Utilizing Downdraft Fixed Bed Reactor for Thermal Upgrading of Sewage Sludge as Fuel by Torrefaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus
2.2. Materials
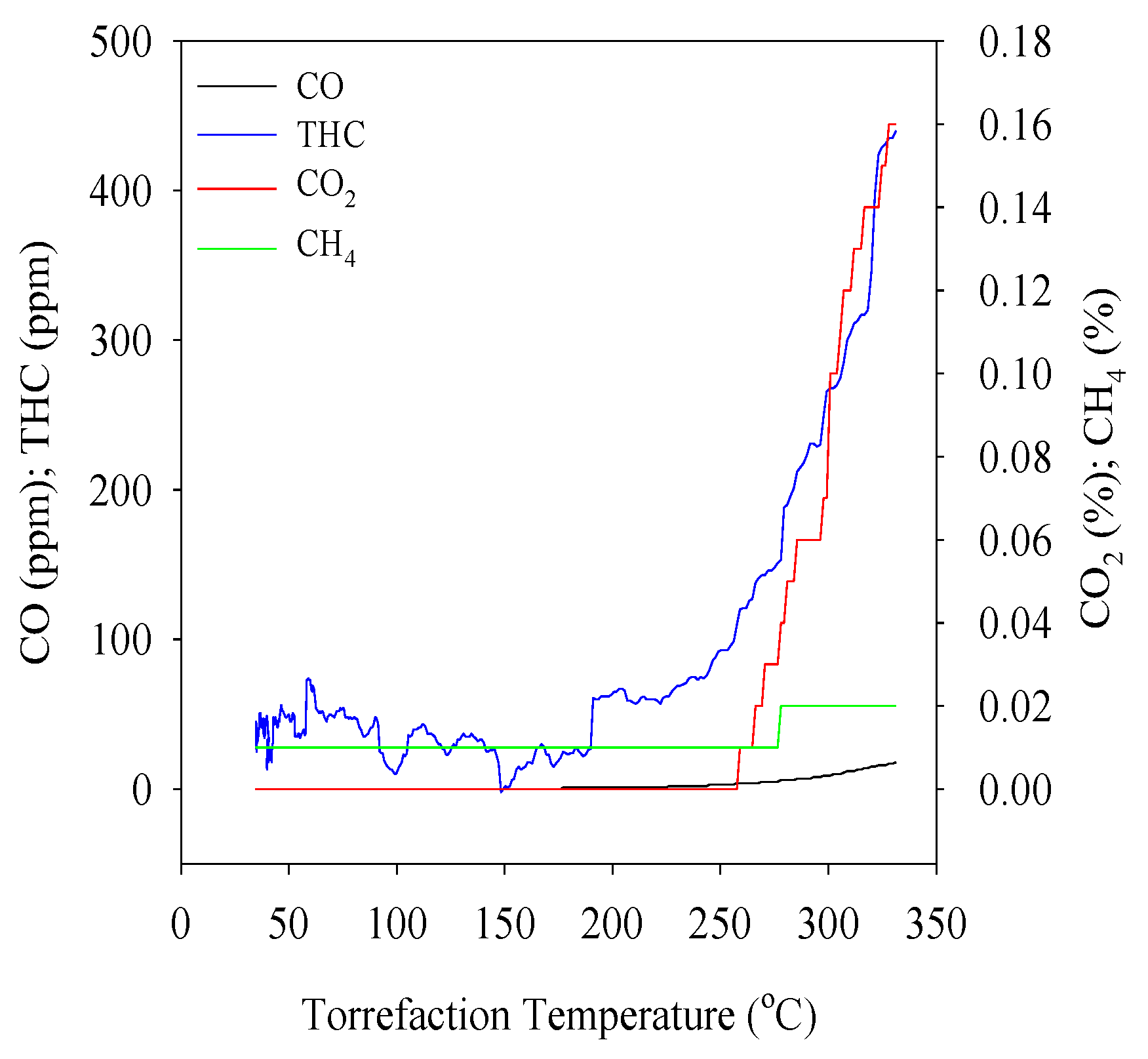
2.3. Methods
2.4. Torrefied Product Analysis
3. Results and Discussion
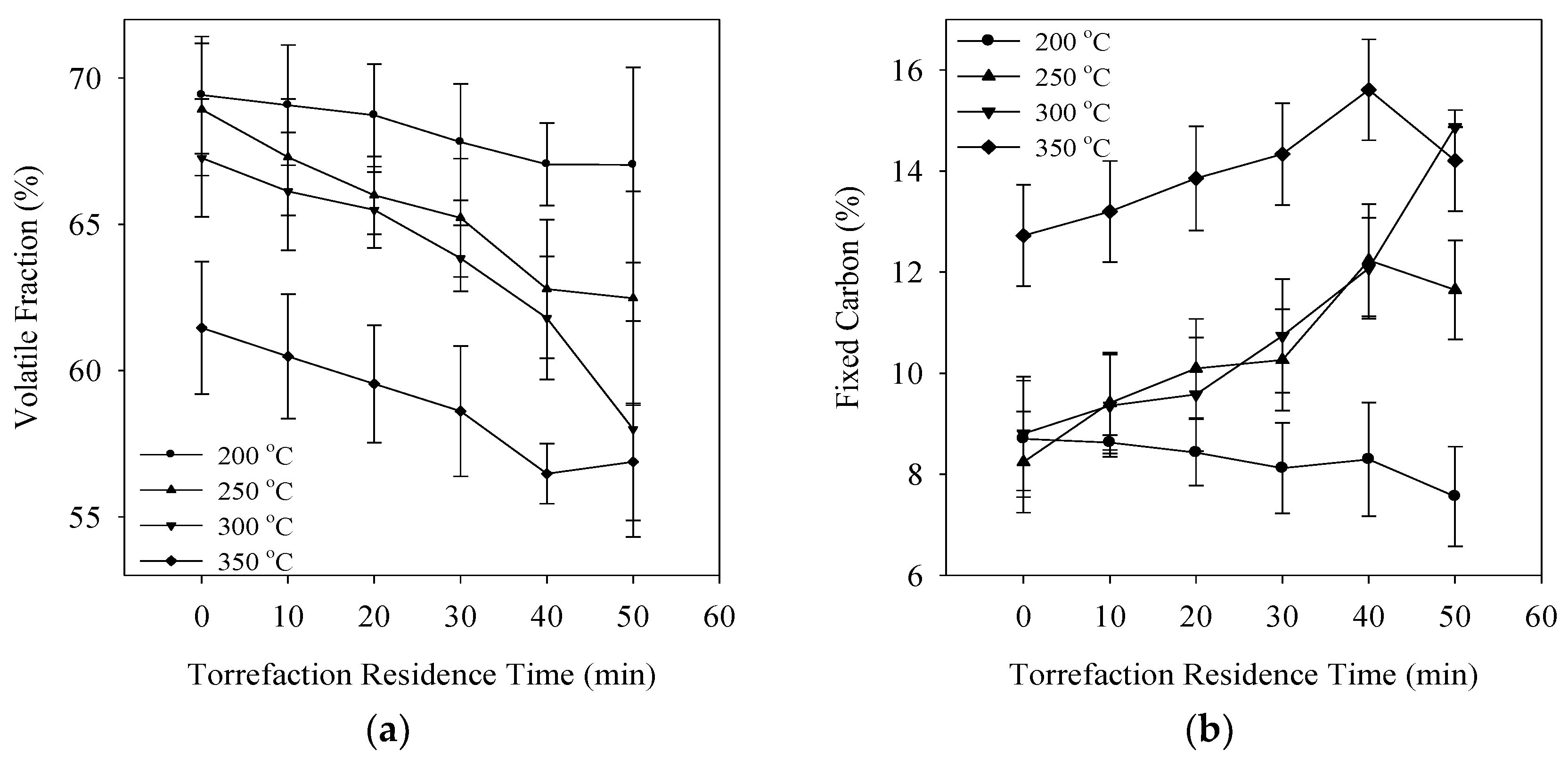
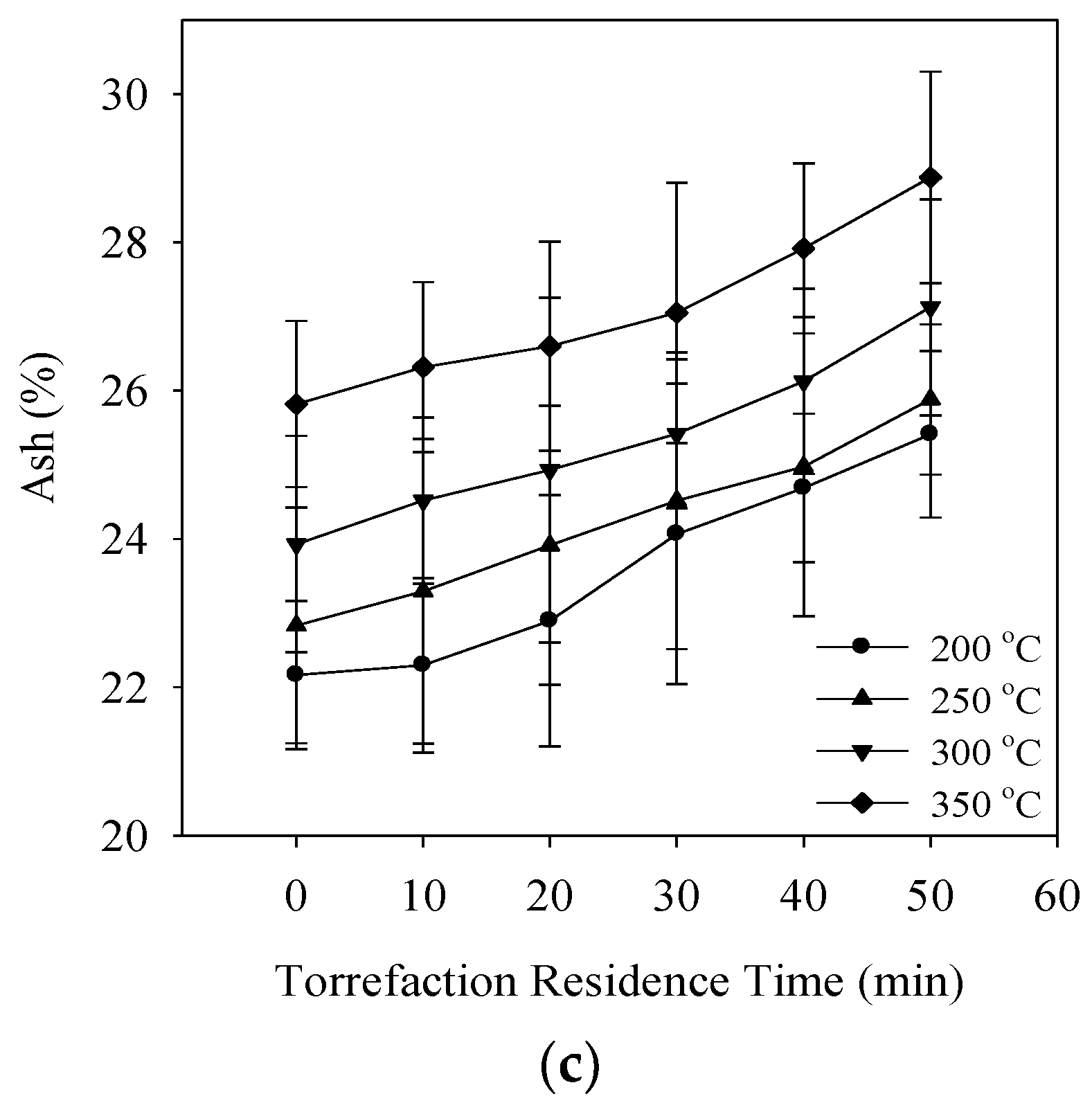
3.1. Degree of Torrefaction
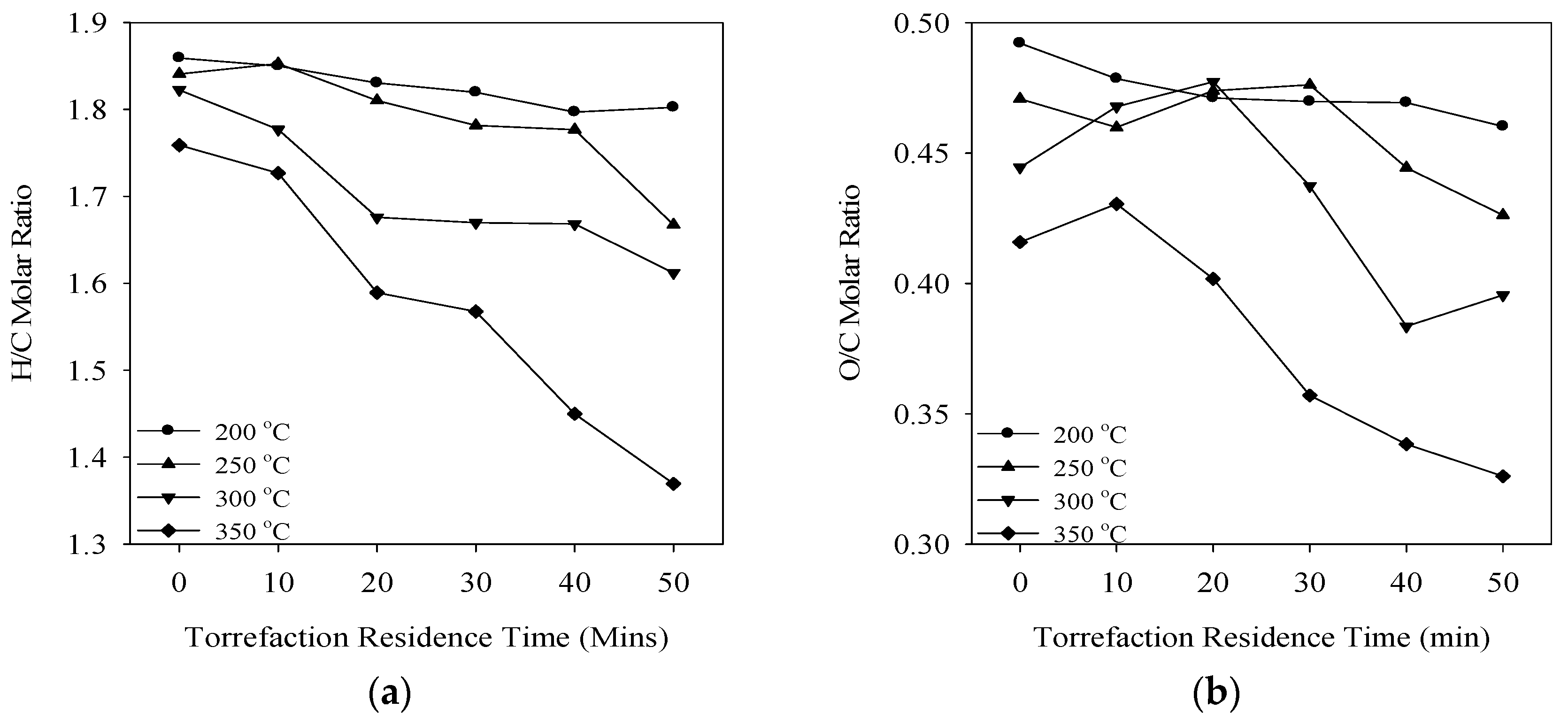
3.2. Torrefaction Index (TI)
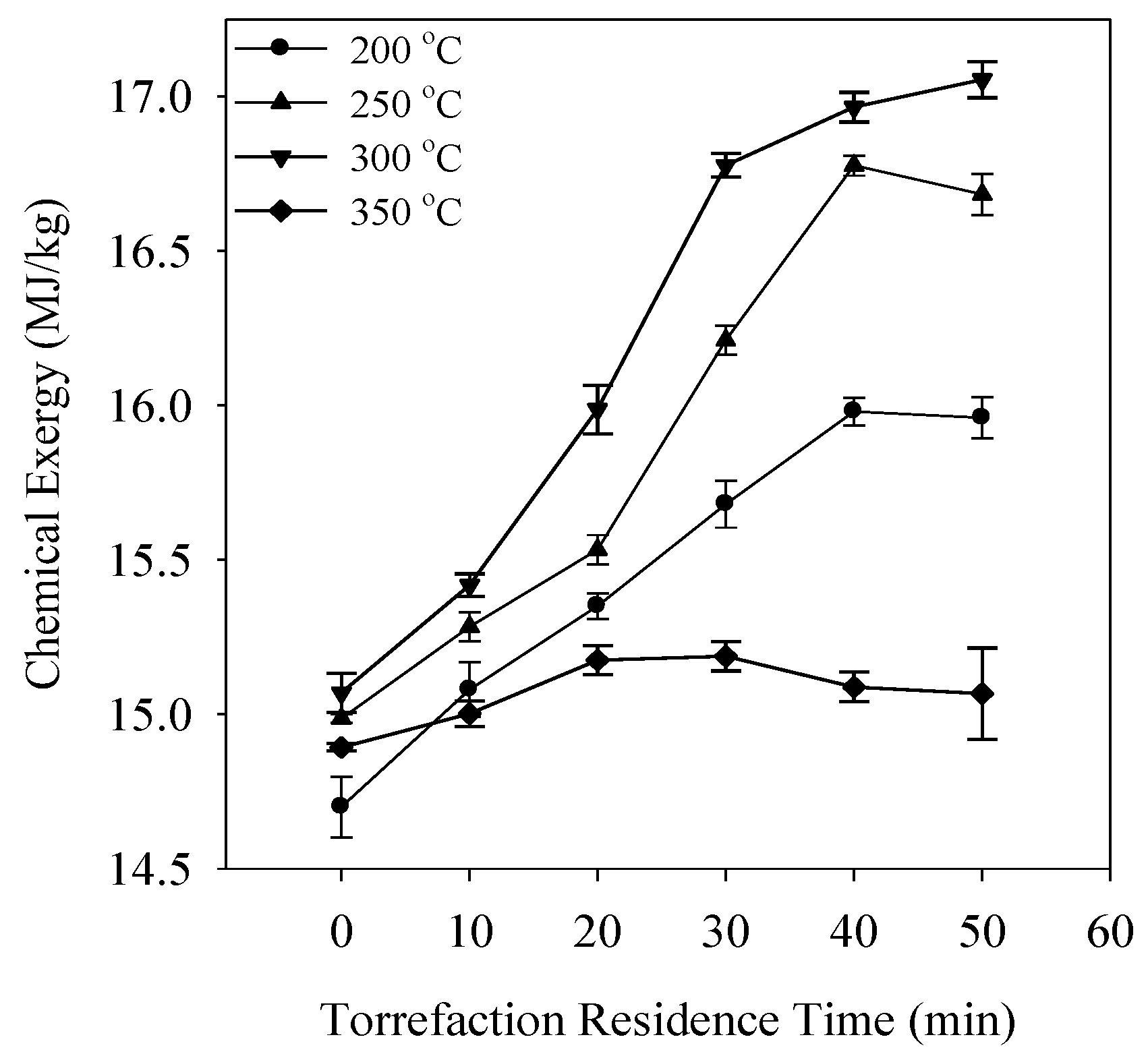
3.3. Chemical Exergy (ech)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Uris, M.; Linares, J.I.; Arenas, E. Techno-economic feasibility assessment of a biomass cogeneration plant based on an Organic Rankine Cycle. Renew. Energy 2014, 66, 707–713. [Google Scholar] [CrossRef]
- Maraver, D.; Sin, A.; Sebastián, F.; Royo, J. Environmental assessment of CCHP (combined cooling heating and power) systems based on biomass combustion in comparison to conventional generation. Energy 2013, 57, 17–23. [Google Scholar] [CrossRef]
