1. Introduction
Nanostructured metallic oxides can be prepared by various methods, such as a combination of the template-based method and sol–gel chemistry [
1,
2,
3,
4], the facile wet chemical method [
5], and the hydrothermal method [
6,
7,
8,
9]. Hydrothermal synthesis has been an interesting technique for preparing materials with different nano-architectures, such as nanowires, nanorods, nanobelts, and nanoflowers [
10,
11,
12], and its main advantage over other soft chemical routes is the ability to control the nanostructures by choosing the proper reaction temperature, time, solvent, and concentration without the need for any major structure-directing agents or templates. The effects of temperature, time, solvent, and concentration on the morphologies of powders prepared by the hydrothermal method have been extensively studied and reported [
13,
14]; however, to our knowledge, there have been few studies on hydrothermal synthesis of rare earth metal oxides for use as radiotracers.
Rare earth metallic oxide radiotracers can be used widely in science and engineering, particularly for industrial use, because they have high activation limits and rare metal elements, although they should be physically and chemically compatible with the material under investigation. A radiotracer for injection is labeled radioactively with a given material that allows it to be monitored as it moves through a system by external radiation detectors [
15,
16]. Radiotracers must be produced relatively easily and cheaply by nuclear reactors and accelerators. Their chemical form is also important because they need to be in the same phase as the process medium, either aqueous or organic, and remain in that intended medium throughout the monitoring process.
Radioisotope nanoparticles (NPs) have been synthesized with core-
198Au shell-silica (
198Au@SiO
2) by neutron irradiation from the HANARO nuclear research reactor at the Korea Atomic Energy Research Institute (Daejeon, Korea) [
17]. These core-shell radioisotope NPs have good physical and mechanical property because of SiO
2 shells in high energy gamma environments; however, the preparation process of
198Au@SiO
2 radioisotope NPs is very complicated because of the capsulation of
197Au NPs using SiO
2 in the presence of anchoring agents. Furthermore, only a very small amount of
198Au@SiO
2 radioisotope NPs could be obtained because the Au NPs used as starting materials are commonly synthesized in amounts under 10
−3 M (mol/L). In addition, core-shell radioisotope NPs with multi gamma-emitting nuclides have been prepared by neutron irradiation at the HANARO research reactor [
18]. The synthesis method of the core-shell radioisotope NPs with gamma-emitting nuclides have low yields. Therefore, synthesis methods with easy, simple, and good yields are needed to prepare radioisotope NPs for their use as radiotracers in various scientific, engineering, and industrial fields.
In order to overcome the above problems, we also prepared four types of radioisotope NPs constructed with Au-ligand frameworks that were synthesized by a self-assembly process at room temperature for 3 h between 1,4-bis((1
H-imidazol-1-yl)methyl)benzene (BIX), 2,6-bis((1
H-imidazol-1-yl)naphthalene (BNX), di(1
H-imidazol-1-yl)dimethylsilane (BIS), 4,4′-bis(pyridin-2-ylmethoxy)biphenyl (BPX), and radioisotope Au
3+ ions, which were prepared by the dissolution of Au-198 isotope in HNO
3/HCl (mol-%, 1/3) mixture solution after neutron irradiation from the HANARO research reactor [
19,
20]; however, the metal-ligand framework-constructed radioisotopes exhibited weak physical and chemical properties.
In this study, nanostructured metallic oxides of MnO2, Sm2O3, and Dy2O3 were synthesized by the hydrothermal process under various reaction conditions. The metallic oxides obtained were characterized by Transmission Electron Microscopy (TEM), Energy Dispersive X-ray Spectrometry (EDXS), Scanning Electron Microscopy (SEM), and X-ray Diffraction (XRD) analysis. Next, after calcination of the as-synthesized metallic oxides, radioisotope metallic oxides were synthesized by neutron irradiation from the HANARO research reactor. Finally, successful synthesis of the nanostructured metallic oxide radioisotopes was confirmed via gamma spectroscopy analysis.
2. Experiments
2.1. Materials
Manganese sulfate monohydrate (MnSO4·H2O), samarium nitrate hydrate (Sm(NO3)3·H2O), dysprosium nitrate hydrate (Dy(NO3)3·H2O), triethylene glycol, pentanol, n-octane, ammonium persulfate ((NH4)2S2O8), cetyltrimethylammonium bromide (CTAB), sodium hydroxide (NaOH), and methanol were obtained from Korea-Sigma-Aldrich, Seoul, Republic of Korea. and used in the treatments. Water was purified in a Milli-Q plus water purification system (Millipore Co. Ltd., Burlington, MA, USA) to a final resistance of 18.2 MΩ cm−1 and degassed prior to each measurement. Other chemicals were of reagent grade.
2.2. Synthesis of Radioisotopes MnO2, Sm2O3, and Dy2O3 NPs by Neutron Irradiation
Manganese dioxide (MnO
2) was synthesized by redox hydrothermal synthesis in aqueous solution at 120 °C for 8 h, as given by the following equation:
In detail, 1.93 g (8.00 mmol) manganese sulfate monohydrate (MnSO4·H2O) and 2.61 g (8.0 mmol) ammonium persulfate ((NH4)2S2O8) were put into 50 mL distilled water at room temperature to form a homogeneous solution, which was then transferred into a 100-mL Teflon-lined stainless-steel autoclave. The sealed solution was kept at 120 °C for 8 h. After the autoclave was cooled down to room temperature naturally, the obtained products were filtered and washed with distilled water to remove any remaining ions. The as-prepared materials were dried overnight in a vacuum oven at 80 °C. In order to remove the organic elements, the as-prepared MnO2 samples were calcined at 900 °C for 2 h under an air atmosphere. Finally, the nanostructured radioisotope MnO2 was synthesized by neutron irradiation using the HANARO research reactor (Korea Atomic Energy Research Institute).
