Interpretation US Elastography in Chronic Hepatitis B with or without Anti-HBV Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Laboratory Assessments
2.3. Serology Tests for Fibrosis
2.4. Histological Assessment
2.5. ARFI Imaging Study
2.6. FibroScan
2.7. Statistics
3. Results
3.1. Patient Demographics
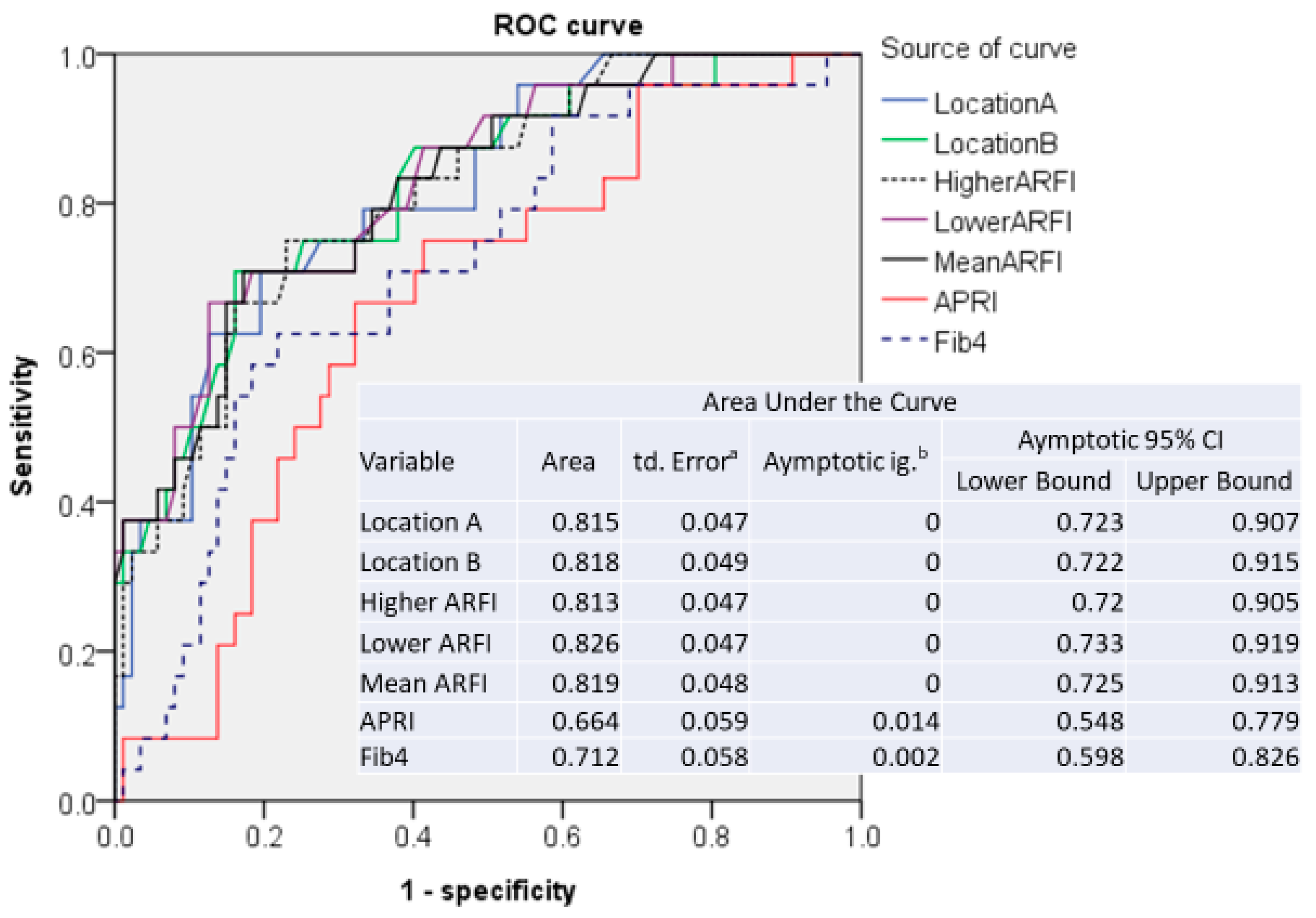
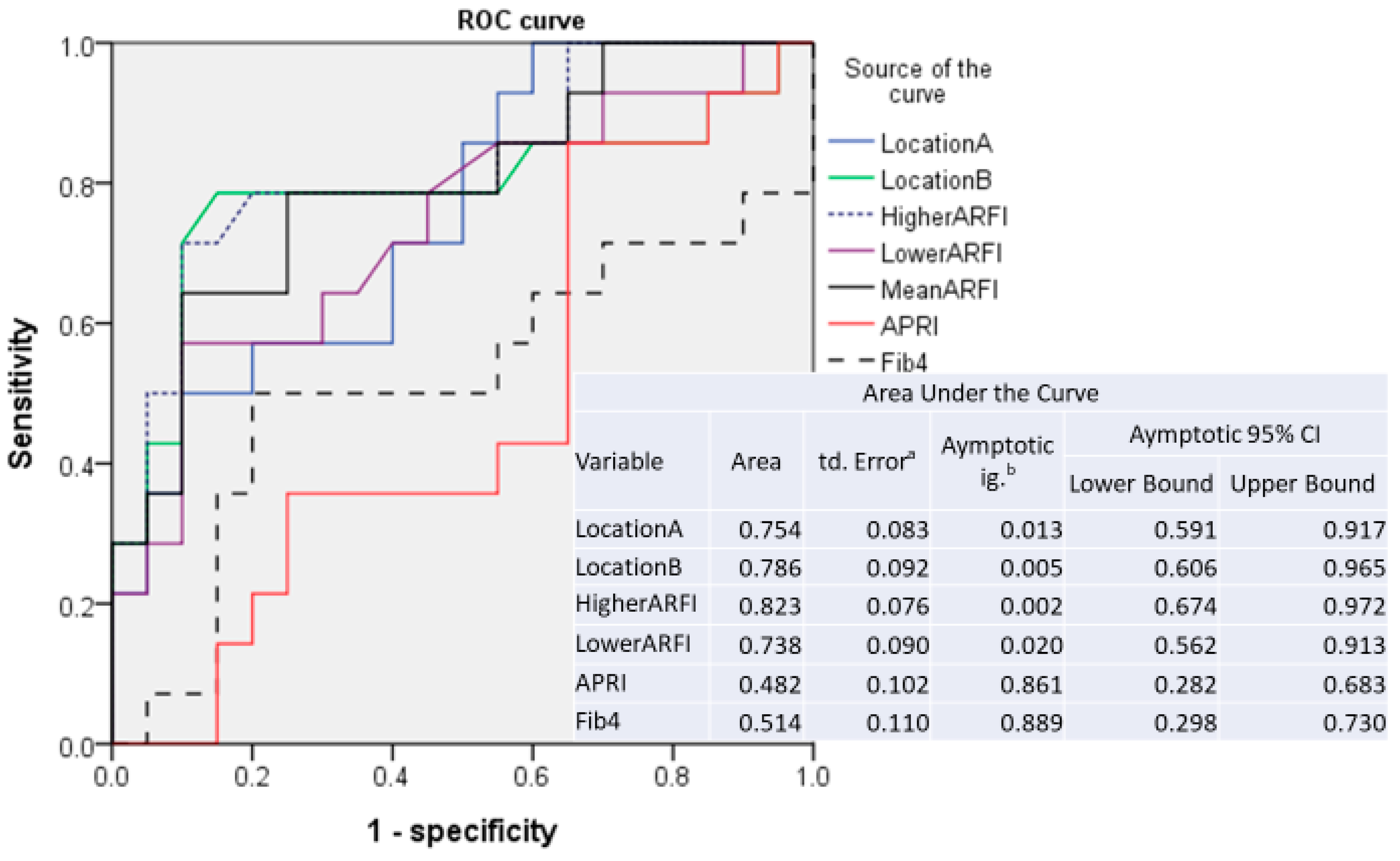
3.2. Sensitivity and Specificity of ARFI Measurements
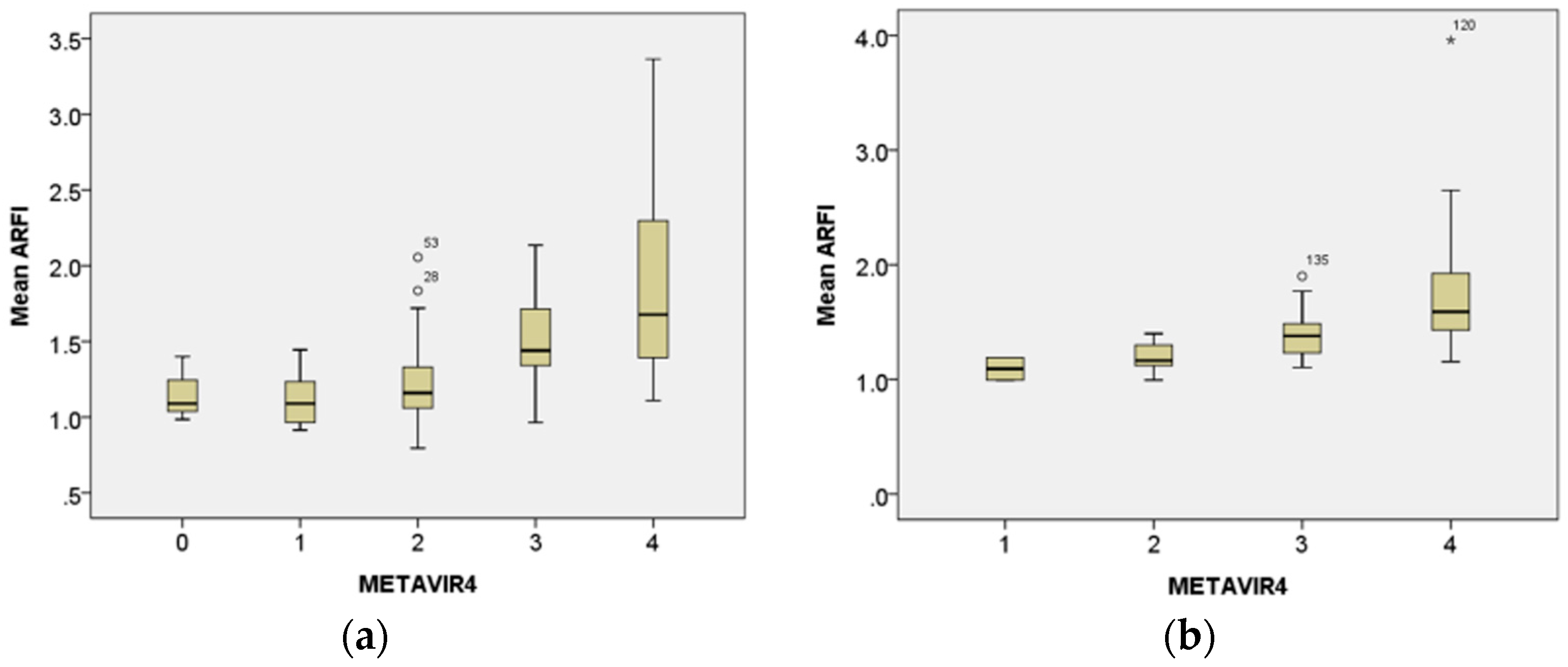
3.3. Correlation of Histological Metavir Scores with Mean ARFI Value
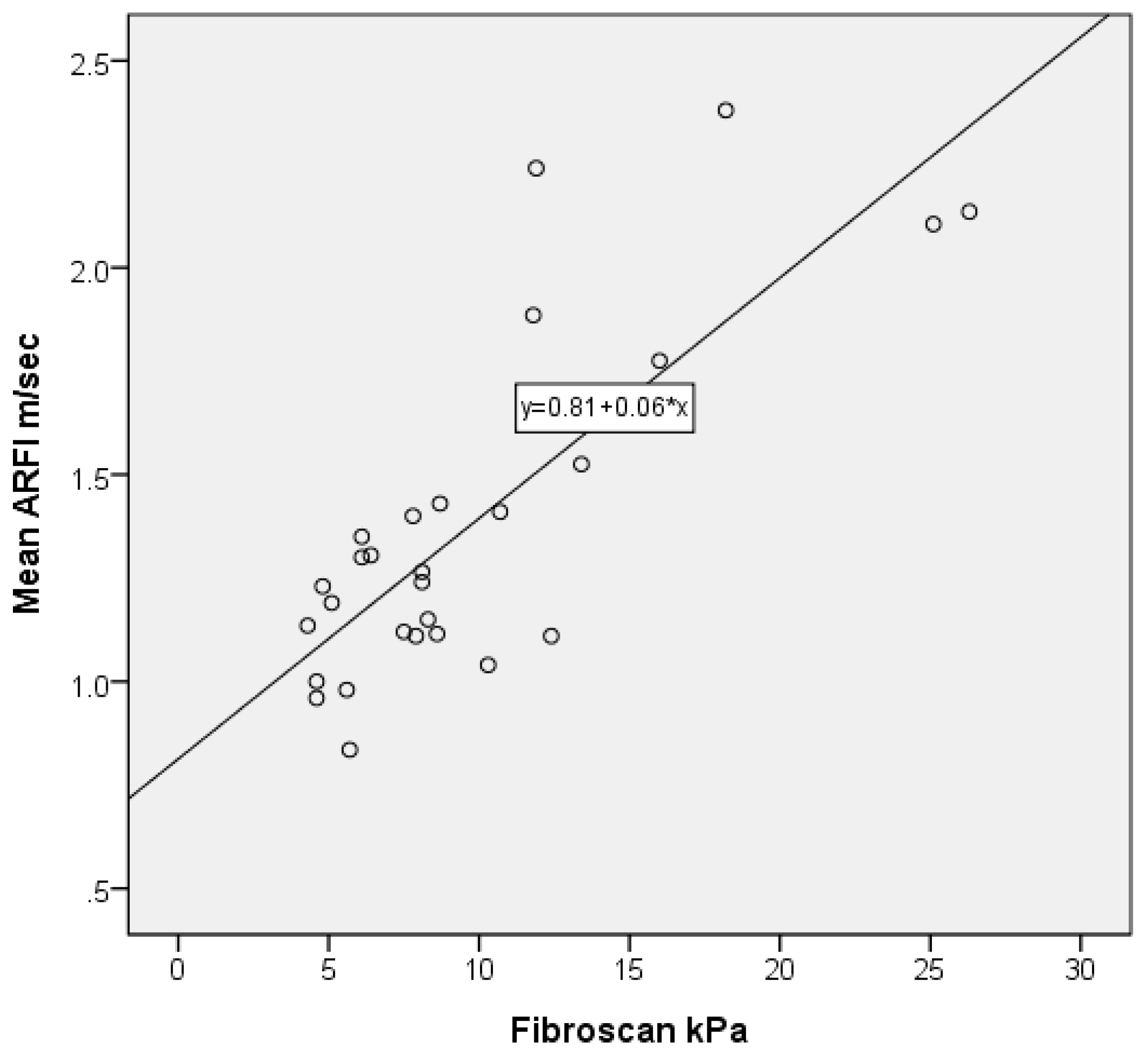
3.4. Correlation of ARFI with FibroScan
3.5. Correlation of ARFI Values with Metavir Score and Treatment Duration
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ARFI | acoustic radiation force impulse |
| ALT | alanine aminotransferase |
| HCV | hepatitis C virus |
| HBV | hepatitis B virus |
| HIV | human immunodeficiency virus |
| INR | international normalized ratio |
| PT | prothrombin time |
| FIB4 | Fibrosis-4 Score |
| ROC | receiver-operating characteristic |
| AUROC | areas under ROC |
References
- Tai, D.I.; Lin, S.M.; Sheen, I.S.; Chu, C.M.; Lin, D.Y.; Liaw, Y.F. Long-term outcome of hepatitis B e antigen-negative hepatitis B surface antigen carriers in relation to changes of alanine aminotransferase levels over time. Hepatology 2009, 49, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Tai, D.I.; Tsay, P.K.; Chen, W.T.; Chu, C.M.; Liaw, Y.F. Relative Roles of HBsAg seroclearance and mortality in the decline of HBsAg prevalence with increasing age. Am. J. Gastroenterol. 2010, 105, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Fattovich, G.; Bortolotti, F.; Donato, F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors. J. Hepatol. 2008, 48, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.F. Natural history of chronic hepatitis B virus infection and long-term outcome under treatment. Liver Int. 2009, 29, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Gkouvatsos, K.; Pantopoulos, K. Chronic hepatitis C and liver fibrosis. World J. Gastroenterol. 