Preparation of Robust Superhydrophobic Halloysite Clay Nanotubes via Mussel-Inspired Surface Modification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Superhydrophobic Surface Modification of the Halloysite Clay Nanotubes (HNTs) by Polydopamine (PDA) Coating and Octadecylamine (ODA) Grifting
2.3. Characterization of Surface Modified HNTs
2.4. Stability Test of the Superhydrophobic HNTs
2.5. The Application of Superhydrophobic HNTs for Solar Thermal Energy Storage
2.6. The Application of Superhydrophobic HNTs for Oil/Water Separation
3. Results and Discussion
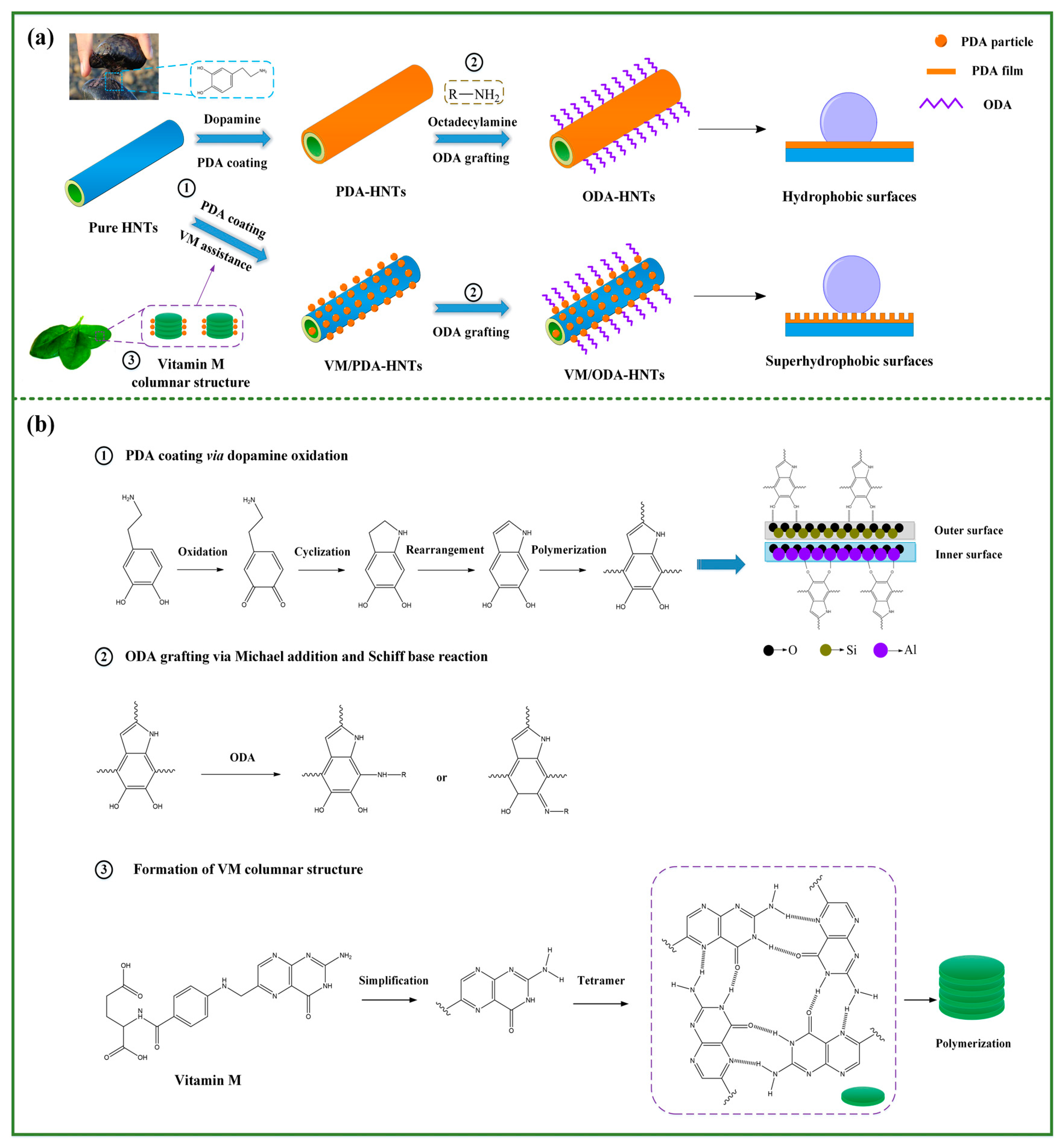
3.1. Preparation Process and Reaction Mechanism
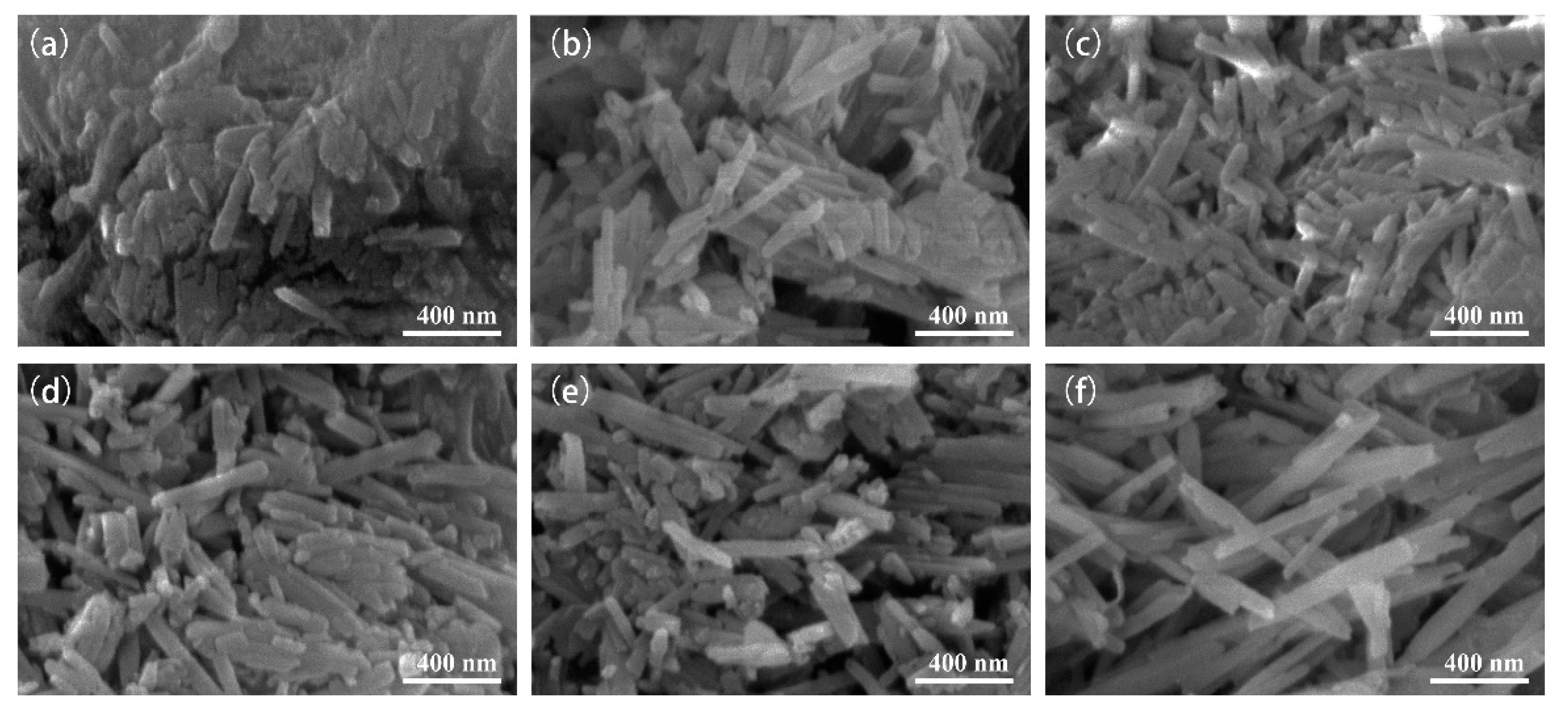
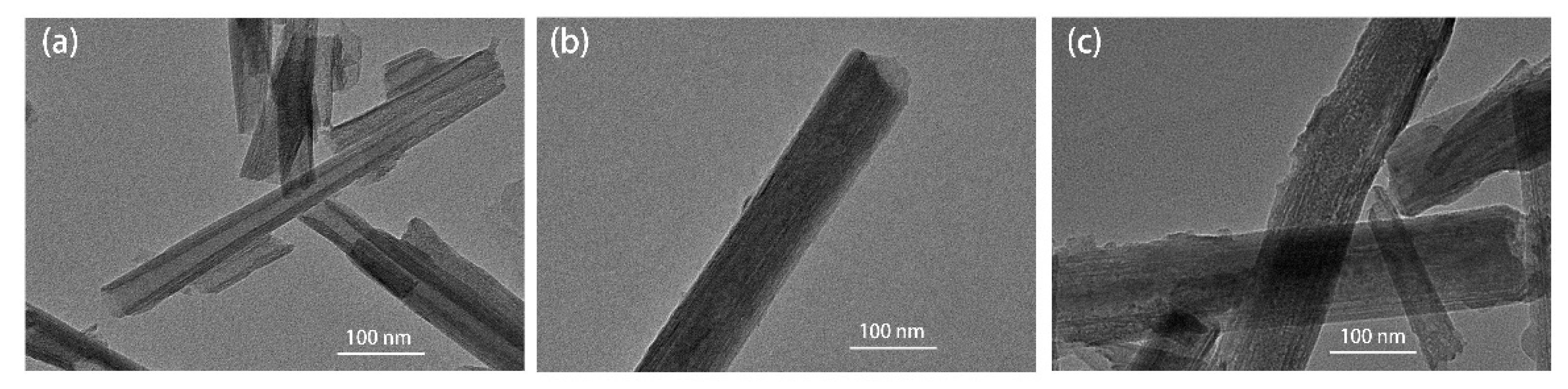
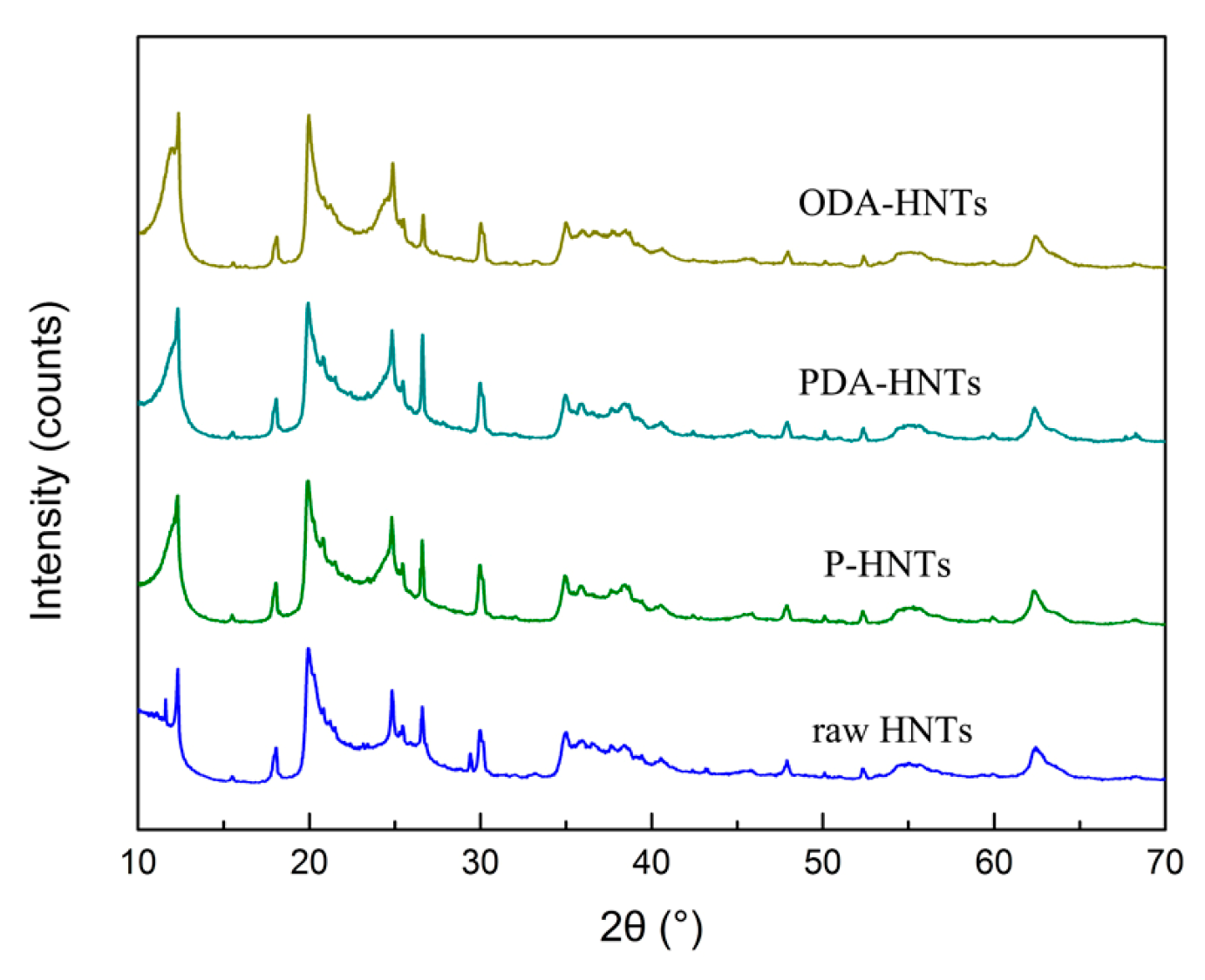
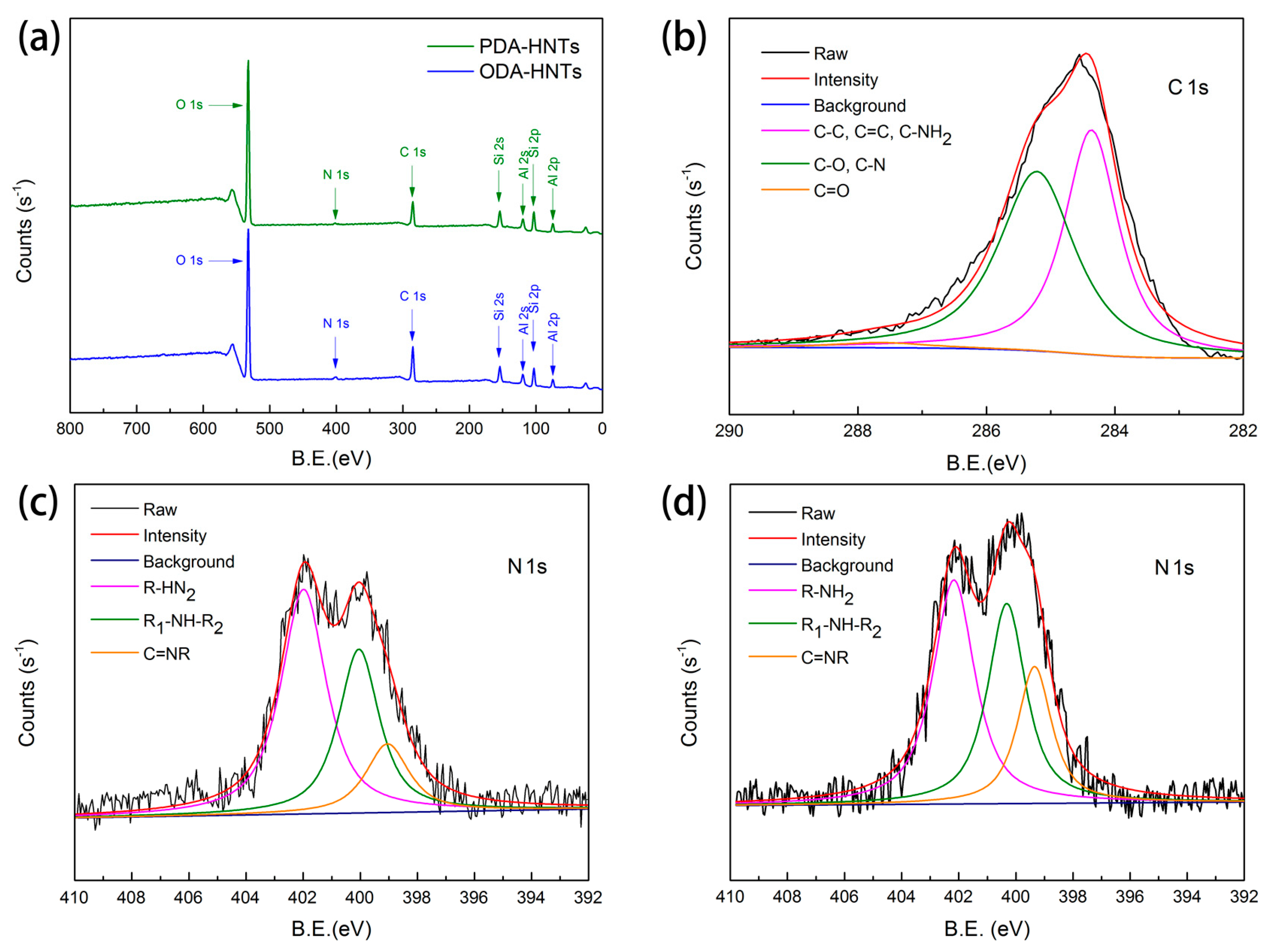
3.2. Micromorphology and Surface Chemical Composition Analysis
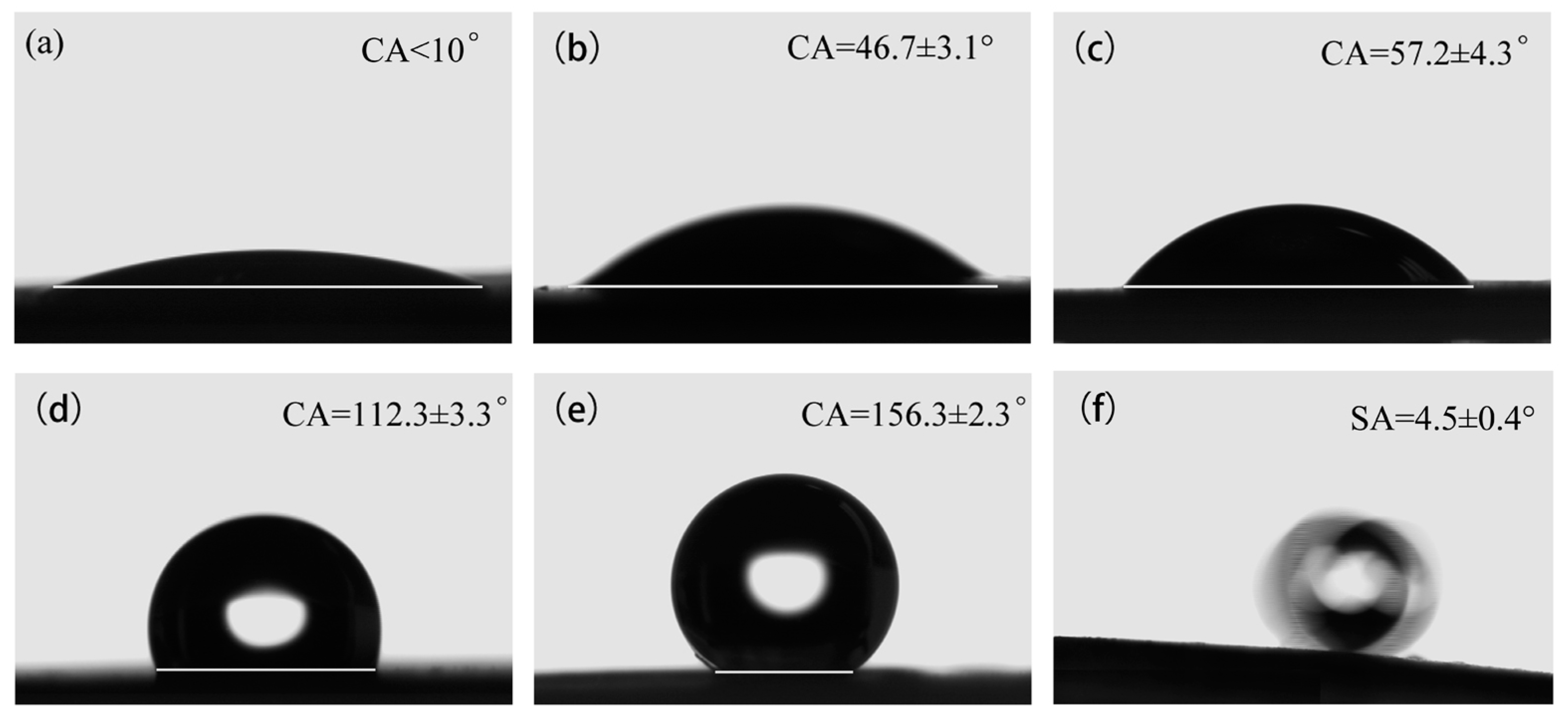
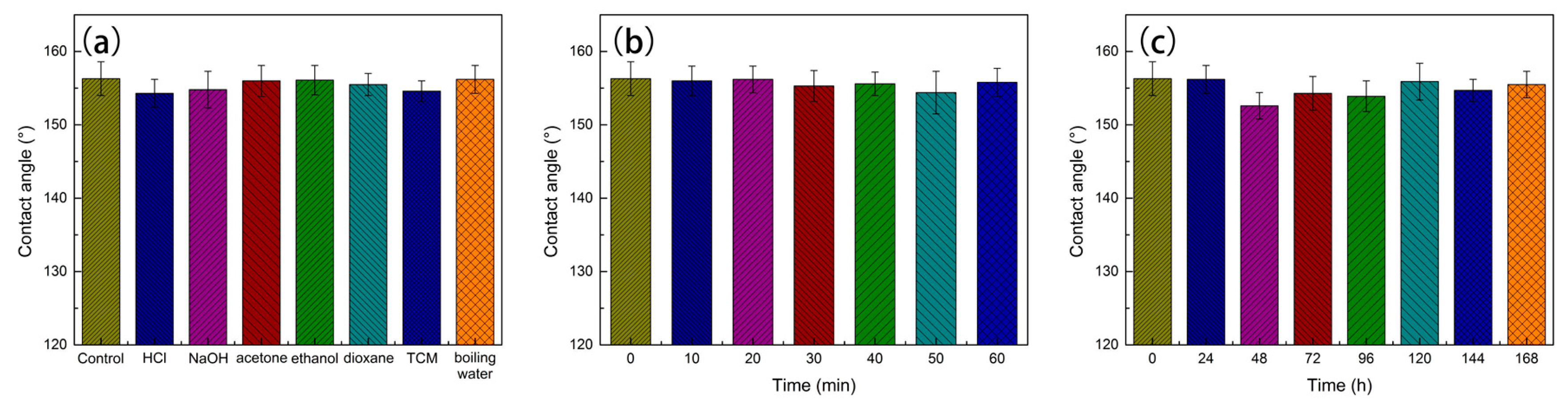
3.3. Superhydrophobic Property and Environmental Durability
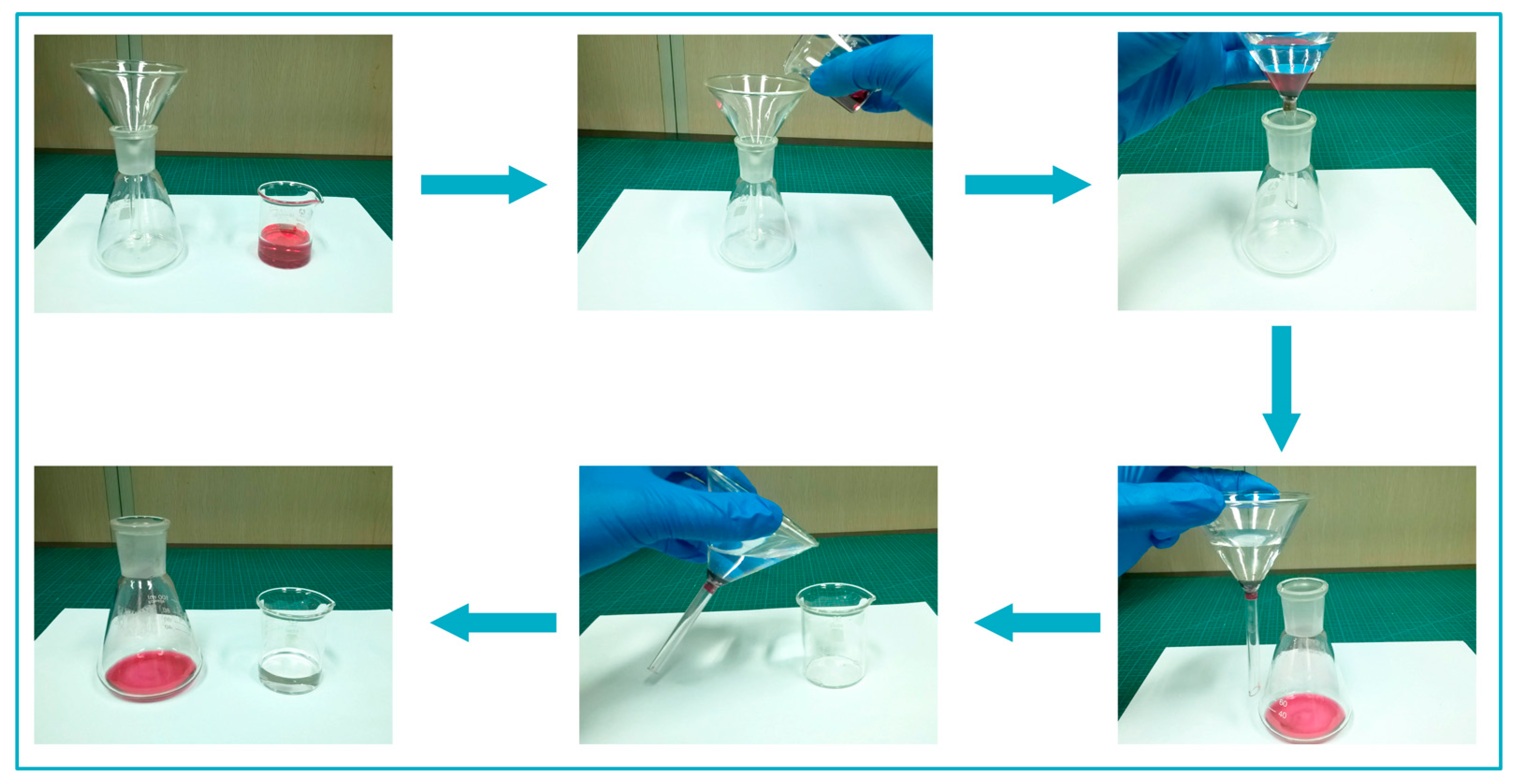
3.4. Application Analysis of Superhydrophobic HNTs
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, Q.; Zhang, W.W.; Dong, C.B.; Sreeprasad, T.S.; Xia, Z.H. Biomimetic self-cleaning surfaces: Synthesis, mechanism and applications. J. R. Soc. Interface 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.Q.; Zhou, Y.H. Fabrication of transparent superhydrophobic porous silica coating for self-cleaning and anti-fogging. Ceram. Int. 2016, 42, 8706–8712. [Google Scholar] [CrossRef]
- Peng, C.Y.; Xing, S.L.; Yuan, Z.Q.; Xiao, J.Y.; Wang, C.Q.; Zeng, J.C. Preparation and anti-icing of superhydrophobic PVDF coating on a wind turbine blade. Appl. Surf. Sci. 2012, 259, 764–768. [Google Scholar] [CrossRef]