Samarium oxide (Sm
2O
3) NPs can be synthesized by the W/O (water/oil) emulsion process and the calcination process as in the following equations:
In the first step, 2.0 mL of 1.5 mol/L Sm(NO3)3 aqueous solution was added to 1.0 g pentanol as a cosurfactant, 8.0 g n-octane as an organic solvent, and the appropriate amount of surfactant cetyltrimethylammonium bromide (CTAB), and then the mixture was agitated until it became a transparent microemulsion. The temperature was maintained by putting the whole setup into a thermostatic water bath. The microemulsion was slowly added to an NaOH aqueous solution under agitation, and this finally led to the formation of a suspension of surfactant-stabilized SmOH. After being stirred for 1 h, the suspension was allowed to stand for 24 h at a specified temperature to obtain as large an amount as possible of surfactant-stabilized SmOH NPs (in the form of a precipitate). The precipitate was then collected by centrifuging and washed several times with pure ethanol and distilled water. The collected products were dried in a vacuum oven at 75 °C for 5 h. In the second step, the dried precipitate (SmOH) was calcined at 500 °C for 6 h in the oxygen state to obtain the primrose yellow Sm2O3 nanoparticles. Finally, nanostructured radioisotope Sm2O3 was synthesized by neutron irradiation (neutron flux: 4.2 × 1013 n·cm−2·s−1) of the as-prepared Sm2O3 powder by the HANARO research reactor (Korea Atomic Energy Research Institute).
Dysprosium oxide (Dy
2O
3) powder can be synthesized by the redox process and the calcination process as in the following equations:
In detail, 5 mmol of Dy(NO3)3·H2O was added to 40 mL of triethylene glycol in a 100-mL three-necked flask, and the solution was stirred magnetically until the precursor was completely dissolved into the solvent. In a separate flask, 15 mmol of NaOH was dissolved in 10 mL of methanol and the resulting solution was added dropwise to the precursor solution. The reaction mixture was heated to 200 °C and refluxed for 24 h with magnetic stirring. Then, the dried precipitate (the precursor) was calcined at 500 °C for 6 h in an air atmosphere to obtain Dy2O3 NPs. Finally, radioisotope Dy2O3 NPs were synthesized by neutron irradiation (neutron flux: 4.2 × 1013 n·cm−2·s−1) of the as-prepared Dy2O3 NPs by the HANARO research reactor (Korea Atomic Energy Research Institute).
