Targeted Molecular Imaging of Pancreatic Cancer with a Miniature Endoscope
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Multifunctional Nanoparticles
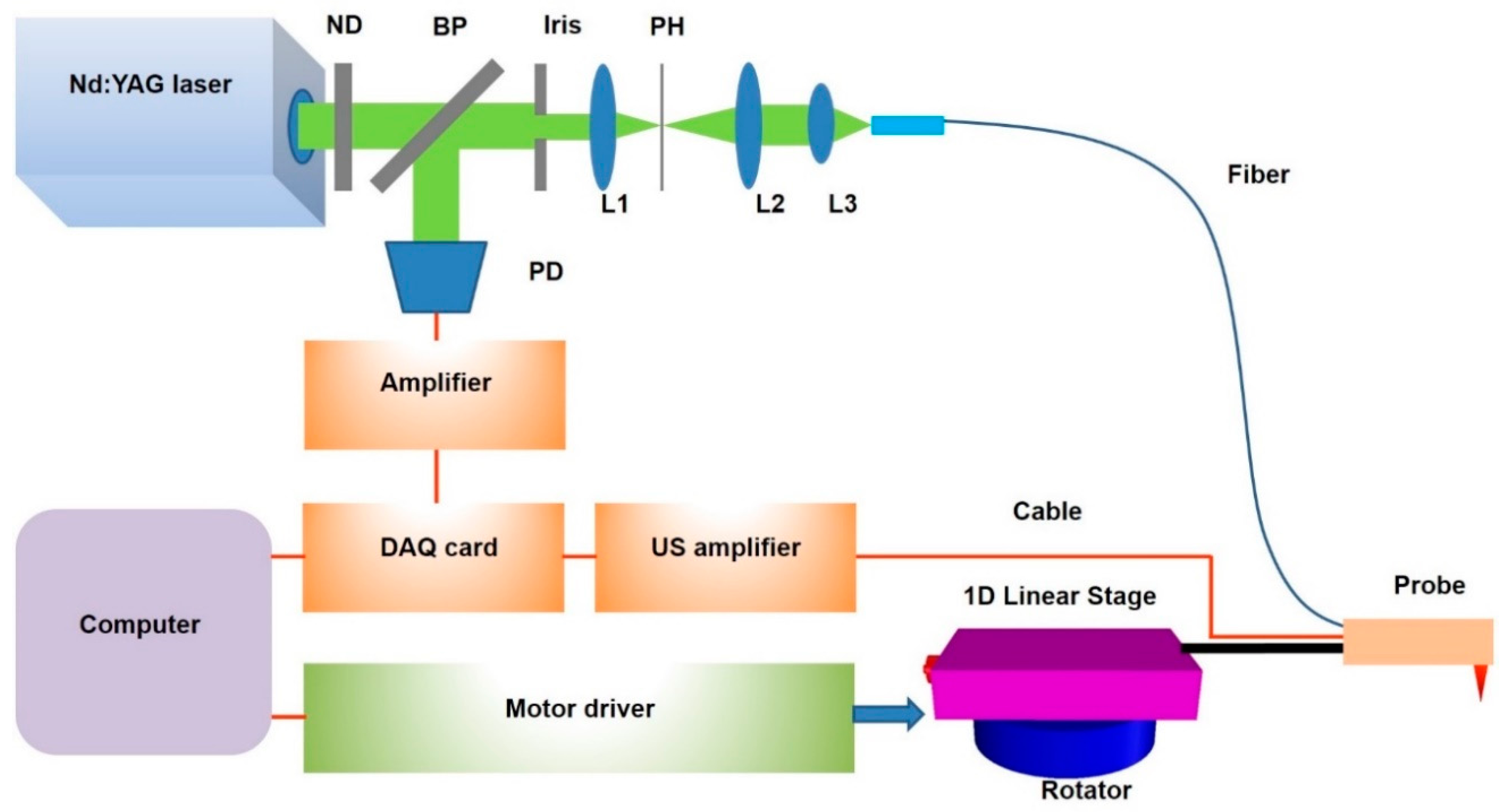
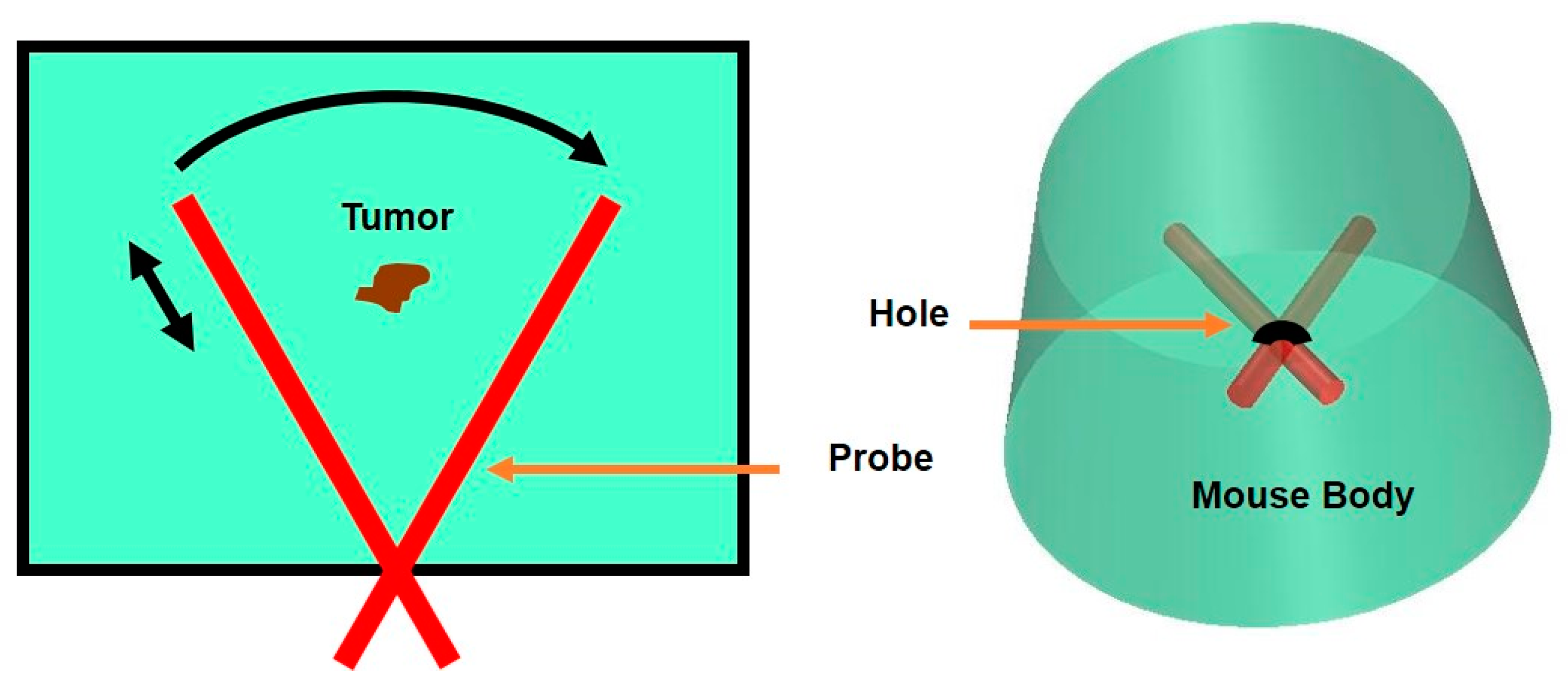
2.2. Miniaturized Endoscopic Imaging System and Scanning Mechanism
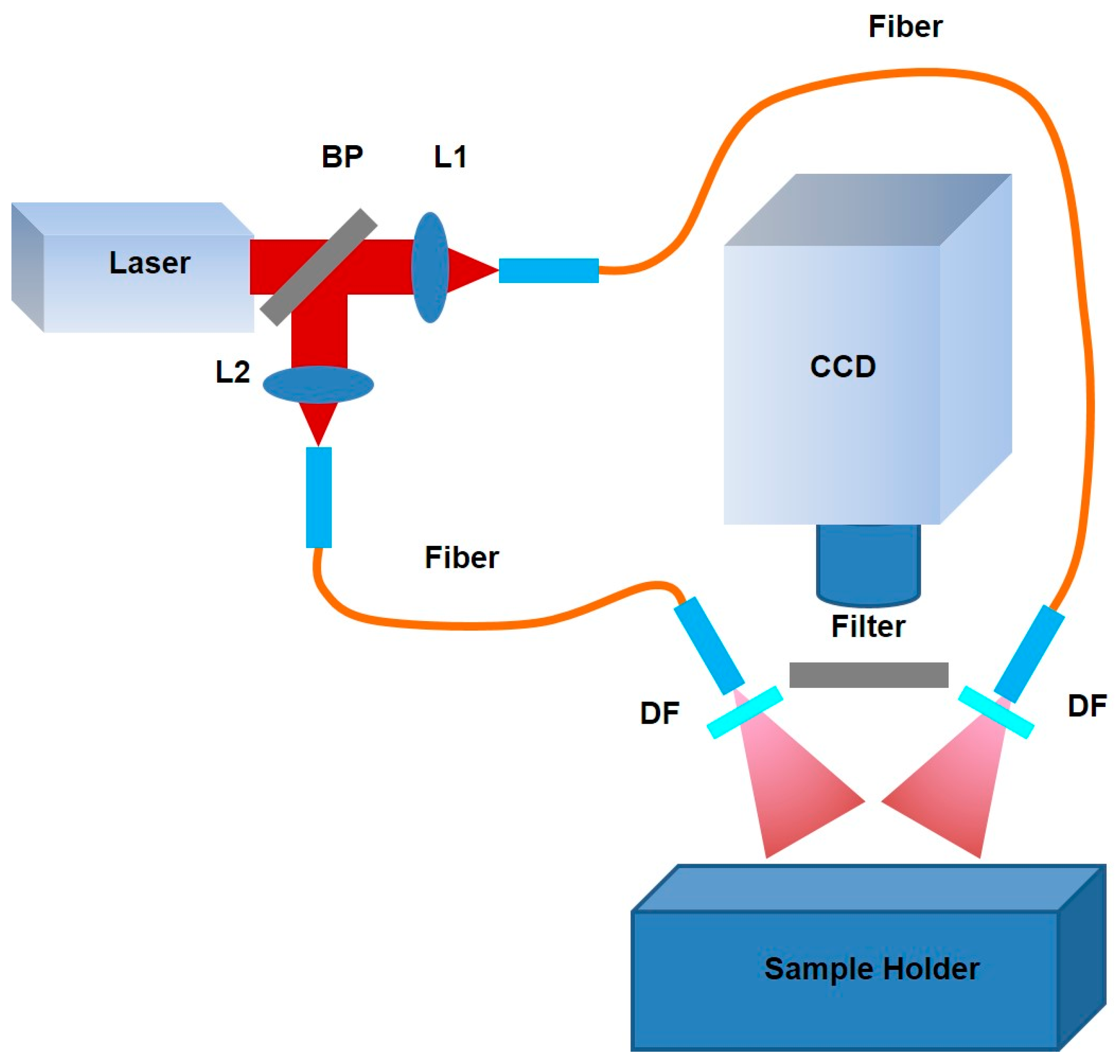
2.3. Near-Infrared Planar Fluorescence Imaging System
2.4. Image Processing
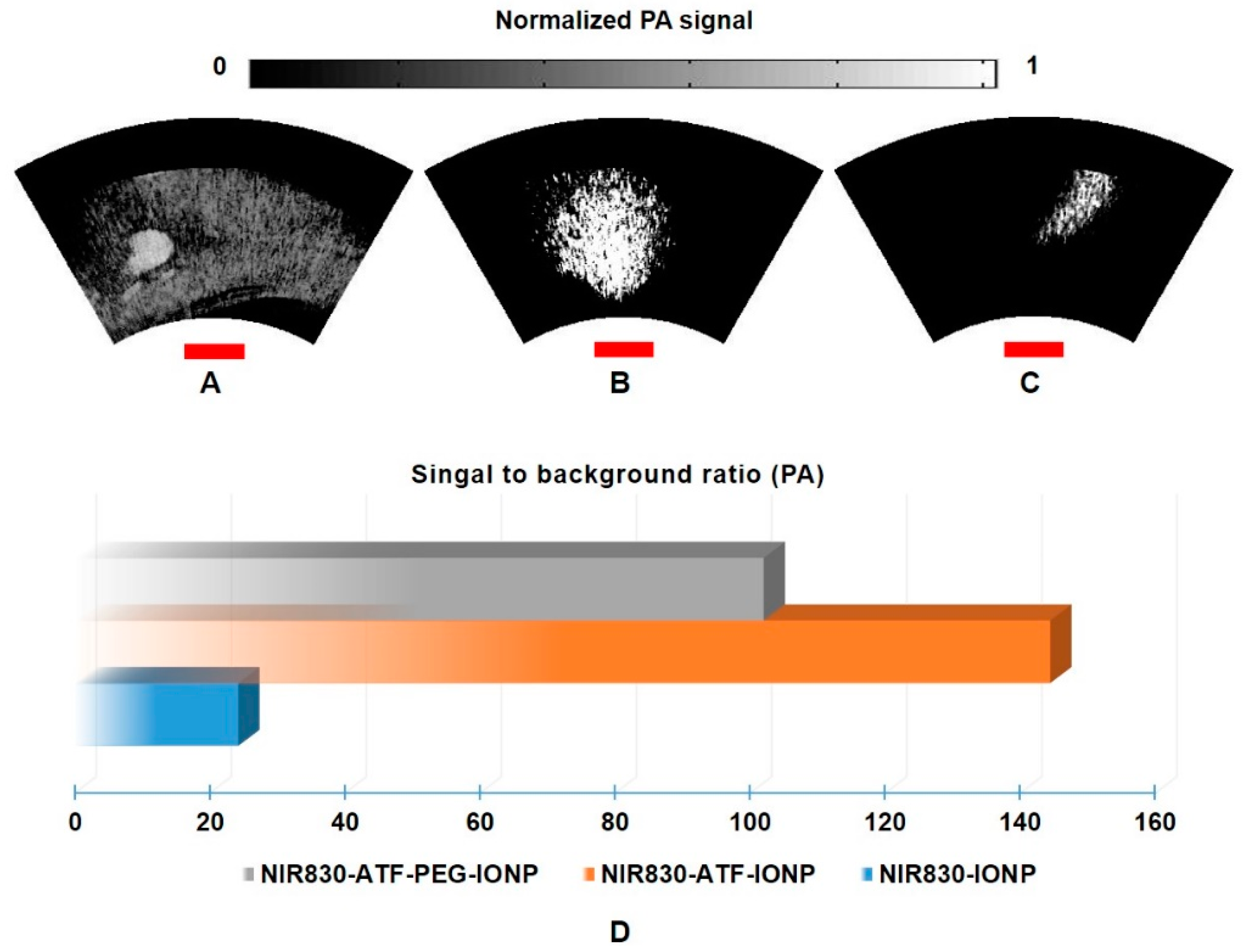
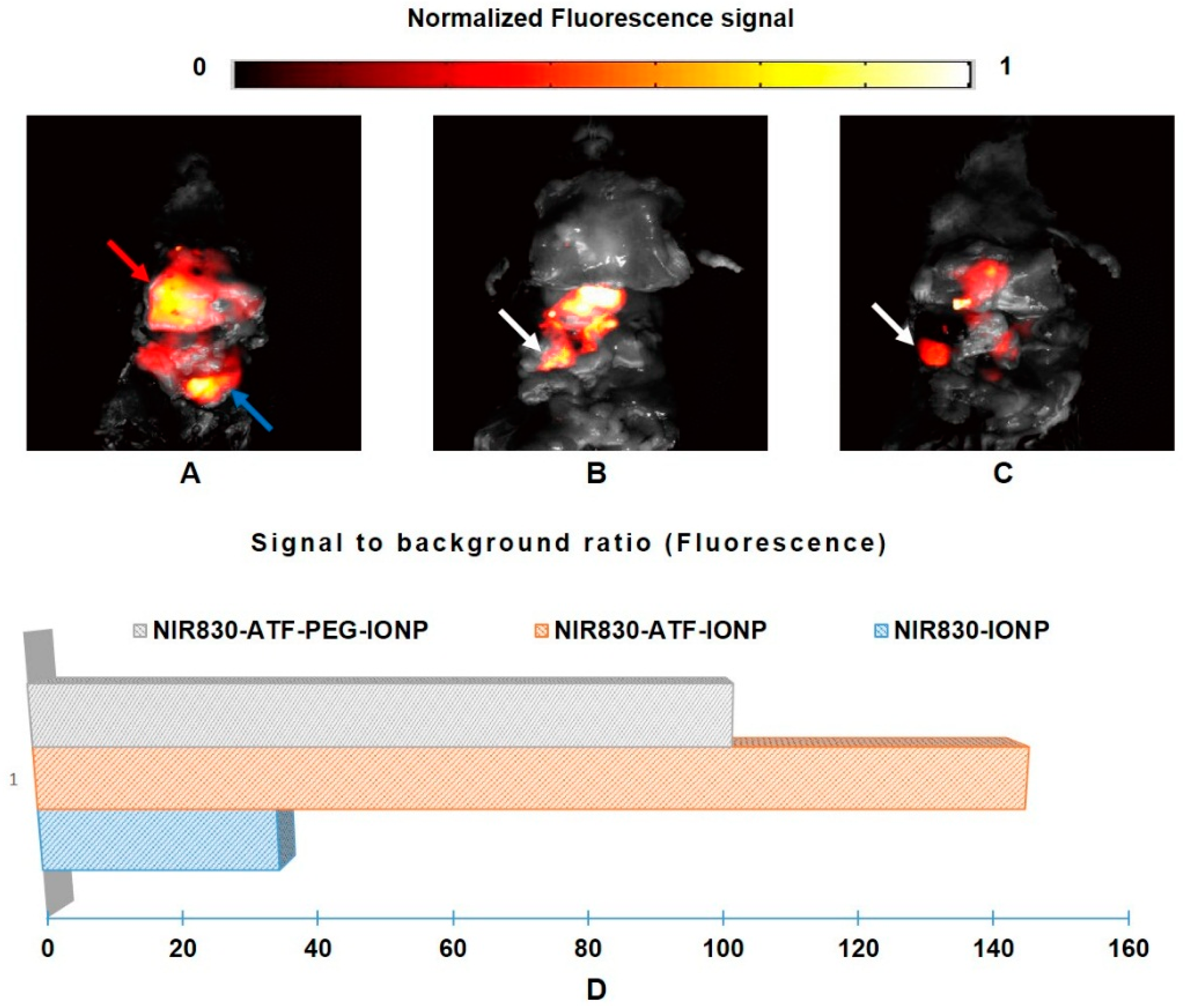
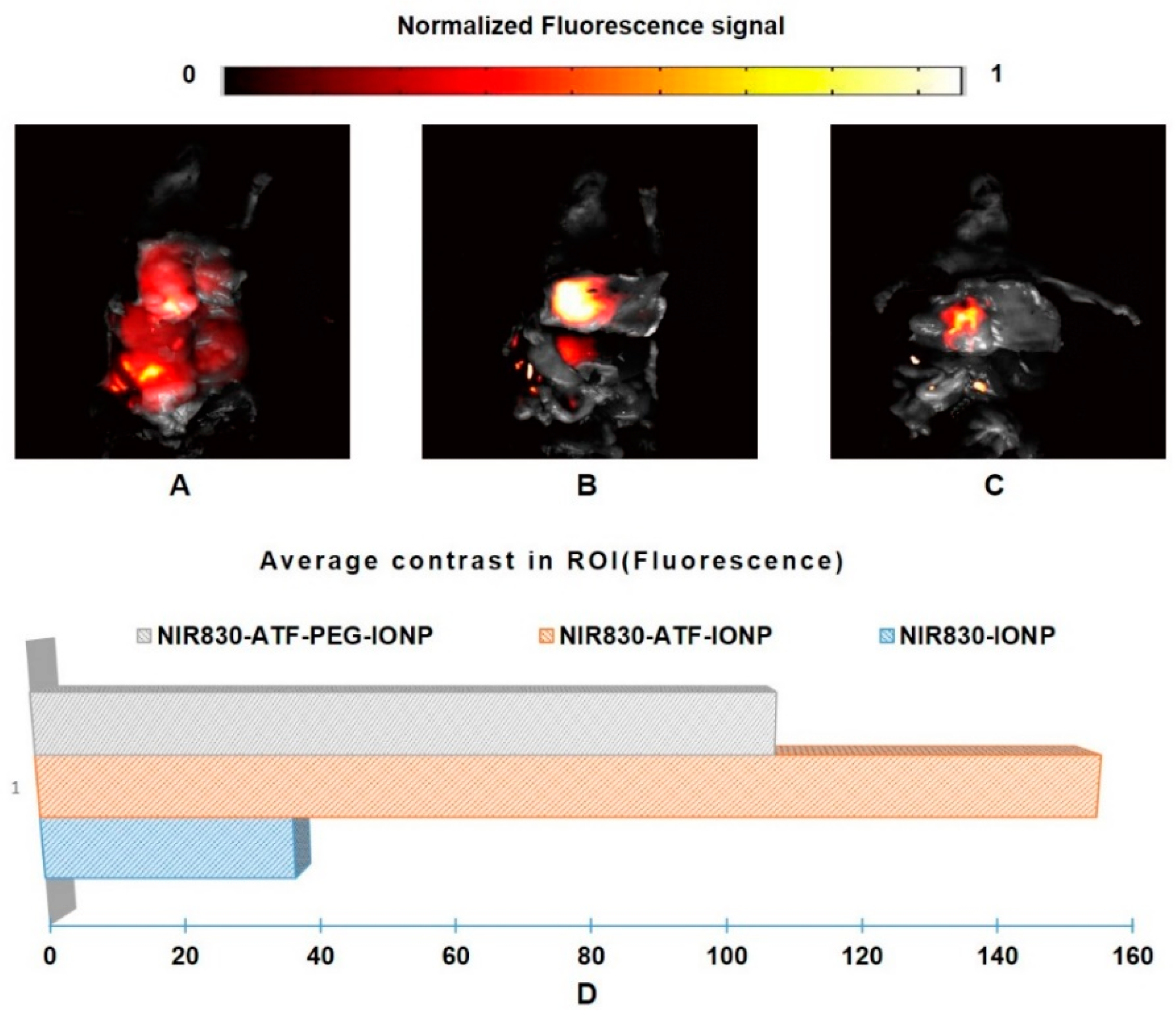
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, S.; Xiong, H.; Jing, X.; Xie, Z.; Chen, X.; Huang, Y. Electrospun pla/mwcnts composite nanofibers for combined chemo-and photothermal therapy. Acta Biomater. 2015, 26, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Dimastromatteo, J.; Brentnall, T.; Kelly, K.A. Imaging in pancreatic disease. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Bares, R.; Klever, P.; Hauptmann, S.; Hellwig, D.; Fass, J.; Cremerius, U.; Schumpelick, V.; Mittermayer, C.; Büll, U. F-18 fluorodeoxyglucose pet in vivo evaluation of pancreatic glucose metabolism for detection of pancreatic cancer. Radiology 1994, 192, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ruf, J.; Hänninen, E.L.; Oettle, H.; Plotkin, M.; Pelzer, U.; Stroszczynski, C.; Felix, R.; Amthauer, H. Detection of recurrent pancreatic cancer: Comparison of fdg-pet with ct/mri. Pancreatology 2005, 5, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Strobel, K.; Heinrich, S.; Bhure, U.; Soyka, J.; Veit-Haibach, P.; Pestalozzi, B.C.; Clavien, P.-A.; Hany, T.F. Contrast-enhanced 18F-FDG PET/CT: 1-stop-shop imaging for assessing the resectability of pancreatic cancer. J. Nucl. Med. 2008, 49, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Serrano, O.K.; Chaudhry, M.A.; Leach, S.D. The role of pet scanning in pancreatic cancer. Adv. Surg. 2010, 44, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, M.; Hayakawa, T.; Mutoh, M.; Imai, T.; Tsuda, K.; Kimura, S.; Umeda, I.O.; Fujii, H.; Wakabayashi, K. In