2014, 20, 11033–11053. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.A.; Sheth, S.G.; Chopra, S. Liver biopsy. N. Engl. J. Med. 2001, 344, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Regev, A.; Berho, M.; Jeffers, L.J.; Milikowski, C.; Molina, E.G.; Pyrsopoulos, N.T.; Feng, Z.Z.; Reddy, K.R.; Schiff, E.R. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am. J. Gastroenterol. 2002, 97, 2614–2618. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Rust, M.; Ong, M.F.; Martens, S.; Sarrazin, C.; Bojunga, J.; Zeuzem, S.; Herrmann, E. Performance of transient elastography for the staging of liver fibrosis: A meta-analysis. Gastroenterology 2008, 134, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Bota, S.; Herkner, H.; Sporea, I.; Salzl, P.; Sirli, R.; Neghinal, A.M.; Peck-Radosavljevic, M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013, 33, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Kircheis, G.; Sagir, A.; Vogt, C.; Vom Dahl, S.; Kubitz, R.; Häussinger, D. Evaluation of acoustic radiation force impulse imaging for determination of liver stiffness using transient elastography as a reference. World J. Gastroenterol. 2012, 18, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, D.Y. Non-invasive diagnosis of hepatitis B virus-related cirrhosis. World J. Gastroenterol. 2014, 20, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Crespo, G.; Fernández-Varo, G.; Mariño, Z.; Casals, G.; Miquel, R.; Martinez, S.M.; Gilabert, R.; Forns, X.; Jimenez, W.; Navasa, M. ARFI, FibroScan, ELF, and their combinations in the assessment of liver fibrosis: A prospective study. J. Hepatol. 2012, 57, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Afdhal, N.H. Vibration-controlled transient elastography: A practical approach to the noninvasive assessment of liver fibrosis. Curr. Opin. Gastroenterol. 