2.3. Characteristics of Radioisotope Metallic Oxide NPs
The morphologies of the as-prepared metallic oxide samples were analyzed by High Resolution Transmission Electron Microscopy (HR-TEM) (JEM-2010F, JEOL Ltd., Tokyo, Japan). An energy dispersive X-ray spectrometer (EDXS) attached to the HR-TEM was used to analyze their chemical compositions. An X-ray Diffractometer (XRD) (D8 ADVANCE, Bruker AXS, Karlsruhe, Germany) was applied to determine the nanostructures. To ensure the chemical purity of the NPs, their gamma spectrum was measured with an HPGe (high purity germanium) detector (EG&G Ortec, 25% relative efficiency, FWHM 1.85 keV at 1332 keV of 60Co) coupled to a 16K-multichannel analyzer.
3. Results and Discussion
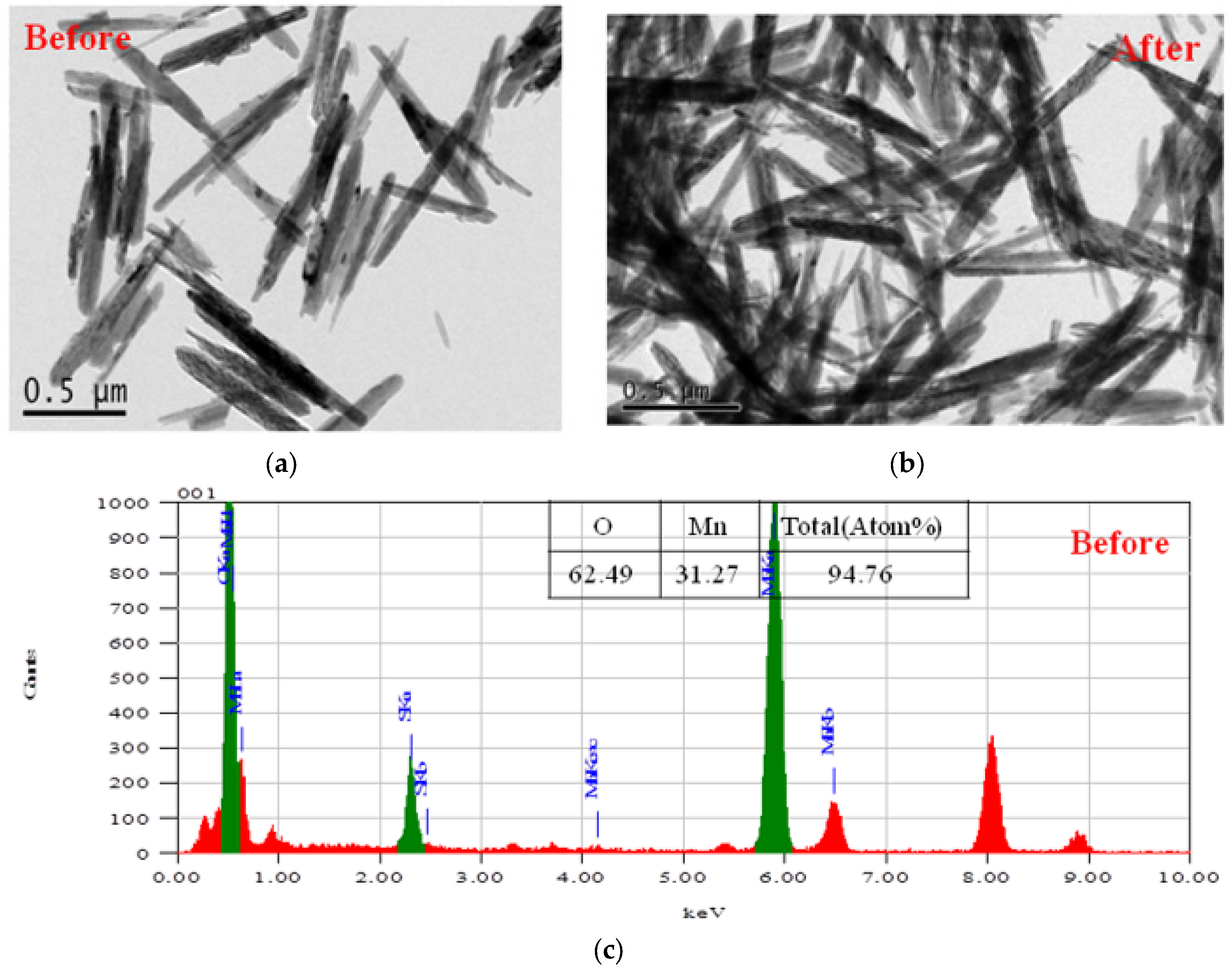
The successful synthesis of MnO
2 NPs by the hydrothermal process is shown by the TEM image and EDS (Energy Dispersive Spectrometry) data of the nanostructured MnO
2 powder (
Figure 1). It can be seen from the TEM images that the nanostructured MnO
2 powder exhibits rod-like structures that consist of nanofibers with a diameter of 50 nm. Form the EDS data, the atomic percentage (%) of Mn element and O element was 31.3% and 62.5%, respectively (
Figure 1c). After neutron irradiation, the morphology of the MnO
2 was not changed. This result means that the nanostructured MnO
2 showed high stability under neutron irradiation.
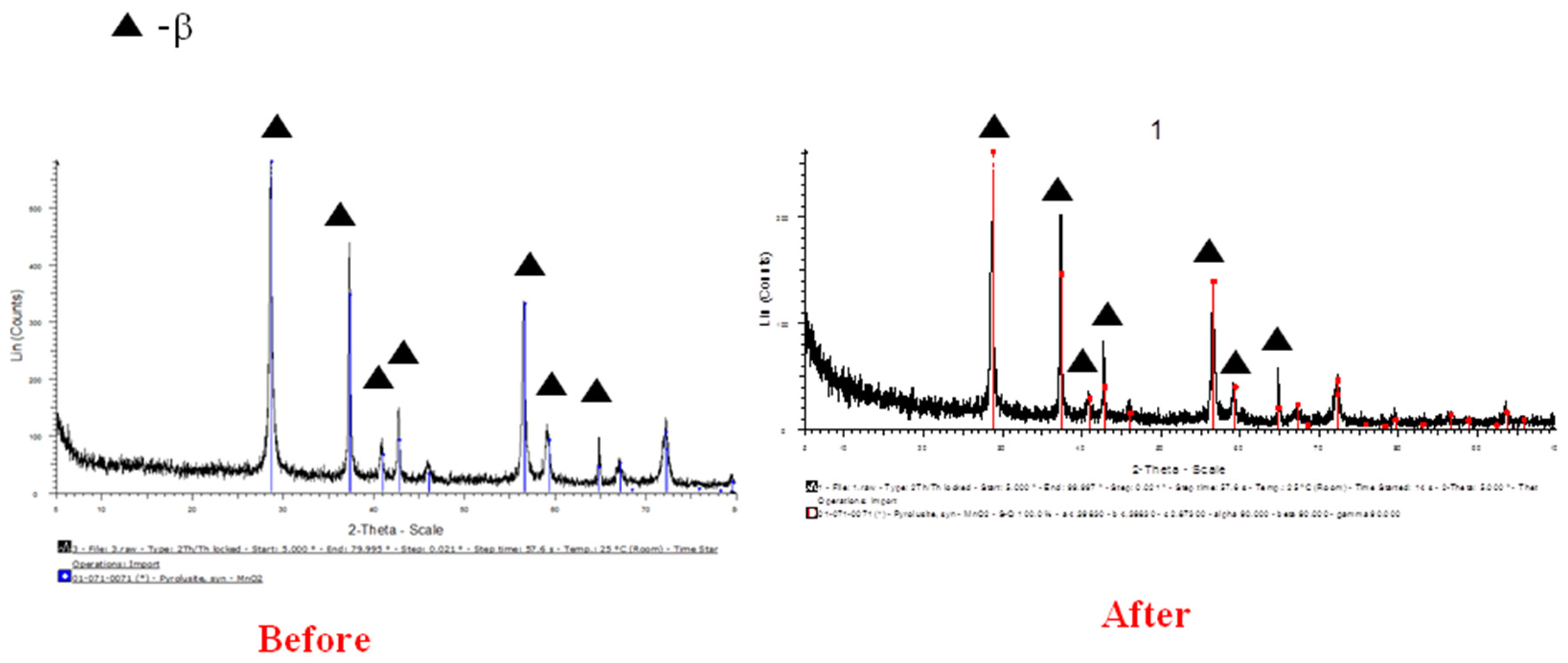
The successful preparation of the MnO
2 NPs before and after neutron irradiation is also shown by the XRD data (
Figure 2). All the peaks assigned by the diffraction of crystalline MnO
2 at 2
Θ = 28.7°, 37.4°, 41.0°, 42.8°, 46.1°, 56.7°, 59.4°, 64.9°, 67.3°, and 72.3° correspond to the (110), (101), (200), (111), (210), (211), (220), (002), (310), and (301) planes (JCPDS card 71-0071). All the diffraction peaks correspond well with standard crystallographic data and can be indexed primarily to the pure tetragonal structure of
β-MnO
2 (JCPDS card 00-024-0735).
