Polyvinyl Alcohol/Lithospermum Erythrorhizon Nanofibrous Membrane: Characterizations, In Vitro Drug Release, and Cell Viability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lithospermum Erythrorhizon Extract
2.3. Preparation of PVA Electrospun Nanofibrous Membranes (PENM)
2.4. Preparation of Lithospermum Erythrorhizon Electrospun Nanofibrous Membranes
2.5. Shikonin Content Test of LENMs
2.6. Encapsulation Efficiency and Drug-Loading Content of LENMs
2.7. In Vitro Drug Release of PENMs
2.8. Scanning Electron Microscopy (SEM) Observation of PENMs and LENMs
2.9. Cell Viability Assay
2.10. Statistical Analysis
3. Results and Discussion
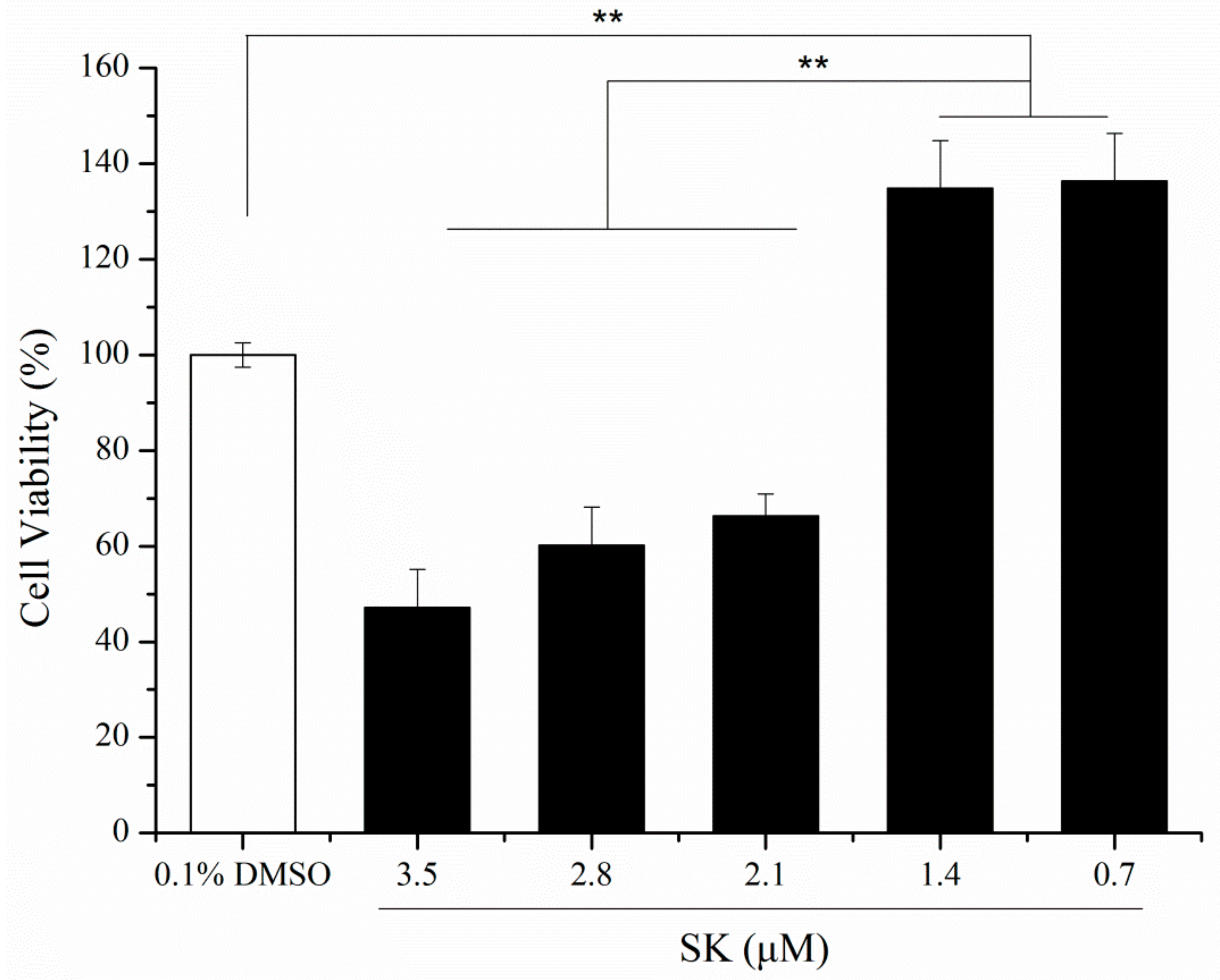
3.1. Purity and In Vitro Cell Viability of SK
3.2. Characterizations of PENMs
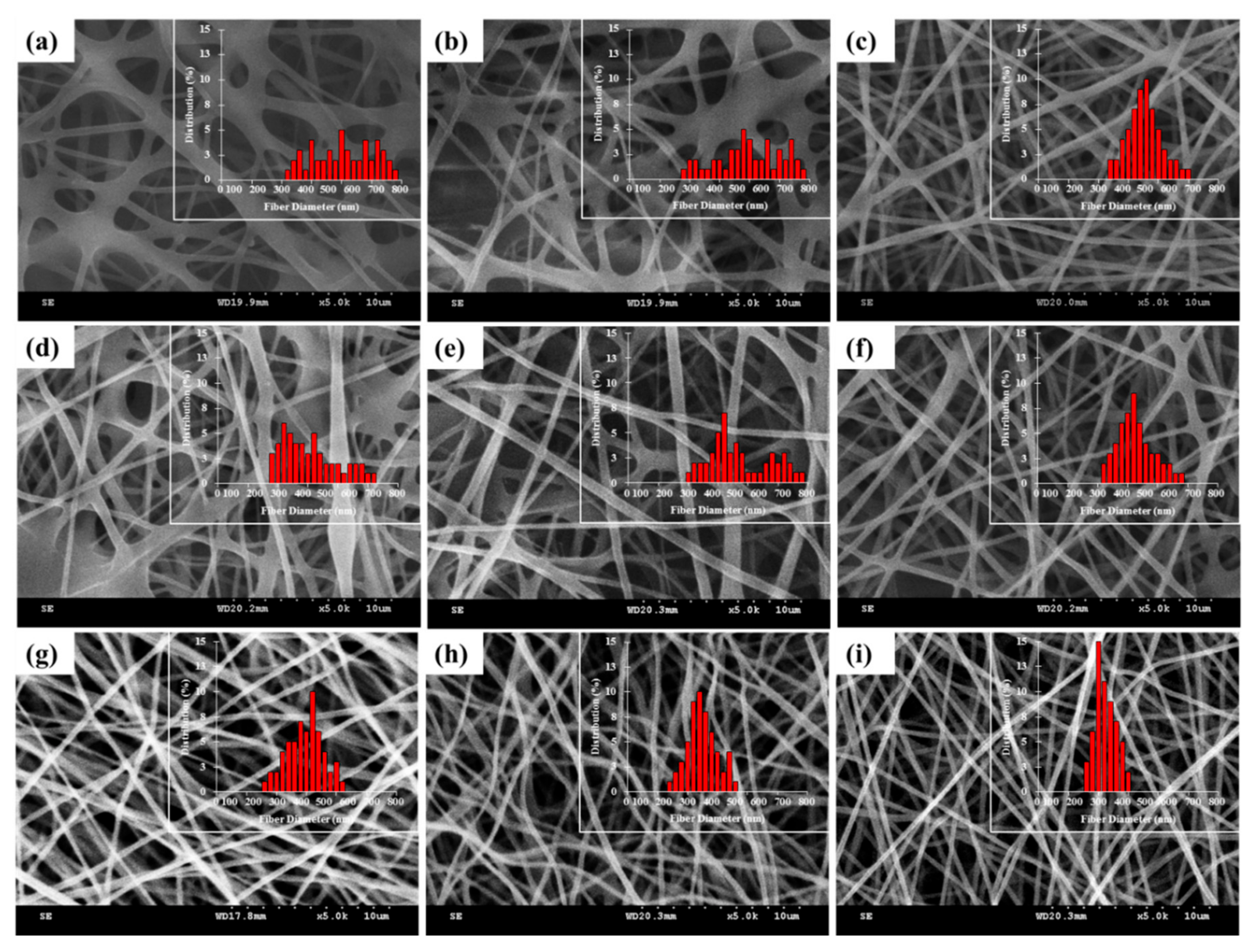
3.3. Morphology of LENMs
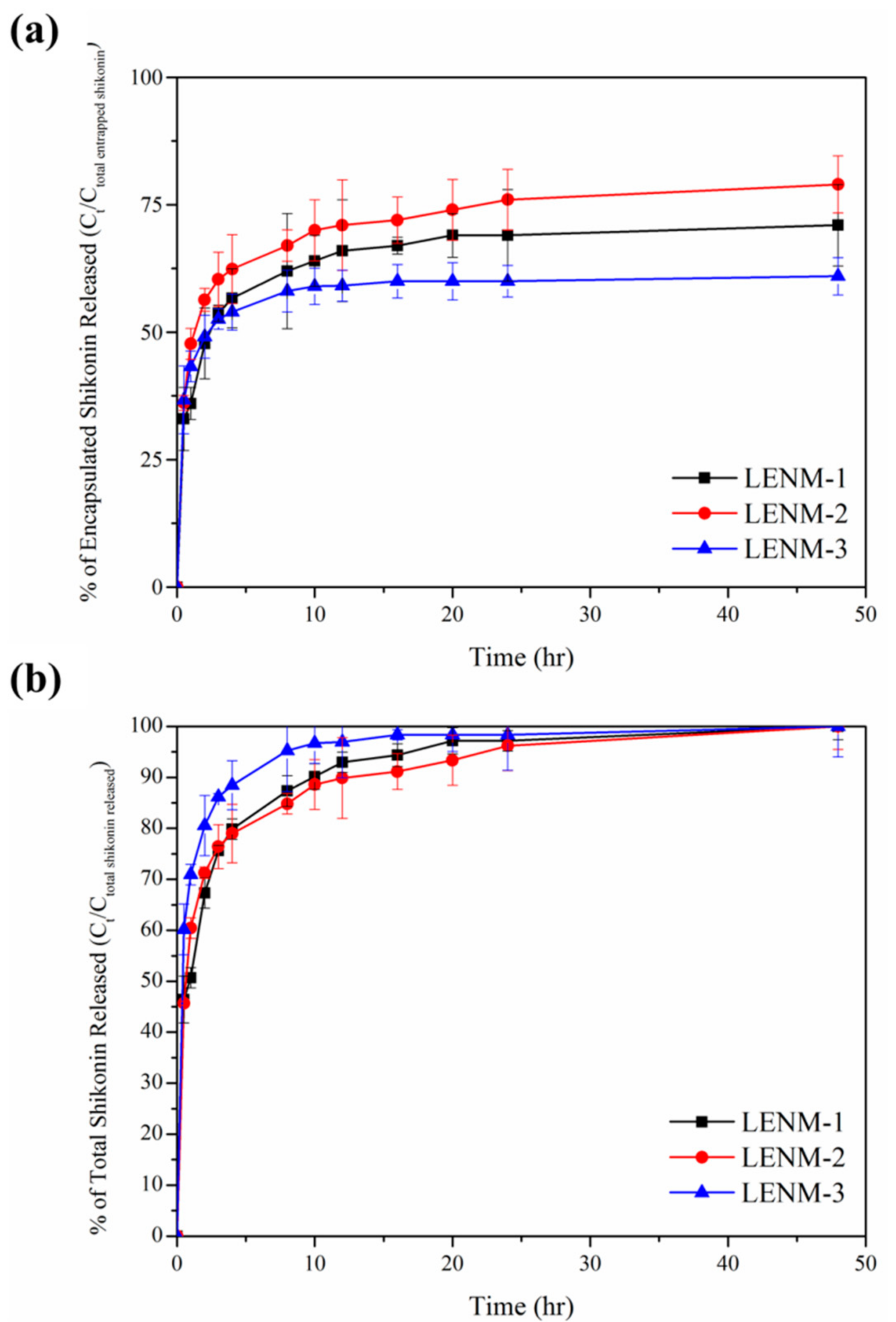
3.4. In Vitro Release of Shikonin
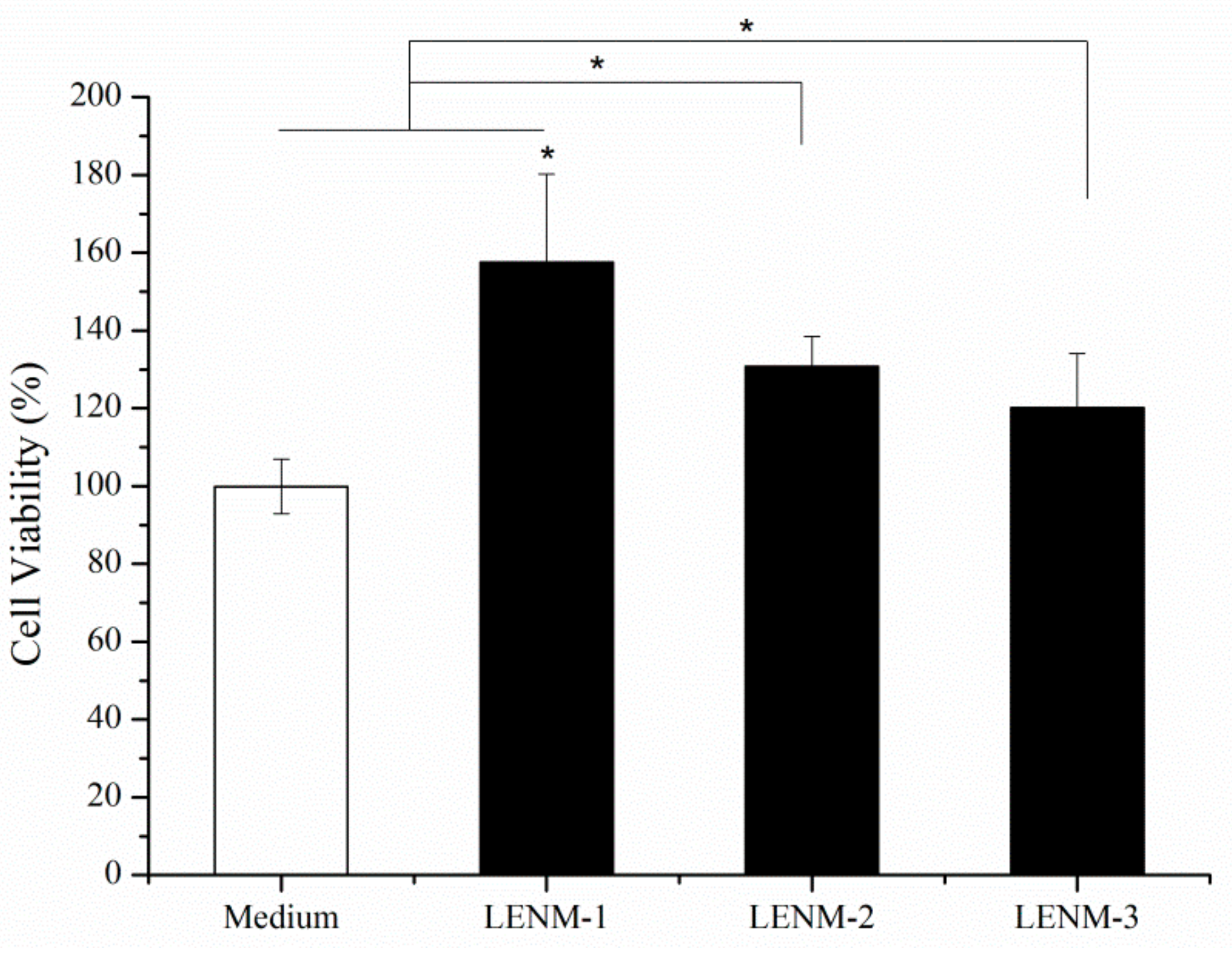
3.5. In Vitro Cell Viability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, Q.; Li, S.Y.; Fu, Z.Z.; Lin, B.H.; Zhou, Z.F.; Wang, Z.Y.; Hua, Y.Q.; Cai, Z.D. Shikonin promotes adriamycin-induced apoptosis by upregulating caspase-3 and caspase-8 in osteosarcoma. Mol. Med. Rep. 2017, 16, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.W.; Chang, C.Y.; Wu, Z.H.; Lin, J.H. The optimal extracting process, manufacturing technique and biological evaluation of Lithospermum erythrorhizon microcapsules. Mater. Sci. Eng. C Mater. 