- Magdziarz, A.; Werle, S. Analysis of the combustion and pyrolysis of dried sewage sludge by TGA and MS. Waste Manag. 2014, 34, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Kijo-Kleczkowska, A.; Środa, K.; Kosowska-Golachowska, M.; Musiał, T.; Wolski, K. Mechanisms and kinetics of granulated sewage sludge combustion. Waste Manag. 2015, 46, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Kandiyoti, R.; Dugwell, D. Trace element behavior during co-combustion of sewage sludge with polish coal. Energy Fuels 2004, 18, 1093–1103. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, W.; Gong, M.; Zhou, H.; Fan, Y.; Amuzu-Sefordzi, B. Interaction between sewage sludge components lignin (phenol) and proteins (alanine) in supercritical water gasification. Int. J. Hydrog. Energy 2015, 40, 9125–9136. [Google Scholar] [CrossRef]
- Xu, C.; Chen, W.; Hong, J. Life-cycle environmental and economic assessment of sewage sludge treatment in china. J. Clean. Prod. 2014, 67, 79–87. [Google Scholar] [CrossRef]
- Somolada, M.C.; Zabaniotou, A.A. Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece. Waste Manag. 2014, 34, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Loow, Y.-L.; New, E.K.; Yang, G.H.; Ang, L.Y.; Foo, L.Y.W.; Wu, T.Y. Potential use of deep eutectic solvents to facilitate lignocellulosic biomass utilization and conversion. Cellulose 2017, 24, 1–28. [Google Scholar] [CrossRef]
- Cremers, M.; Koppejan, J.; Middelkamp, J.; Witkamp, J.; Sokhansanj, S.; Melin, S. Status Overview of Torrefaction Technologies. A Review of the Commercialisation Status of Biomass Torrefaction. Available online: http://www.ieabcc.nl/publications/IEA_Bioenergy_T32_Torrefaction_update_2015b.pdf (accessed on 28 September 2017).
- Van der Stelt, M.; Gerhauser, H.; Kiel, J.; Ptasinski, K. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Shankar Tumuluru, J.; Sokhansanj, S.; Hess, J.R.; Wright, C.T.; Boardman, R.D. A review on biomass torrefaction process and product properties for energy applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Hellwig, M. Basics of the Combustion of Wood and Straw; Elsevier Applied Science Publishers: London, UK, 1985; pp. 793–798. [Google Scholar]