A neutron activation analysis of the prepared nanoparticles was carried out at the HANARO research reactor (neutron flux: 4.2 × 10
13 n·cm
−2·s
−1), a nuclear research reactor at the Atomic Energy Research Institute. A 3.7-mg sample was activated for 20 s, and the gamma spectrum was then measured after cooling for 10 min. The peaks in the gamma spectrum were assigned to the characteristic gamma energies of Mn-56 (
Figure 3). The results showed that the radioisotope was successfully synthesized by neutron irradiation after hydrothermal synthesis of the nanostructured MnO
2 NPs.
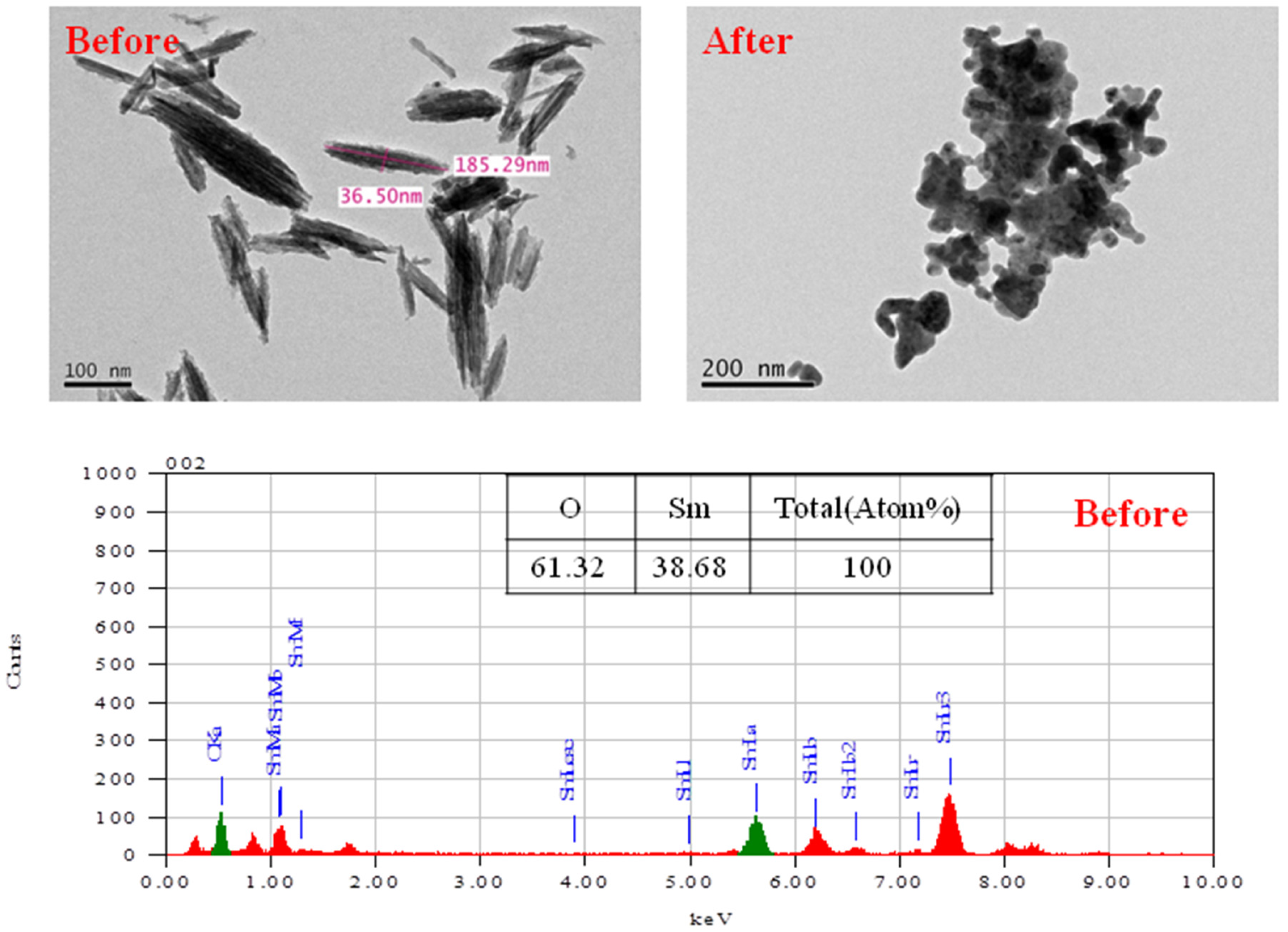
In order to obtain nanostructured Sm
2O
3 powder, first nanostructured SmOH was synthesized by the W/O emulsion process. Then, to obtain pure Sm
2O
3 powder, the SmOH powder was calcinated at 900 °C for 2 h under an air atmosphere. The Sm
2O
3 powder was successfully synthesized by the microemulsion process and the calcination process, as shown by the TEM image and EDS data of the Sm
2O
3 powder after calcinations (
Figure 4). All of the Sm
2O
3 nanopowder had a rod shape with the dimensions of 36 × 185 nm. As shown by the EDS data, the percentage (%) of Sm element and O element was 31.3% and 62.5%, respectively. After the neutron irradiation, the morphology of the Sm
2O
3 powder was changed from a rod-like structure to an amorphous structure.