vivo SPECT imaging with 111In-DOTA-c (RGDFK) to detect early pancreatic cancer in a hamster pancreatic carcinogenesis model. J. Nucl. Med. 2012, 53, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, H.; Ma, T.; Sun, X.; Shi, J.; Jia, B.; Sun, Y.; Zhan, J.; Zhang, H.; Zhu, Z. Integrin αvβ6–targeted SPECT imaging for pancreatic cancer detection. J. Nucl. Med. 2014, 55, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Adamek, H.E.; Albert, J.; Breer, H.; Weitz, M.; Schilling, D.; Riemann, J.F. Pancreatic cancer detection with magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography: A prospective controlled study. Lancet 2000, 356, 190–193. [Google Scholar] [CrossRef]
- Romijn, M.G.; Stoker, J.; van Eijck, C.H.; van Muiswinkel, J.M.; Torres, C.G.; Laméris, J.S. Mri with mangafodipir trisodium in the detection and staging of pancreatic cancer. J. Magn. Reson. Imaging 2000, 12, 261–268. [Google Scholar] [CrossRef]
- Bipat, S.; Phoa, S.S.S.; van Delden, O.M.; Bossuyt, P.M.; Gouma, D.J.; Laméris, J.S.; Stoker, J. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: A meta-analysis. J. Comput. Assist. Tomogr. 2005, 29, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Mao, H.; Cao, Z.; Wang, Y.A.; Peng, X.; Wang, X.; Sajja, H.K.; Wang, L.; Duan, H.; Ni, C. Molecular imaging of pancreatic cancer in an animal model using targeted multifunctional nanoparticles. Gastroenterology 2009, 136, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Qian, W.P.; Wang, L.; Wang, Y.A.; Staley, C.A.; Satpathy, M.; Nie, S.; Mao, H.; Yang, L. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and mri of pancreatic cancer. ACS Nano 2013, 7, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, M.; Wang, J.; Nardin, S.R.; Nassirpour, R.; Yang, M.; Baranov, E.; Jiang, P.; Moossa, A.; Hoffman, R.M. Real-time optical imaging of primary tumor growth and multiple metastatic events in a pancreatic cancer orthotopic model. Cancer Res. 2002, 62, 1534–1540. [Google Scholar] [PubMed]