- Dhungana, A.; Dutta, A.; Basu, P. Torrefaction of non-lignocellulose biomass waste. Can. J. Chem. Eng. 2012, 90, 186–195. [Google Scholar] [CrossRef]
- Ábrego, J.; Sánchez, J.L.; Arauzo, J.; Fonts, I.; Gil-Lalaguna, N.; Atienza-Martínez, M. Technical and energetic assessment of a three-stage thermochemical treatment for sewage sludge. Energy Fuels 2013, 27, 1026–1034. [Google Scholar] [CrossRef]
- Atienza-Martínez, M.; Mastral, J.F.; Ábrego, J.; Ceamanos, J.; Gea, G. Sewage sludge torrefaction in an auger reactor. Energy Fuels 2014, 29, 160–170. [Google Scholar] [CrossRef]
- Atienza-Martínez, M.; Fonts, I.; Ábrego, J.; Ceamanos, J.; Gea, G. Sewage sludge torrefaction in a fluidized bed reactor. Chem. Eng. J. 2013, 222, 534–545. [Google Scholar] [CrossRef]
- Kuo, P.; Wu, W.; Chen, W. Gasification performances of raw and torrefied biomass in a downdraft fixed bed gasifier using thermodynamic analysis. Fuel 2014, 117, 1231–1241. [Google Scholar] [CrossRef]
- Kotas, T.J. The Exergy Method of Thermal Plant Analysis; Krieger Publishing Company: Boston, MA, USA, 1995. [Google Scholar]