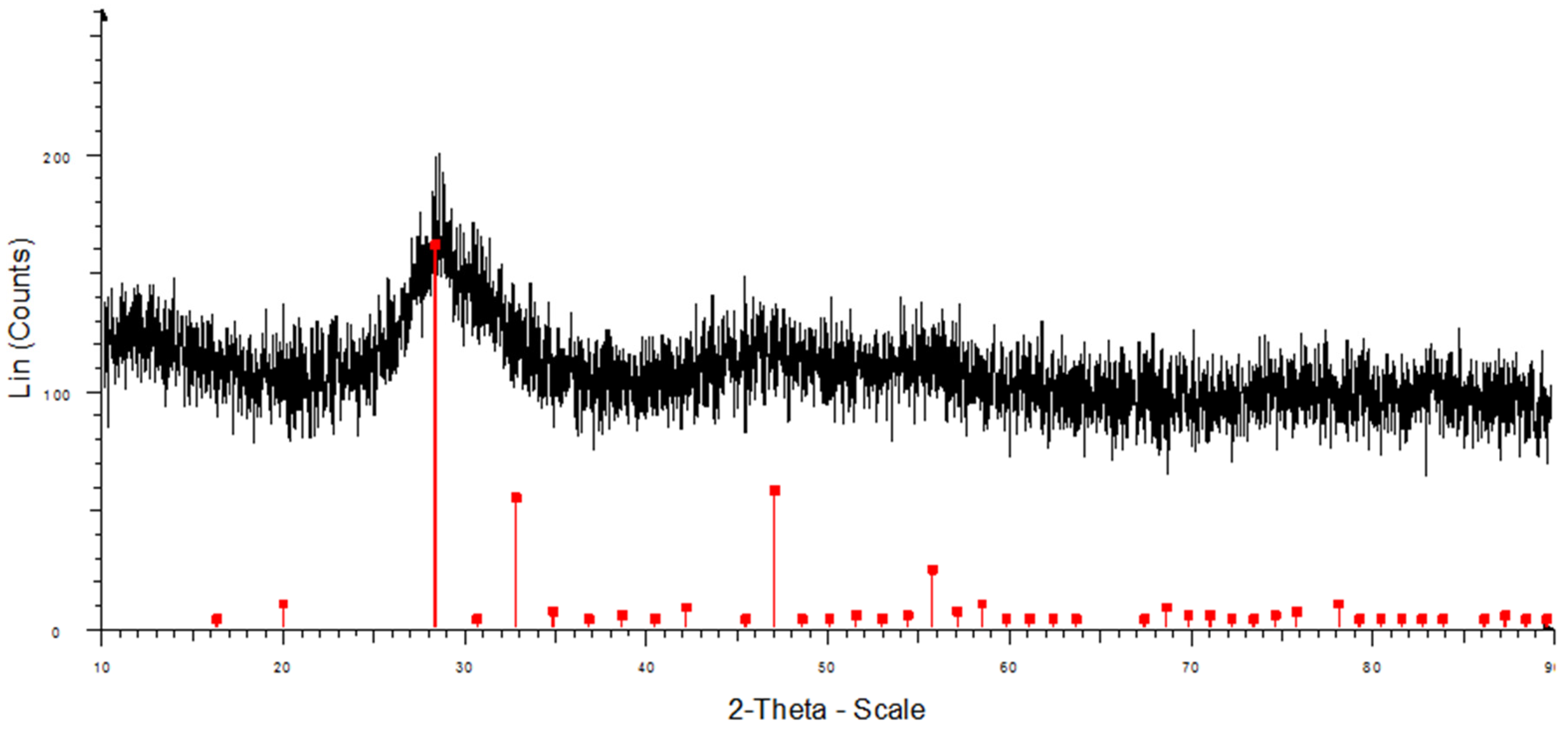
XRD data of the Sm
2O
3 powder after the calcination process (
Figure 5) showed that the diffraction peaks of the Sm
2O
3 powder were very weak, indicating an amorphous structure. All the peaks assigned by the diffraction of crystalline Sm
2O
3 at 2
Θ = 19.9, 28.3, 32.8, 47.0, and 55.7° correspond to the (211), (222), (400), (440), and (622) planes (JCPDS card 43-1029). Evident broad peaks were a mixture of the amorphous and nanocrystalline nature of the samples.