2015, 48, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Seo, Y.C.; No, R.H.; Lee, H.Y. Improved cosmetic activity by optimizing the Lithospermum erythrorhizon extraction process. Cytotechnology 2015, 67, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Chen, R.Z.; Wang, C.Z. Experiment study on ultrahigh pressure extraction of ginsenosides. J. Food Eng. 2007, 79, 1–5. [Google Scholar]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Ballis, A.C. Alkannins and Shikonins: A New Class of Wound Healing Agents. Curr. Med. Chem. 2008, 15, 3248–3267. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Zhou, P.; Huang, H.B.; Chen, D.; Ma, N.F.; Cui, Q.Z.C.; Shen, S.X.; Dong, W.H.; Zhang, X.Y.; Lian, W.; et al. Shikonin exerts antitumor activity via proteasome inhibition and cell death induction in vitro and in vivo. Int. J. Cancer 2009, 124, 2450–2459. [Google Scholar] [CrossRef] [PubMed]
- Staniforth, V.; Wang, S.Y.; Shyur, L.F.; Yang, N.S. Shikonins, phytocompounds from Lithospermum erythrorhizon, inhibit the transcriptional activation of human tumor necrosis factor alpha promoter in vivo. J. Biol. Chem. 2004, 279, 5877–5885. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hayashi, S.; Umezaki, M.; Yamamoto, T.; Kageyama-Yahara, N.; Kondo, T.; Kadowaki, M. Shikonin, a constituent of Lithospermum erythrorhizon exhibits anti-allergic effects by suppressing orphan nuclear receptor Nr4a family gene expression as a new prototype of calcineurin inhibitors in mast cells. Chem. Biol. Interact. 2014, 224, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, D.Y.; Kim, Y.B.; Lee, S.W.; Cha, S.W.; Park, H.W.; Kim, G.S.; Kwon, D.Y.; Lee, M.H.; Han, S.H. The Mechanism Underlying the Antibacterial Activity of Shikonin against Methicillin-Resistant Staphylococcus aureus. Evid. Based Complement. Altern. 2015, 2015, 520578. [Google Scholar]
- He, J. Complex of shikonin and beta-cyclodextrins by using supercritical carbon dioxide. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 249–255. [Google Scholar] [CrossRef]
- Kontogiannopoulos, K.N.; Assimopoulou, A.N.; Dimas, K.; Papageorgiou, V.P. Shikonin-loaded liposomes as a new drug delivery system: Physicochemical characterization and in vitro cytotoxicity. Eur. J. Lipid Sci. Technol. 2011, 113, 1113–1123. [Google Scholar] [CrossRef]
- Alexis, F.; Rhee, J.W.; Richie, J.P.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. New frontiers in nanotechnology for cancer treatment. Urol. Oncol. Semin. Orig. Investig. 2008, 26, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Shabafrooz, V.; Mozafari, M.; Vashaee, D.; Tayebi, L. Electrospun Nanofibers: From Filtration Membranes to Highly Specialized Tissue Engineering Scaffolds. J. Nanosci. Nanotechnol. 