- Kongkeaw, N.; Patumsawad, S. Thermal upgrading of biomass as a fuel by torrefaction. In Proceedings of the International Conference on Environmental Engineering and Applications, Shanghai, China, 19 August 2011; pp. 38–42. [Google Scholar]
- Strandberg, M.; Olofsson, I.; Pommer, L.; Wiklund-Lindstrom, S.; Aberg, K.; Nordin, A. Effects of temperature and residence time on continuous torrefaction of spruce wood. Fuel Process. Technol. 2015, 134, 387–398. [Google Scholar] [CrossRef]
- Barta-Rajnai, E.; Wang, L.; Sebestyen, Z.; Barta, Z.; Khalil, R.; Skreiberg, O.; Gronli, M.; Jakab, E.; Czegeny, Z. Effect of Temperature and Duration of Torrefaction on the Thermal Behavior of Stem Wood, Bark, and Stump of Spruce. Energy Proced. 2017, 105, 551–556. [Google Scholar] [CrossRef]
- Zanzi, R.; Ferro, D.T.; Torres, A.; Soler, P.B.; Bjornbom, E. Biomass torrefaction. In Proceedings of the 6th Asia-Pacific International Symposium on Combustion and Energy Utilization, Kuala Lumpur, Malaysia, 20–22 May 2002. [Google Scholar]