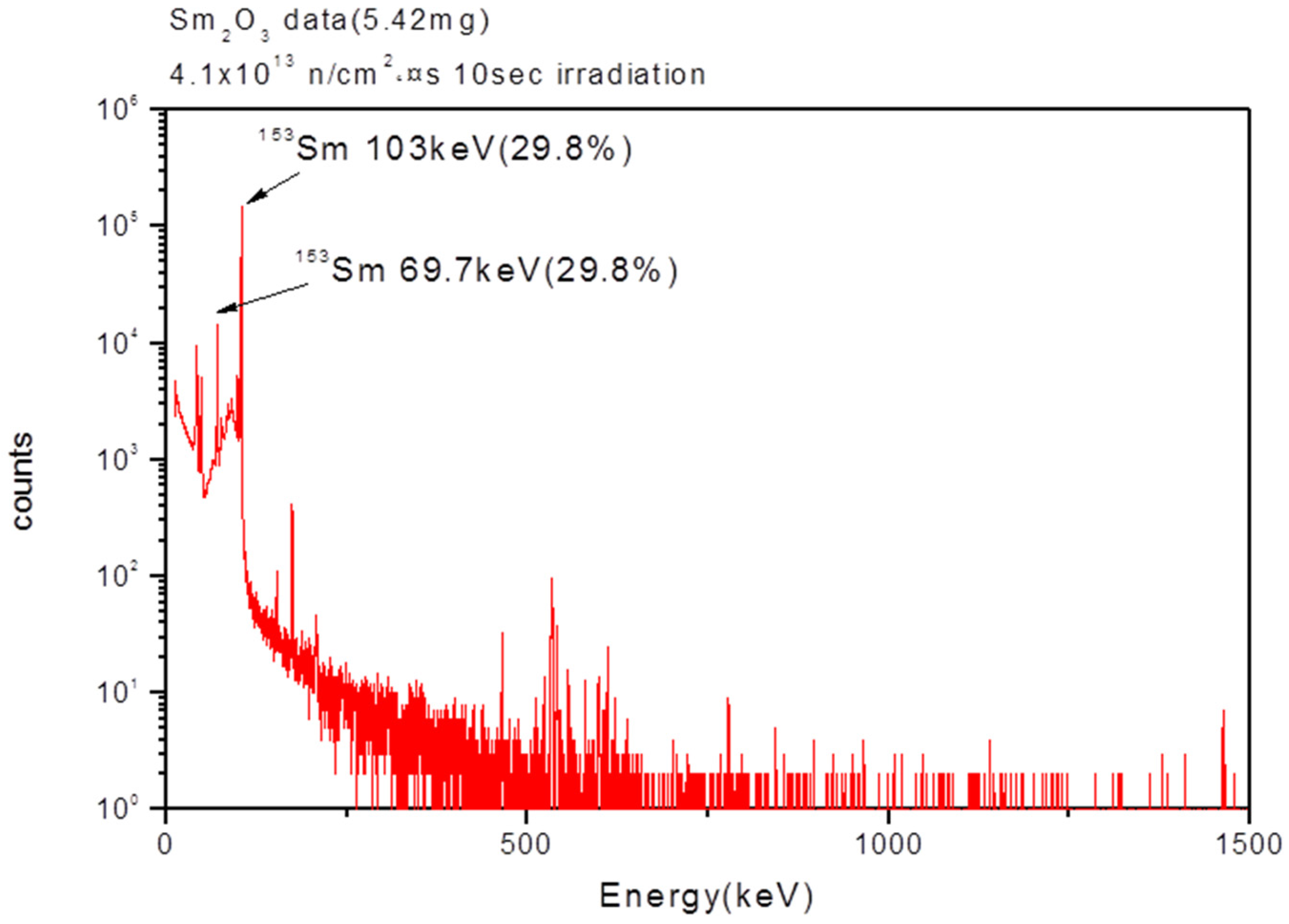
For gamma spectroscopy analysis, a 5.42 mg sample of the as-prepared Sm
2O
3 powder was activated for 10 s by neutron irradiation, and the gamma spectrum was then measured after cooling for 10 min. The peaks in the gamma spectrum were assigned to the characteristic gamma peaks at 103 keV and 69.7 keV of Sm-153 (
Figure 6). These results showed that the radioisotope Sm
2O
3 NPs were successfully synthesized by the neutron irradiation of the as-prepared Sm
2O
3 NPs that were synthesized by the microemulsion process and the calcination process.
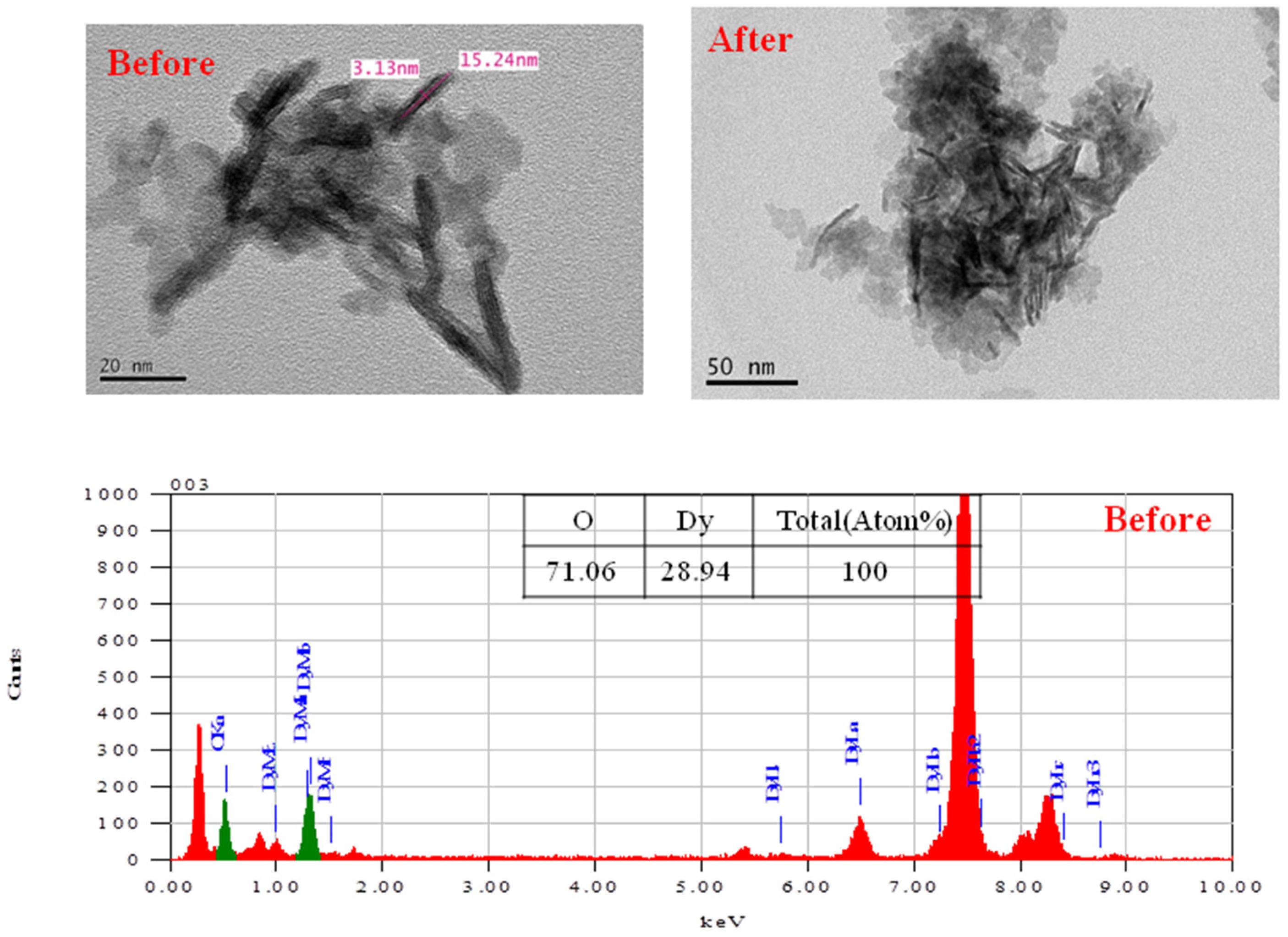
The successful synthesis of the Dy
2O
3 powder with nanorod structures obtained by the redox process and the calcination process is shown by the results of the TEM image and EDS data (
Figure 7). The morphology of Dy
2O