2014, 14, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Gokceoren, A.T. Optical and Electrical Investigation of the Electrospun Poly(N-vinyl carbazole) Fibers. Fiber Polym. 2015, 16, 86–94. [Google Scholar] [CrossRef]
- Marega, C.; Maculan, J.; Rizzi, G.A.; Saini, R.; Cavaliere, E.; Gavioli, L.; Cattelan, M.; Giallongo, G.; Marigo, A.; Granozzi, G. Polyvinyl alcohol electrospun nanofibers containing Ag nanoparticles used as sensors for the detection of biogenic amines. Nanotechnology 2015, 26, 075501. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, H.Y.; Park, S.H.; Park, J.H.; Kim, J.H.; Min, B.H.; Kim, M.S. Preparation and Evaluation of Dexamethasone-Loaded Electrospun Nanofiber Sheets as a Sustained Drug Delivery System. Materials 2016, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.C.; Xiang, G.Q.; Jiang, A.L.; Liu, Y.P.; Wu, L.L.; Dong, X.W.; Duan, S. Antioxidant properties of components extracted from puccoon (Lithospermum erythrorhizon Sieb. et Zucc.). Food Chem. 2000, 69, 143–146. [Google Scholar] [CrossRef]
- Kontogiannopoulos, K.N.; Assimopoulou, A.N.; Tsivintzelis, I.; Panayiotou, C.; Papageorgiou, V.P. Electrospun fiber mats containing shikonin and derivatives with potential biomedical applications. Int. J. Pharm. 