2015, 31, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, G.; Filice, C.; Castera, L.; Choi, B.I.; Sporea, I.; Wilson, S.R.; Cosgrove, D.; Dietrich, C.F.; Amy, D.; Bamber, J.C.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography Part 3: Liver. Ultrasound Med. Biol. 2015, 41, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.D.; Qiu, L.Y.; Liu, D.; Qian, L.X. Acoustic radiation force impulse elastography for non-invasive evaluation of hepatic fibrosis in chronic hepatitis B and C patients: A systematic review and meta-analysis. Med. Ultrason. 2017, 19, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Tai, D.I.; Tsay, P.K.; Jeng, W.J.; Weng, C.C.; Huang, S.F.; Huang, C.H.; Lin, S.M.; Chiu, C.T.; Chen, W.T.; Wan, Y.L. Differences in liver fibrosis between patients with chronic hepatitis B and C: Evaluation by acoustic radiation force impulse measurements at 2 locations. J. Ultrasound Med. 2015, 34, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Li, Y.F.; Lai, H.C.; Kao, J.T.; Peng, C.Y.; Chuang, P.H.; Su, W.P.; Chiang, I.P. Effects of patient factors on noninvasive liver stiffness measurement using acoustic radiation force impulse elastography in patients with chronic hepatitis C. BMC Gastroenterol. 2012, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Bota, S.; Sporea, I.; Sirli, R.; Popescu, A.; Danila, M.; Costachescu, D. Intra- and interoperator reproducibility of acoustic radiation force impulse (ARFI) elastography—preliminary results. Ultrasound Med. Biol. 2012, 38, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Salvatore, V.; Di Donato, R.; D’Onofrio, M.; Gualandi, S.; Gallotti, A.; Sagrini, E. Accuracy of VirtualTouch Acoustic Radiation Force Impulse (ARFI) imaging for theet al diagnosis of cirrhosis during liver ultrasonography. Ultraschall Med. Eur. J. Ultrasound 2011, 32, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Bui, K.T.; Corte, C.; Lee, A.; Ngu, M.C.; Pattullo, V. Longer duration of transient elastography predicts unreliable liver stiffness measurements. Eur. J. Gastroenterol. Hepatol. 