3 powder exhibited nanorod-like structures with dimensions of 3 × 15 nm in the TEM image. As EDS data shows, the percentage (%) of the Dy element and O element was 28.9% and 71.1%, respectively. After neutron irradiation, the morphology was not changed. From this result, the prepared Dy
2O
3 was shown to have high stability under neutron irradiation.
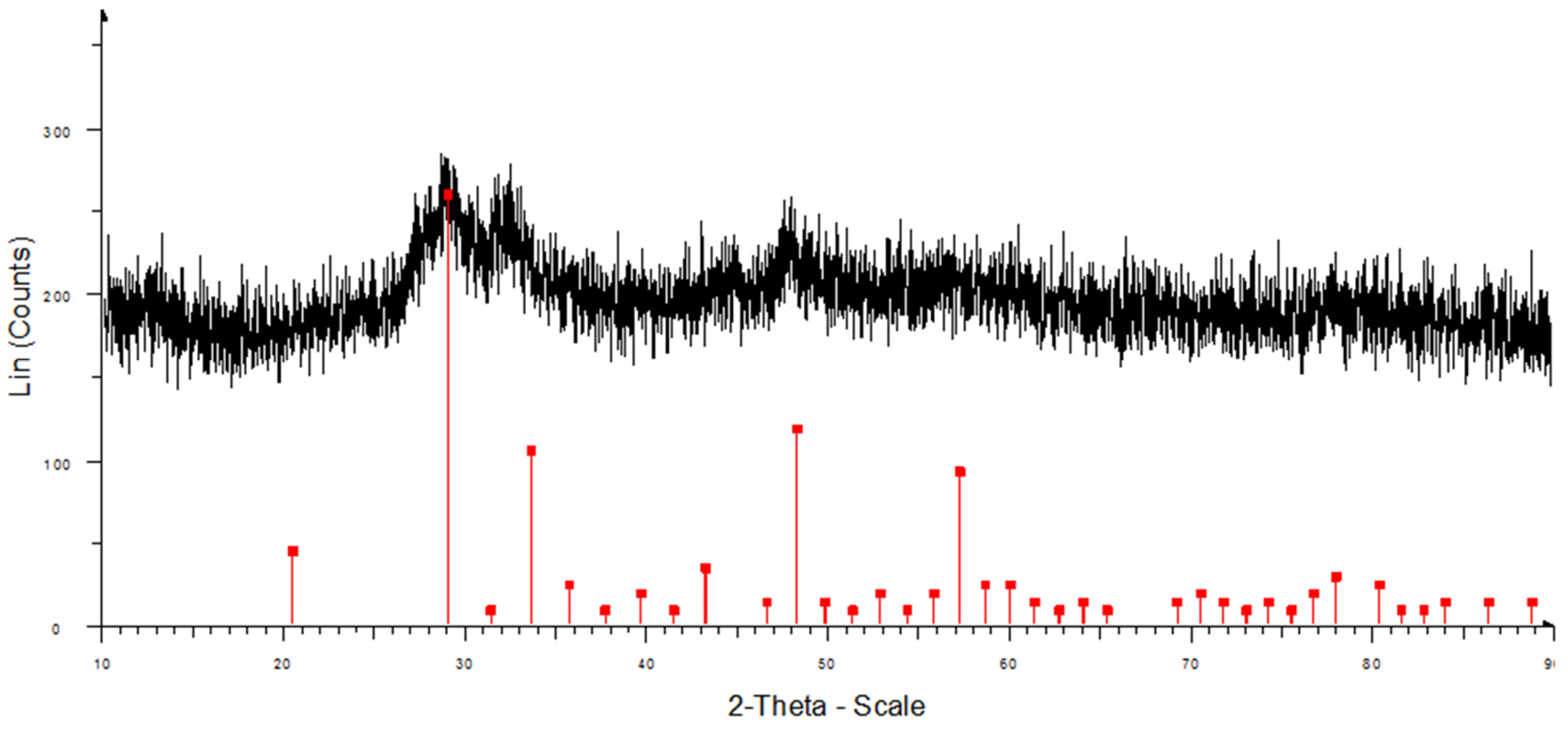
XRD data of the Dy
2O
3 powder after the calcination process (
Figure 8) showed that the diffraction peaks of the Dy
2O
3 powder were very weak, indicating an amorphous structure after the calcination process. All the peaks assigned by the diffraction of crystalline Dy
2O
3 at 2
Θ = 20.4°, 29.0°, 31.4°, 33.6°, 43.2°, 48.2°, and 57.3° correspond to the (211), (222), (321), (400), (431), (440), and (622) planes (JCPDS card 22-0612). Evident broad peaks were a mixture of the amorphous and nanocrystalline nature of the samples.