2011, 409, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Furumura, M.; Gouya, T.; Iwanaga, A.; Teye, K.; Numata, S.; Karashima, T.; Li, X.G.; Hashimoto, T. Shikonin Promotes Skin Cell Proliferation and Inhibits Nuclear Factor-kappa B Translocation via Proteasome Inhibition In Vitro. Chin. Med. J. Peking 2015, 128, 2228–2233. [Google Scholar]
- Andujar, I.; Rios, J.L.; Giner, R.M.; Recio, M.C. Shikonin promotes intestinal wound healing in vitro via induction of TGF-beta release in IEC-18 cells. Eur. J. Pharm. Sci. 2013, 49, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.; Park, J.; Kim, S.H.; Uchida, Y.; Holleran, W.M.; Cho, Y. Water Extract of Gromwell (Lithospermum erythrorhizon) Enhances Migration of Human Keratinocytes and Dermal Fibroblasts with Increased Lipid Synthesis in an In Vitro Wound Scratch Model. Skin Pharmacol. Phys. 2012, 25, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kim, H.Y.; Bang, H.J.; Jung, Y.H.; Lee, S.G. The change of bead morphology formed on electrospun polystyrene fibers. Polymer 2003, 44, 4029–4034. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded nanofibers formed during electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Hsu, C.M.; Shivkumar, S. Nano-sized beads and porous fiber constructs of poly(epsilon-caprolactone) produced by electrospinning. J. Mater. Sci. 2004, 39, 3003–3013. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.H.; Yu, J.Y.; Zeng, H.M. Controlling numbers and sizes of beads in electrospun nanofibers. Polym. Int. 2008, 57, 632–636. [Google Scholar] [CrossRef]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer-polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]

| Extraction | Temperature (°C) | 60 | 40 | 25 | 10 | −10 |
|---|---|---|---|---|---|---|
| Time (days) | 1 | 1 | 1 | 7 | 7 | |
| Lithospermum erythrorhizon extract (mg/mL) | 6.24 ± 0.85 | 3.30 ± 0.32 | 1.93 ± 0.13 | 2.6 ± 0.11 | 2.00 ± 0.04 * | |
| SK (mg/mL) | 2.17 | 1.15 | 0.78 | 1.04 | 1.07 ** | |
| Purity of SK (%) | 34.8 | 34.9 | 40.4 | 40.2 | 53.8 | |
| Sample | Field Strength (kV/cm) | Flow Rate (mL/h) | Fiber Diameter (nm) | Average Diameter (nm) | |
|---|---|---|---|---|---|
| PENM-1 | 2.5 | 0.5 | 400–1000 | 683 | ±196 |
| PENM-2 | 2.5 | 0.75 | 300–980 | 585 | ±157 |
| PENM-3 | 2.5 | 1.0 | 360–620 | 481 | ±60 |
| PENM-4 | 2.0 | 0.5 | 250–660 | 418 | ±111 |
| PENM-5 | 2.0 | 0.75 | 320–960 | 544 | ±156 |
| PENM-6 | 2.0 | 1.0 | 320–620 | 443 | ±73 |
| PENM-7 | 1.5 | 0.5 | 230–570 | 411 | ±70 |
| PENM-8 | 1.5 | 0.75 | 250–490 | 350 | ±58 |
| PENM-9 | 1.5 | 1.0 | 240–390 | 316 | ±37 ** |
| Samples | Amount of Water Based PVA Solution (%) | Amount of Ethanol Based LE Solution (%) | Viscosity (cP) | Electrical Conductivity (µS/cm) | Average Diameter (nm) |