- Katz, M.H.; Takimoto, S.; Spivack, D.; Moossa, A.; Hoffman, R.M.; Bouvet, M. An imageable highly metastatic orthotopic red fluorescent protein model of pancreatic cancer. Clin. Exp. Metastasis 2004, 21, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Homan, K.A.; Souza, M.; Truby, R.; Luke, G.P.; Green, C.; Vreeland, E.; Emelianov, S. Silver nanoplate contrast agents for in vivo molecular photoacoustic imaging. ACS Nano 2012, 6, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.E.; Wang, T.Y.; Willmann, J.K. Acoustic and photoacoustic molecular imaging of cancer. J. Nucl. Med. 2013, 54, 1851–1854. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.V.; Huang, J.S.; Yin, W.; Albeituni, S.; Rush, J.; Khanal, A.; Yan, J.; Ceresa, B.P.; Frieboes, H.B.; McNally, L.R. Targeted noninvasive imaging of egfr-expressing orthotopic pancreatic cancer using multispectral optoacoustic tomography. Cancer Res. 2014, 74, 6271–6279. [Google Scholar] [CrossRef] [PubMed]
- England, C.G.; Hernandez, R.; Eddine, S.B.Z.; Cai, W. Molecular imaging of pancreatic cancer with antibodies. Mol. Pharm. 2015, 13, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V.; Razansky, D. Molecular imaging by means of multispectral optoacoustic tomography (MSOT). Chem. Rev. 2010, 110, 2783–2794. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wang, B.; Ji, L.; Jiang, H. 4-D photoacoustic tomography. Sci. Rep. 2013, 3, 1113. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Chen, X. Structural and functional photoacoustic molecular tomography aided by emerging contrast agents. Chem. Soc. Rev. 2014, 43, 7132–7170. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Ahmad, M.; Hu, X.; Cheng, Z.; Xing, L. Label-free photoacoustic cell-tracking in real-time. X Acoust. Imaging Sens. 2014, 1, 18–22. [Google Scholar] [CrossRef]