- Wang, Z.W.; Su, Y.L.; Li, Q.; Liu, Y.; She, Z.X.; Chen, F.N.; Li, L.Q.; Zhang, X.X.; Zhang, P. Researching a highly anti-corrosion superhydrophobic film fabricated on AZ91D magnesium alloy and its anti-bacteria adhesion effect. Mater. Charact. 2015, 99, 200–209. [Google Scholar] [CrossRef]
- Cao, W.T.; Liu, Y.J.; Ma, M.G.; Zhu, J.F. Facile preparation of robust and superhydrophobic materials for self-cleaning and oil/water separation. Colloids Surf. A 2017, 529, 18–25. [Google Scholar] [CrossRef]
- Liang, W.D.; Wu, Y.; Sun, H.X.; Zhu, Z.Q.; Chen, P.S.; Yang, B.P.; Li, A. Halloysite clay nanotubes based phase change material composites with excellent thermal stability for energy saving and storage. RSC Adv. 2016, 6, 19669–19675. [Google Scholar] [CrossRef]
- Fan, L.; Li, B.C.; Wang, Q.; Wang, A.Q.; Zhang, J.P. Superhydrophobic gated polyorganosilanes/halloysite nanocontainers for sustained drug release. Adv. Mater. Interfaces 2014, 1. [Google Scholar] [CrossRef]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2006, 39, 644–652. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Shah, S.M.; Zulfiqar, U.; Hussain, S.Z.; Ahmad, I.; Habib-ur-Rehman; Hussain, I.; Subhani, T. A durable superhydrophobic coating for the protection of wood materials. Mater. Lett. 2017, 203, 17–20. [Google Scholar] [CrossRef]
- Liu, L.; Lei, J.L.; Li, L.J.; Zhang, R.; Mi, N.Y.; Chen, H.R.; Huang, D.; Li, N.B. A facile method to fabricate the superhydrophobic magnetic sponge for oil-water separation. Mater. Lett. 2017, 195, 66–70. [Google Scholar] [CrossRef]
- Cao, H.; Gu, W.H.; Fu, J.Y.; Liu, Y.; Chen, S.G. Preparation of superhydrophobic/oleophilic copper mesh for oil-water separation. Appl. Surf. Sci. 2017, 412, 599–605. [Google Scholar] [CrossRef]