2015, 27, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Lampertico, P.; de Lédinghen, V.; Chang, P.E.; Kim, S.U.; Chen, Y.P.; Chan, H.L.Y.; Mangia, G.; Foucher, J.; Chow, W.C.; et al. Probability-based interpretation of liver stiffness measurement in untreated chronic hepatitis B patients. Dig. Dis. Sci. 2015, 60, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Mellema, D.C.; Sheedy, S.P.; Meixner, D.D.; Karshen, R.M.; Urban, M.W.; Manduca, A.; Sanchez, W.; Callstrom, M.R.; Greenleaf, J.F.; et al. Performance of 2-Dimensional Ultrasound Shear Wave Elastography in Liver Fibrosis Detection Using Magnetic Resonance Elastography as the Reference Standard: A Pilot Study. J. Ultrasound Med. 2016, 35, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Poynard, T. An algorithm for the grading of activity in chronic hepatitis C: The METAVIR Cooperative Study Group. Hepatology 1996, 24, 289–393. [Google Scholar] [CrossRef] [PubMed]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.M.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. Eur. J. Ultrasound 2017, 38, e16–e47. [Google Scholar] [CrossRef] [PubMed]
- Tai, D.I. Reply. Ultrasound Med. 2016, 35, 668. [Google Scholar] [CrossRef] [PubMed]
- Miailhes, P.; Pradat, P.; Chevallier, M.; Lacombe, K.; Bailly, F.; Cotte, L.; Trabaud, M.-A.; Boibieux, A.; Bottero, J.; Trepo, C.; et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J. Viral. Hepat. 2011, 18, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Maida, I.; Soriano, V.; Castellares, C.; Ramos, B.; Sotgiu, G.; Martin-Carbonero, L.; Barreiro, P.; Rivas, P.; Fonzalez-Lahoz, J.; Nunez, M. Liver fibrosis in HIV-infected patients with chronic hepatitis B extensively exposed to antiretroviral therapy with anti-HBV activity. HIV Clin. Trials 2006, 7, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Shouval, D.; Lok, A.S.; Chang, T.T.; Cheinquer, H.; Goodman, M.D.; Deheertogh, D.; Wilber, R.; Zink, R.C.; Cross, A.; et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 2006, 354, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T.; Gish, R.G.; de Man, R.; Gadano, A.; Sollano, J.; Chao, Y.C.; Lok, A.S.; Han, K.H.; Goodman, Z.; Zhu, J.; et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 2006, 354, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Viganò, M.; Massironi, S.; Lampertico, P.; Iavarone, M.; Paggi, S.; Pozzi, R.; Conte, D.; Colombo, M. Transient elastography assessment of the liver stiffness dynamics during acute hepatitis B. Eur. J. Gastroenterol. Hepatol. 2010, 22, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Verveer, C.; Zondervan, P.E.; ten Kate, F.J.W.; Hansen, B.E.; Janssen, H.L.A. Evaluation of transient elastography for fibrosis assessment compared with large biopsies in chronic hepatitis B and C. Liver Int. 2012, 32, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.F.; Tai, D.I.; Chu, C.M.; Pao, C.C.; Chen, T.J. Acute exacerbation in chronic type B hepatitis: Comparison between HBeAg and antibodypositive patients. Hepatology 1987, 7, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Knop, V.; Hoppe, D.; Welzel, T.; Vermehren, J.; Herrmann, E.; Vermehren, A.; Friedrich-Rust, M.; Sarrazin, C.; Zeuzem, S.; Welker, M.-W. Regression of fibrosis and portal hypertension in HCV-associated cirrhosis and sustained virologic response after interferon-free antiviral therapy. J. Viral. Hepat. 2016, 23, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Ahn, H.S.; Kim, S.G.; Lee, Y.N.; Kim, Y.S.; Jeong, S.W.; Jang, J.Y.; Lee, S.H.; Kim, H.S.; Kim, B.S. The usefulness of transient elastography, acoustic-radiation-force impulse elastography, and real-time elastography for the evaluation of liver fibrosis. Clin. Mol. Hepatol. 2013, 19, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, M.; Wang, R.; Liu, Y.; Zhang, D.; Liu, L.; Zhou, G. Comparison of acoustic radiation force impulse imaging and transient elastography for non-invasive assessment of liver fibrosis in patients with chronic hepatitis B. Ultrasound Med. Biol. 2015, 41, 7–14. [Google Scholar] [CrossRef] [PubMed]