- Iroba, K.L.; Baik, O.; Tabil, L.G. Torrefaction of biomass from municipal solid waste fractions II: Grindability characteristics, higher heating value, pelletability and moisture adsorption. Biomass Bioenergy 2017, 106, 8–20. [Google Scholar] [CrossRef]
- Nimlos, M.N.; Brooking, E.; Looker, M.; Evans, R. Biomass torrefaction studies with a molecular beam mass spectrometer. Am. Chem. Soc. Div. Fuel Chem. 2003, 48, 590–591. [Google Scholar]
- Chen, W.; Lu, K.; Lee, W.; Liu, S.; Lin, T. Non-oxidative and oxidative torrefaction characterization and SEM observations of fibrous and ligneous biomass. Appl. Energy 2014, 114, 104–113. [Google Scholar] [CrossRef]
- Park, J.; Meng, J.; Lim, K.H.; Rojas, O.J.; Park, S. Transformation of lignocellulosic biomass during torrefaction. J. Anal. Appl. Pyrol. 2013, 100, 199–206. [Google Scholar] [CrossRef]
- Bridgeman, T.; Jones, J.; Shield, I.; Williams, P. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Sadaka, S.; Negi, S. Improvements of biomass physical and thermochemical characteristics via torrefaction process. Environ. Prog. Sustain. Energy 2009, 28, 427–434. [Google Scholar] [CrossRef]