- Wang, L.; Xi, G.H.; Wan, S.J.; Zhao, C.H.; Liu, X.D. Asymmetrically superhydrophobic cotton fabrics fabricated by mist polymerization of lauryl methacrylate. Cellulose 2014, 21, 2983–2994. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, S.J.; Kim, Y.H. Improvement of interfacial adhesion of incorporated halloysite-nanotubes in fiber-reinforced epoxy-based composites. Appl. Sci. 2017, 7, 441. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Halloysite nanotubes as support for metal-based catalysts. J. Mater. Chem. A 2017, 5, 13276–13293. [Google Scholar] [CrossRef]
- Rizzo, C.; Arrigo, R.; D’Anna, F.; Di Blasi, F.; Dintcheva, N.T.; Lazzara, G.; Parisi, F.; Riela, S.; Spinelli, G.; Massaro, M. Hybrid supramolecular gels of fmoc-f/halloysite nanotubes: Systems for sustained release of camptothecin. J. Mater. Chem. B 2017, 5, 3217–3229. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S.; Cavallaro, G.; Colletti, C.G.; Milioto, S.; Noto, R.; Lazzara, G. Ecocompatible halloysite/cucurbit[8]uril hybrid as efficient nanosponge for pollutants removal. Chemistryselect 2016, 1, 1773–1779. [Google Scholar] [CrossRef]
- Massaro, M.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Covalently modified halloysite clay nanotubes: Synthesis, properties, biological and medical applications. J. Mater. Chem. B 2017, 5, 2867–2882. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.Y.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112, 75–93. [Google Scholar] [CrossRef]
- Wu, H.; Watanabe, H.; Ma, W.; Fujimoto, A.; Higuchi, T.; Uesugi, K.; Takeuchi, A.; Suzuki, Y.; Jinnai, H.; Takahara, A. Robust liquid marbles stabilized with surface-modified halloysite nanotubes. Langmuir 2013, 29, 14971–14975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shi, Z.W.; Chen, L.S.; Jiang, Y.J.; Xu, C.Y.; Wu, Z.H.; Wang, Y.Y.; Peng, C.S. Porous superhydrophobic and superoleophilic surfaces prepared by template assisted chemical vapor deposition. Surf. Coat. Technol. 2017, 315, 385–390. [Google Scholar] [CrossRef]