| Category | Non-Treatment | Treatment | p Value # |
|---|---|---|---|
| Total No | 112 | 34 | |
| Age (year) | 52.34 ± 10.80 | 55.05 ± 10.41 | |
| Male (%) | 85 (75.9) | 28 (82.4) | |
| Liver cancer (%) | 55 (49.1) | 19 (55.9) | |
| Ultrasound spleen index (cm2) | 16.58 ± 7.10 | 16.84 ± 5.80 | |
| Ultrasound fibrosis score | 6.43 ± 1.38 | 7.26 ± 1.05 | 0.001 |
| GGT (glutamyl transpetidase) (U/L) | 60.9 ± 71.68 | 56.48 ± 53.90 | |
| AST (U/L) | 73 ± 111 | 49 ± 48 | |
| ALT (U/L) | 92 ± 193 | 56 ± 75 | |
| Bilirubin (mg/DL) | 0.97 ± 1.22 | 0.76 ± 0.36 | |
| Platelet (109/L) | 187.33 ± 65.10 | 161.62 ± 47.52 | 0.034 |
| Prothrombin time (* INR) | 1.07 ± 0.08 | 1.08 ± 0.06 | |
| Body height (cm) | 164.56 ± 7.51 | 164.81 ± 10.47 | |
| Body weight (kg) | 69.38 ± 10.47 | 69.45 ± 13.30 | |
| Histology | |||
| Ishak inflammatory score | 3.89 ± 2.45 | 2.76 ± 1.74 | 0.020 |
| Confluence necrosis (No.) | 3 (2.7) | 1 (2.9) | |
| Metavir fibrosis score 0 | 3 (2.7) | 0 (0) | |
| Metavir fibrosis score 1 | 12 (10.7) | 2 (5.9) | |
| Metavir fibrosis score 2 | 43 (38.4) | 5 (14.7) | |
| Metavir fibrosis score 3 | 30 (26.8) | 13 (38.2) | |
| Metavir fibrosis score 4 | 24 (21.4) | 14 (41.2) | 0.026 |
| Type of ARFI | AUROC | Cut-Off (m/sec) | Sensitivity | 1-Specificity |
|---|---|---|---|---|
| Patients without anti-HBV treatment (N = 112) | ||||
| Location A | 0.816 | 1.505 | 0.708 | 0.193 |
| Location B | 0.818 | 1.515 | 0.708 | 0.159 |
| Higher | 0.813 | 1.515 | 0.750 | 0.227 |
| Lower | 0.827 | 1.460 | 0.708 | 0.182 |
| Mean | 0.820 | 1.523 | 0.708 | 0.170 |
| Patients without anti-HBV treatment and ALT level <5x ULN (N = 99) | ||||
| Location A | 0.816 | 1.415 | 0.739 | 0.263 |
| Location B | 0.815 | 1.460 | 0.739 | 0.263 |