- Deng, J.; Wang, G.; Kuang, J.; Zhang, Y.; Luo, Y. Pretreatment of agricultural residues for co-gasification via torrefaction. J. Anal. Appl. Pyrol. 2009, 86, 331–337. [Google Scholar] [CrossRef]
- Mani, S. Integrating biomass torrefaction with thermo-chemical conversion processes. In Proceedings of the AIChE Annual Meeting, Nashville, TN, USA, 8–13 November 2009; pp. 8–13. [Google Scholar]
- Tumuluru, J.S.; Wright, C.T.; Boardman, R.D.; Hess, R.J.; Sokhansanj, S. Review on biomass torrefaction process and product properties and design of moving bed torrefaction system model development. In Proceedings of the ASABE Annual Meeting, Louisville, Kentucky, 7–10 August 2011. [Google Scholar]
- Bergman, P.C.; Boersma, A.; Zwart, R.; Kiel, J. Torrefaction for Biomass Co-Firing in Existing Coal-FiredPower Stations. Energy Centre of Netherlands, Report No.ECN-C-05-013. Available online: https://www.researchgate.net/profile/Robin_Zwart/publication/204978559_Torrefaction_for_Biomass_Co-Firing_in_Existing_Coal-Fired_Power_Stations/links/09e41511c9aaf0e1c7000000.pdf (accessed on 5 October 2017).