- Kavalenka, M.N.; Vulliers, F.; Lischker, S.; Zeiger, C.; Hopf, A.; Rohrig, M.; Rapp, B.E.; Worgull, M.; Holscher, H. Bioinspired air-retaining nanofur for drag reduction. ACS Appl. Mater. Interfaces 2015, 7, 10651–10655. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.Y.; Chen, G.; Yang, J.; Hu, J.; Song, H.J.; Zhao, Y.T. A simple approach to fabricate stable superhydrophobic glass surfaces. Appl. Surf. Sci. 2013, 266, 105–109. [Google Scholar] [CrossRef]
- Menga, N.; Di Mundo, R.; Carbone, G. Soft blasting of fluorinated polymers: The easy way to superhydrophobicity. Mater. Des. 2017, 121, 414–420. [Google Scholar] [CrossRef]
- Su, D.; Huang, C.Y.; Hu, Y.; Jiang, Q.W.; Zhang, L.; Zhu, Y.F. Preparation of superhydrophobic surface with a novel sol-gel system. Appl. Surf. Sci. 2011, 258, 928–934. [Google Scholar] [CrossRef]
- Li, C.P.; Li, X.Y.; Duan, X.L.; Li, G.J.; Wang, J.Q. Halloysite nanotube supported Ag nanoparticles heteroarchitectures as catalysts for polymerization of alkylsilanes to superhydrophobic silanol/siloxane composite microspheres. J. Colloid Interface Sci. 2014, 436, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.X.; Jia, Z.X.; Liu, F.; Jia, D.M.; Guo, B.C. Tailoring the wettability of polypropylene surfaces with halloysite nanotubes. J. Colloid Interface Sci. 2010, 350, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.H.; Meng, Y.; Yang, Z.X.; Cheng, H.T.; Zhang, S.B.; Song, W. Mussel-inspired polydopamine modification of bamboo fiber and its effect on the properties of bamboo fiber/polybutylene succinate. BioResources 2017, 12, 8419–8442. [Google Scholar] [CrossRef]