| Higher | 0.793 | 1.485 | 0.739 | 0.257 |
| Lower | 0.827 | 1.460 | 0.696 | 0.184 |
| Mean | 0.818 | 1.523 | 0.696 | 0.171 |
| Patients without anti-HBV treatment and ALT level <2x ULN (N = 79) | ||||
| Location A | 0.789 | 1.415 | 0.706 | 0.258 |
| Location B | 0.761 | 1.460 | 0.647 | 0.274 |
| Higher | 0.764 | 1.520 | 0.647 | 0.258 |
| Lower | 0.793 | 1.460 | 0.647 | 0.177 |
| Mean | 0.775 | 1.523 | 0.647 | 0.177 |
| Patients with anti-HBV treatment (N = 34) | ||||
| Location A | 0.754 | 1.495 | 0.571 | 0.200 |
| Location B | 0.786 | 1.480 | 0.786 | 0.150 |
| Higher | 0.823 | 1.515 | 0.768 | 0.200 |
| Lower | 0.737 | 1.490 | 0.571 | 0.100 |
| Mean | 0.796 | 1.420 | 0.786 | 0.250 |
| ARFI (m/sec) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Age | Sex | HCC | ALT | Durg | Treatment | METAVIR | # Mean of | * Predicted | ** Compared |
| (Mo) | Two Locations | Metavir Score | with Histology Metavir Score | |||||||
| T1 | 55.7 | M | 1 | 48 | ETV | 2 | 2 | 1.17 | 2 | equal |
| T2 | 48.3 | M | 0 | 335 | Pegasy | 5 | 2 | 1.4 | 3 | higher |
| T3 | 52.8 | M | 0 | 103 | ETV | 6 | 2 | 1.3 | 2 | equal |
| T4 | 47.3 | M | 0 | 50 | ETV | 12 | 3 | 1.5 | 4 | higher |
| T5 | 30.2 | M | 0 | 46 | ETV | 12 | 4 | 1.84 | 4 | equal |
| T6 | 50.3 | M | 0 | 100 | TDF | 13 | 3 | 1.41 | 3 | equal |
| T7 | 45.2 | M | 0 | 39 | ETV | 14 | 4 | 1.59 | 4 | equal |
| T8 | 71.3 | F | 0 | 172 | ETV | 18 | 4 | 3.96 | 4 | equal |
| T9 | 52.1 | M | 0 | 23 | ETV | 19 | 3 | 1.77 | 4 | higher |
| T10 | 64.1 | F | 1 | 23 | ETV | 21 | 3 | 1.38 | 3 | equal |
| T11 | 55.3 | M | 1 | 45 | ETV | 22 | 4 | 1.73 | 4 | equal |
| T12 | 53.0 | M | 1 | 19 | ADVTBV | 26 | 4 | 1.59 | 4 | equal |
| T13 | 57.1 | M | 1 | 42 | ETV | 28 | 3 | 1.15 | 1 | lower |
| T14 | 60.9 | M | 1 | 16 | ETV | 29 | 4 | 1.93 | 4 | equal |
| T15 | 65.6 | F | 0 | 10 | ETV | 31 | 3 | 1.49 | 4 | higher |
| T16 | 64.0 | F | 1 | 27 | TDF | 31 | 4 | 2.65 | 4 | equal |
| T17 | 45.9 | M | 0 | 26 | ETV | 31 | 4 | 2.12 | 4 | equal |