- Kumar, S.; Gupta, R.; Lee, Y.; Gupta, R.B. Cellulose pretreatment in subcritical water: Effect of temperature on molecular structure and enzymatic reactivity. Bioresour. Technol. 2010, 101, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- White, R.H.; Dietenberger, M. Wood Products: Thermal Degradation and Fire; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- He, C.; Wang, K.; Giannis, A.; Yang, Y.; Wang, J. Products evolution during hydrothermal conversion of dewatered sewage sludge in sub-and near-critical water: Effects of reaction conditions and calcium oxide additive. Int. J. Hydrog. Energy 2015, 40, 5776–5787. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Assima, G.P.; Beaudoin, G.; Larachi, F. Biomass torrefaction and CO2 capture using mining wastes—A new approach for reducing greenhouse gas emissions of co-firing plants. Fuel 2014, 115, 749–757. [Google Scholar] [CrossRef]
- Shang, L.; Ahrenfeldt, J.; Holm, J.K.; Sanadi, A.R.; Barsberg, S.; Thomsen, T.; Stelte, W.; Henriksen, U.B. Changes of chemical and mechanical behavior of torrefied wheat straw. Biomass Bioenergy 2012, 40, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Basu, P.; Kulshreshtha, A.; Acharya, B. An index for quantifying the degree of torrefaction. BioResources 2017, 12, 1749–1766. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J. Optimization of biomass torrefaction conditions by the gain and loss method and regression model analysis. Bioresour. Technol. 2014, 172, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, M.; Klika, V.; Vágner, P.; Maršík, F. Generalization of exergy analysis. Appl. Energy 2015, 137, 158–172. [Google Scholar] [CrossRef]


| Elemental Analysis (wt. %) * | C | 37.82 |
| H | 5.82 | |
| N | 4.14 | |
| O | 25.12 | |
| S | 1.44 | |
| Others | 25.66 | |
| Proximate Analysis (wt. %) ** | Moisture (%) | 80.12 |
| Volatile Content (%) | 12.87 | |
| Ash (%) | 5.50 | |
| Fixed Carbon (%) | 1.51 | |
| HHV (MJ/Kg) * | 15.81 | |
| Torrefaction Regimes | Temperature (°C) | Torrefaction Index (TI) [40] | TI (Obtained in this Study) |
|---|---|---|---|
| Light | 200 to 235 | 0.93 to 0.95 | 0.89–0.96 |
| Medium | 235 to 275 | 0.95 to 0.97 | 0.94–0.99 |
| Severe | 275 to 300 | 0.97 to 1.0 | 0.96–1.0 |
| - | 350 | - | 0.94–0.92 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karki, S.; Poudel, J.; Oh, S.C. Utilizing Downdraft Fixed Bed Reactor for Thermal Upgrading of Sewage Sludge as Fuel by Torrefaction. Appl. Sci. 2017, 7, 1189. https://doi.org/10.3390/app7111189
Karki S, Poudel J, Oh SC. Utilizing Downdraft Fixed Bed Reactor for Thermal Upgrading of Sewage Sludge as Fuel by Torrefaction. Applied Sciences. 2017; 7(11):1189. https://doi.org/10.3390/app7111189
Chicago/Turabian StyleKarki, Sujeeta, Jeeban Poudel, and Sea Cheon Oh. 2017. "Utilizing Downdraft Fixed Bed Reactor for Thermal Upgrading of Sewage Sludge as Fuel by Torrefaction" Applied Sciences 7, no. 11: 1189. https://doi.org/10.3390/app7111189