- Liu, Y.L.; Ai, K.L.; Lu, L.H. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.R.; Fan, H.L.; Zha, D.A.; Wang, L.; Jin, Z.X. Characterizations of the formation of polydopamine-coated halloysite nanotubes in various pH environments. Langmuir 2016, 32, 10377–10386. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Liu, J.D.; Wang, J.T.; Zhang, Y.W.; Zhang, B.; Zhang, Y.T.; Xiang, X.; Chen, R.F. Surface modification of halloysite nanotubes with dopamine for enzyme immobilization. ACS Appl. Mater. Interfaces 2013, 5, 10559–10564. [Google Scholar] [CrossRef] [PubMed]
- Yah, W.O.; Xu, H.; Soejima, H.; Ma, W.; Lvov, Y.; Takahara, A. Biomimetic dopamine derivative for selective polymer modification of halloysite nanotube lumen. J. Am. Chem. Soc. 2012, 134, 12134–12137. [Google Scholar] [CrossRef] [PubMed]
- Di Mundo, R.; Palumbo, F.; D’Agostino, R. Nanotexturing of polystyrene surface in fluorocarbon plasmas: From sticky to slippery superhydrophobicity. Langmuir 2008, 24, 5044–5051. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Xu, Y.C.; Liu, Y.Y.; Shao, L. A novel mussel-inspired strategy toward superhydrophobic surfaces for self-driven crude oil spill cleanup. J. Mater. Chem. A 2015, 3, 12171–12178. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.J.; Wang, Y.X.; Long, Y.H.; Zhao, N.; Xu, J. Combination of bioinspiration: A general route to superhydrophobic particles. J. Am. Chem. Soc. 2012, 134, 9879–9881. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Pan, Q.M. Mussel-inspired direct immobilization of nanoparticles and application for oil-water separation. ACS Nano 2014, 8, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Li, S.H.; Ge, M.Z.; Wang, L.N.; Xing, T.L.; Chen, G.Q.; Liu, X.F.; Al-Deyab, S.S.; Zhang, K.Q.; Chen, T.; et al. Robust superhydrophobic TiO2@fabrics for UV shielding, self-cleaning and oil-water separation. J. Mater. Chem. A 2015, 3, 2825–2832. [Google Scholar] [CrossRef]