| T18 | 51.5 | M | 1 | 26 | ETV | 32 | 3 | 1.29 | 3 | lower |
| T19 | 41.3 | M | 1 | 26 | ETV | 32 | 4 | 1.43 | 2 | lower |
| T20 | 49.3 | M | 0 | 35 | TDF | 36 | 3 | 1.47 | 1 | lower |
| T21 | 68.4 | F | 1 | 19 | TDF | 36 | 4 | 1.16 | 2 | lower |
| T22 | 45.4 | M | 1 | 45 | TDF | 36 | 3 | 1.29 | 3 | equal |
| T23 | 45.0 | M | 0 | 316 | TDF | 37 | 4 | 1.9 | 4 | equal |
| T24 | 68.1 | M | 0 | 16 | ETV | 39 | 1 | 0.995 | 0 | lower |
| T25 | 59.7 | M | 0 | 34 | TDF | 39 | 3 | 1.36 | 3 | equal |
| T26 | 42.0 | M | 0 | 19 | ETV | 41 | 3 | 1.105 | 1 | lower |
| T27 | 60.3 | M | 1 | 50 | TDF | 44 | 2 | 1.12 | 1 | lower |
| T28 | 63.0 | F | 1 | 16 | TBV | 48 | 2 | 0.995 | 0 | lower |
| T29 | 73.9 | M | 1 | 13 | TBV | 52 | 4 | 1.52 | 4 | equal |
| T30 | 62.7 | M | 1 | 26 | ETV | 55 | 3 | 1.23 | 2 | lower |
| T31 | 38.5 | M | 1 | 26 | ETV | 58 | 4 | 1.18 | 1 | lower |
| T32 | 50.4 | M | 1 | 24 | ETV | 60 | 1 | 1.19 | 1 | equal |
| T33 | 63.7 | M | 1 | 21 | ETV | 68 | 3 | 1.115 | 1 | lower |
| T34 | 69.5 | M | 1 | 68 | ETV | 71 | 4 | 1.435 | 3 | lower |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-H.; Wan, Y.-L.; Hsu, T.-H.; Huang, S.-F.; Yu, M.-C.; Lee, W.-C.; Tsui, P.-H.; Chen, Y.-C.; Lin, C.-Y.; Tai, D.-I. Interpretation US Elastography in Chronic Hepatitis B with or without Anti-HBV Therapy. Appl. Sci. 2017, 7, 1164. https://doi.org/10.3390/app7111164
Lee C-H, Wan Y-L, Hsu T-H, Huang S-F, Yu M-C, Lee W-C, Tsui P-H, Chen Y-C, Lin C-Y, Tai D-I. Interpretation US Elastography in Chronic Hepatitis B with or without Anti-HBV Therapy. Applied Sciences. 2017; 7(11):1164. https://doi.org/10.3390/app7111164
Chicago/Turabian StyleLee, Cheng-Han, Yung-Liang Wan, Tse-Hwa Hsu, Shiu-Feng Huang, Ming-Chin Yu, Wei-Chen Lee, Po-Hsiang Tsui, Yi-Cheng Chen, Chun-Yen Lin, and Dar-In Tai. 2017. "Interpretation US Elastography in Chronic Hepatitis B with or without Anti-HBV Therapy" Applied Sciences 7, no. 11: 1164. https://doi.org/10.3390/app7111164






