.; Hao, Y. Trifluoromethyl-substituted 9,9′-bianthracene derivative as host material for highly efficient blue OLED. Opt. Mater. Express 2015, 5, 2468–2477. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Z.; Li, Z.; Jiao, B.; Li, L.; Ma, L.; Wang, D.; Zhou, G.; Hou, X. Highly efficient deep-blue organic electroluminescent devices (CIEy ≈ 0.08) doped with fluorinated 9,9′-bianthracene derivatives (fluorophores). J. Mater. Chem. C 2013, 1, 8117–8127. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 6th ed.; Elsevier: Waltham, MA, USA, 2009; pp. 61–79. [Google Scholar]
- Shin, M.-G.; Kim, S.O.; Park, H.Y.; Park, S.J.; Yu, H.S.; Kim, Y.-H.; Kwon, S.-K. Synthesis and characterization of ortho-twisted asymmetric anthracene derivatives for blue organic light emitting diodes (OLEDs). Dyes Pigments 2012, 92, 1075–1082. [Google Scholar] [CrossRef]
- Park, J.-W.; Kang, P.; Park, H.; Oh, H.-Y.; Yang, J.-H.; Kim, Y.-H.; Kwon, S.-K. Synthesis and properties of blue-light-emitting anthracene derivative with diphenylamino-fluorene. Dyes Pigments 2010, 85, 93–98. [Google Scholar] [CrossRef]
| Compound | λabs 1 (nm) | λem 1 (nm) | Eg 2 (eV) | Eox 3 (V) | HOMO 4 (eV) | LUMO 5 (eV) | Tg (°C) | Td5 (°C) | Φ 6 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 365, 384, 405 | 429 | 2.98 | 1.11 | −5.51 | −2.53 | 65 | 262 | 0.24 |
| 2 | 367, 386, 407 | 439 | 2.93 | 1.22 | −5.62 | −2.69 | 121 | 387 | 0.21 |
| 3 | 367, 386, 407 | 439 | 2.93 | 1.15 | −5.55 | −2.62 | 118 | 358 | 0.22 |
| Compound | Turn-on Voltage 1 (V) | Operating Voltage 2 (V) | Current Efficiency 3 (cd·A−1) | Power Efficiency 3 (lm·W−1) | EQE 3 (%) | CIE (x, y) |
|---|---|---|---|---|---|---|
| 1 (8%) | 6.0 | 7.4 | 2.1 | 0.9 | 0.8 | (0.25, 0.51) |
| 2 (8%) | 7.8 | 9.4 | 1.4 | 0.5 | 0.5 | (0.25, 0.51) |
| 3 (8%) | 8.5 | 9.8 | 1.1 | 0.4 | 0.4 | (0.27, 0.52) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, S.W.; Lee, K.M.; Lee, J.-E.; Yoo, J.; Yi, Y.; Kwon, H.; Lee, H.; Park, M.H.; Chung, Y. Synthesis and Electroluminescence Properties of 3-(Trifluoromethyl)phenyl-Substituted 9,10-Diarylanthracene Derivatives for Blue Organic Light-Emitting Diodes. Appl. Sci. 2017, 7, 1109. https://doi.org/10.3390/app7111109
Kwak SW, Lee KM, Lee J-E, Yoo J, Yi Y, Kwon H, Lee H, Park MH, Chung Y. Synthesis and Electroluminescence Properties of 3-(Trifluoromethyl)phenyl-Substituted 9,10-Diarylanthracene Derivatives for Blue Organic Light-Emitting Diodes. Applied Sciences. 2017; 7(11):1109. https://doi.org/10.3390/app7111109
Chicago/Turabian StyleKwak, Sang Woo, Kang Mun Lee, Ji-Eun Lee, Jisu Yoo, Yeonjin Yi, Hyoshik Kwon, Hyunbok Lee, Myung Hwan Park, and Yongseog Chung. 2017. "Synthesis and Electroluminescence Properties of 3-(Trifluoromethyl)phenyl-Substituted 9,10-Diarylanthracene Derivatives for Blue Organic Light-Emitting Diodes" Applied Sciences 7, no. 11: 1109. https://doi.org/10.3390/app7111109