- Atluri, R.; Hedin, N.; Garcia-Bennett, A.E. Nonsurfactant supramolecular synthesis of ordered mesoporous silica. J. Am. Chem. Soc. 2009, 131, 3189–3191. [Google Scholar] [CrossRef] [PubMed]
- Atluri, R.; Iqbal, M.N.; Bacsik, Z.; Hedin, N.; Villaescusa, L.A.; Garcia-Bennett, A.E. Self-assembly mechanism of folate-templated mesoporous silica. Langmuir 2013, 29, 12003–12012. [Google Scholar] [CrossRef] [PubMed]
- Gaaz, T.S.; Sulong, A.B.; Kadhum, A.A.H.; Nassir, M.H.; Al-Amiery, A.A. Impact of sulfuric acid treatment of halloysite on physico-chemic property modification. Materials 2016, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Zhang, B.; Zhai, R.; Xiang, X.; Liu, J.; Chen, R. Natural nanotube-based biomimetic porous microspheres for significantly enhanced biomolecule immobilization. ACS Sustain. Chem. Eng. 2014, 2, 396–403. [Google Scholar] [CrossRef]
- Hebbar, R.S.; Isloor, A.M.; Ananda, K.; Ismail, A.F. Fabrication of polydopamine functionalized halloysite nanotube/polyetherimide membranes for heavy metal removal. J. Mater. Chem. A 2016, 4, 764–774. [Google Scholar] [CrossRef]
- Zhu, X.; Li, H.; Liu, H.; Peng, W.; Zhong, S.; Wang, Y. Halloysite-based dopamine-imprinted polymer for selective protein capture. J. Sep. Sci. 2016, 39, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Gaaz, T.S.; Sulong, A.B.; Kadhum, A.A.H.; Nassir, M.H.; Al-Amiery, A.A. Surface improvement of halloysite nanotubes. Appl. Sci. 2017, 7, 291. [Google Scholar] [CrossRef]
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.R.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of halloysite clay nanotubes by grafting with gamma-aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Kang, H.J.; Liu, X.R.; Zhang, S.F.; Li, J.Z. Functionalization of halloysite nanotubes (HNTs) via mussel-inspired surface modification and silane grafting for HNTs/soy protein isolate nanocomposite film preparation. RSC Adv. 2017, 7, 24140–24148. [Google Scholar] [CrossRef]