|---|---|---|---|---|---|
| PENM-9 | 100 | 0 | 1562 ± 31 | 551 | 316 ± 37 |
| LENM-1 | 90 | 10 | 1297 ± 83 | 403 | 279 ± 52 |
| LENM-2 | 80 | 20 | 1088 ± 72 | 288 | 272 ± 51 |
| LENM-3 | 70 | 30 | 760 ± 71 | 217 | 177 ± 45 ** |
| Sample | Shikonin | Total Released SK (48 h, %) | Drug Release at 60 min (%) | |
|---|---|---|---|---|
| EE % | DLC % | |||
| LENM-1 | 92.5 ± 9.5 | 0.15 ± 0.02 | 71 ± 7.9 | 36 ± 3.1 |
| LENM-2 | 89.2 ± 2.2 | 0.21 ± 0.01 | 79 ± 5.5 * | 47 ± 3.4 |
| LENM-3 | 66.0 ± 3.5 | 0.22 ± 0.01 | 61 ± 3.6 * | 43 ± 3.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, C.-W.; Wu, Z.-H.; Lee, M.-C.; Chen, Y.-S.; Lin, J.-H. Polyvinyl Alcohol/Lithospermum Erythrorhizon Nanofibrous Membrane: Characterizations, In Vitro Drug Release, and Cell Viability. Appl. Sci. 2017, 7, 1143. https://doi.org/10.3390/app7111143
Lou C-W, Wu Z-H, Lee M-C, Chen Y-S, Lin J-H. Polyvinyl Alcohol/Lithospermum Erythrorhizon Nanofibrous Membrane: Characterizations, In Vitro Drug Release, and Cell Viability. Applied Sciences. 2017; 7(11):1143. https://doi.org/10.3390/app7111143
Chicago/Turabian StyleLou, Ching-Wen, Zong-Han Wu, Mong-Chuan Lee, Yueh-Sheng Chen, and Jia-Horng Lin. 2017. "Polyvinyl Alcohol/Lithospermum Erythrorhizon Nanofibrous Membrane: Characterizations, In Vitro Drug Release, and Cell Viability" Applied Sciences 7, no. 11: 1143. https://doi.org/10.3390/app7111143