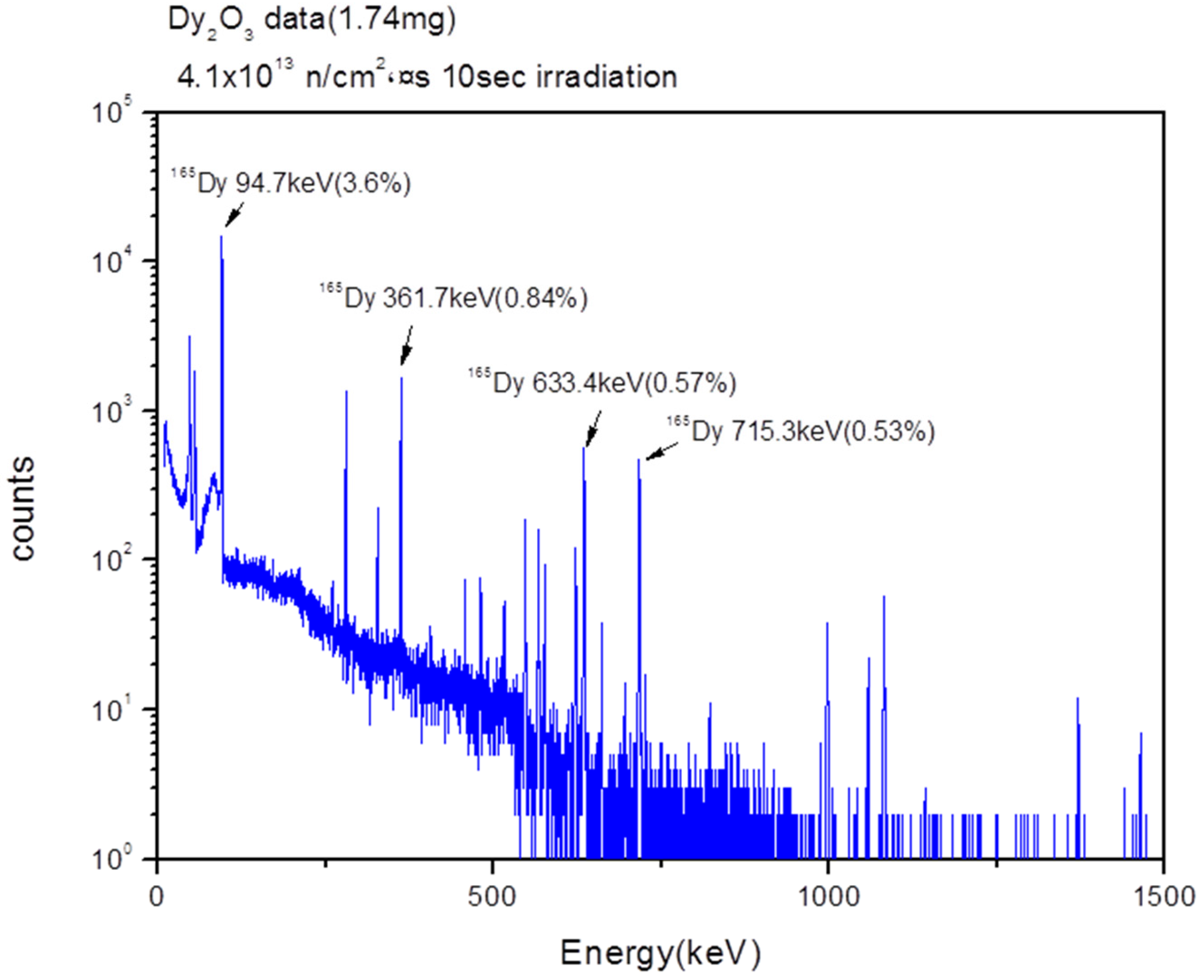
For gamma spectroscopy analysis, a 1.74-mg sample of the as-prepared Dy
2O
3 powder was activated for 10 s by neutron irradiation, and the gamma spectrum was then measured after cooling for 10 min. The peaks in the gamma spectrum were assigned to the characteristic gamma peaks at 94.7 keV, 361.7 keV, 633.4 keV, and 715.3 keV of Dy-165 (
Figure 9). These results showed that the radioisotope Dy
2O
3 nanorods were successfully synthesized by the neutron irradiation of the as-prepared Dy
2O
3 nanorods that were synthesized by the redox process and the calcination process. We expect that the radioisotope Dy
2O
3 nanorods can be used as radiotracers in scientific, environmental, engineering, and industrial fields.