- Raman, V.S.; Rooj, S.; Das, A.; Stöckelhuber, K.W.; Simon, F.; Nando, G.B.; Heinrich, G. Reinforcement of solution styrene butadiene rubber by silane functionalized halloysite nanotubes. J. Macromol. Sci. Part A Pure Appl. Chem. 2013, 50, 1091–1106. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wu, X.; Zhang, X.; Zhou, Z.; Lu, C. Dual functional biocomposites based on polydopamine modified cellulose nanocrystal for Fe3+-pollutant detecting and autoblocking. ACS Sustain. Chem. Eng. 2016, 4, 5667–5673. [Google Scholar] [CrossRef]
- Wang, P.; Du, M.L.; Zhang, M.; Zhu, H.; Bao, S.Y.; Zou, M.L.; Yang, T.T. Facile fabrication of AuNPs/PANI/HNTs nanostructures for high-performance electrochemical sensors towards hydrogen peroxide. Chem. Eng. J. 2014, 248, 307–314. [Google Scholar] [CrossRef]
- Liu, C.Y.; Wang, S.L.; Shi, J.Y.; Wang, C.Y. Fabrication of superhydrophobic wood surfaces via a solution-immersion process. Appl. Surf. Sci. 2011, 258, 761–765. [Google Scholar] [CrossRef]
- Yu, H.; Gao, J.; Chen, Y.; Zhao, Y. Preparation and properties of stearic acid/expanded graphite composite phase change material for low-temperature solar thermal application. J. Therm. Anal. Calorim. 2016, 124, 87–92. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martinez, M.; Cabeza, L.F.; Ines Fernandez, A. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef]
- Kenisarin, M.M.; Kenisarina, K.M. Form-stable phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2012, 16, 1999–2040. [Google Scholar] [CrossRef]
- Mei, D.; Zhang, B.; Liu, R.; Zhang, H.; Liu, J. Preparation of stearic acid/halloysite nanotube composite as form-stable PCM for thermal energy storage. Int. J. Energy Res. 2011, 35, 828–834. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Abdullayev, E.; Vasiliev, A.; Lvov, Y. Halloysite nanotubule clay for efficient water purification. J. Colloid Interface Sci. 2013, 406, 121–129. [Google Scholar] [CrossRef] [PubMed]



| No. | Labels | HNTs (g) | VM (mg/mL) | DA (mg/mL) | ODA (mmol/L) |
|---|---|---|---|---|---|
| 1 | P-HNTs | 5 | - | - | - |
| 2 | PDA-HNTs | 5 | - | 2 | - |
| 3 | VM/PDA-HNTs | 5 | 1 | 2 | - |
| 4 | ODA-HNTs | 5 | - | 2 | 10 |
| 5 | VM/ODA-HNTs | 5 | 1 | 2 | 10 |
| Samples | W/% | Heating | Cooling | ||||
|---|---|---|---|---|---|---|---|
| Onset T/°C | Peak T/°C | Latent Heat/J g−1 | Onset T/°C | Peak T/°C | Latent Heat/J g−1 | ||
| SA | 100 | 52.81 | 56.62 | 184.30 | 52.96 | 51.15 | 177.90 |
| SA/P-HNTs | 46.2 | 51.03 | 54.52 | 68.12 | 54.12 | 52.45 | 60.75 |
| SA/S-HNTs | 58.6 | 51.52 | 54.82 | 88.42 | 54.43 | 52.15 | 79.37 |
| SA/S-HNTs 300 cycles | 58.0 | 51.31 | 54.74 | 85.23 | 54.02 | 52.20 | 75.61 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Wang, M.; Tang, M.; Hong, G.; Gao, J.; Chen, Y. Preparation of Robust Superhydrophobic Halloysite Clay Nanotubes via Mussel-Inspired Surface Modification. Appl. Sci. 2017, 7, 1129. https://doi.org/10.3390/app7111129
Meng Y, Wang M, Tang M, Hong G, Gao J, Chen Y. Preparation of Robust Superhydrophobic Halloysite Clay Nanotubes via Mussel-Inspired Surface Modification. Applied Sciences. 2017; 7(11):1129. https://doi.org/10.3390/app7111129
Chicago/Turabian StyleMeng, Yang, Mingjie Wang, Mengfei Tang, Gonghua Hong, Jianmin Gao, and Yao Chen. 2017. "Preparation of Robust Superhydrophobic Halloysite Clay Nanotubes via Mussel-Inspired Surface Modification" Applied Sciences 7, no. 11: 1129. https://doi.org/10.3390/app7111129