- Qin, H.; Zhou, T.; Yang, S.; Xing, D. Fluorescence quenching nanoprobes dedicated to in vivo photoacoustic imaging and high-efficient tumor therapy in deep-seated tissue. Small 2015, 11, 2675–2686. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, H.; Yuan, Z. Two schemes for quantitative photoacoustic tomography based on monte carlo simulation. Med. Phys. 2016, 43, 3987–3997. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Dai, X.; Jiang, H. Wearable scanning photoacoustic brain imaging in behaving rats. J. Biophotonics 2016, 9, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Coleman, J.E.; Dai, X.; Jiang, H. Wearable 3-D photoacoustic tomography for functional brain imaging in behaving rats. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xi, L.; Samuelson, S.; Xie, H.; Yang, L.; Jiang, H. Handheld miniature probe integrating diffuse optical tomography with photoacoustic imaging through a mems scanning mirror. Biomed. Opt. Express 2013, 4, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Xi, L.; Duan, C.; Yang, H.; Xie, H.; Jiang, H. Miniature probe integrating optical-resolution photoacoustic microscopy, optical coherence tomography, and ultrasound imaging: Proof-of-concept. Opt. Lett. 2015, 40, 2921–2924. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Yang, H.; Jiang, H. In vivo photoacoustic imaging of vasculature with a low-cost miniature light emitting diode excitation. Opt. Lett. 2017, 42, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Grobmyer, S.R.; Zhou, G.; Qian, W.; Yang, L.; Jiang, H. Molecular photoacoustic tomography of breast cancer using receptor targeted magnetic iron oxide nanoparticles as contrast agents. J. Biophotonics 2014, 7, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Qian, W.; Cao, Z.; Wang, L.; Bozeman, E.N.; Ward, C.; Yang, B.; Selvaraj, P.; Lipowska, M.; Wang, Y.A. Theranostic nanoparticles carrying doxorubicin attenuate targeting ligand specific antibody responses following systemic delivery. Theranostics 2015, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Yang, H.; Shan, T.; Xie, H.; Berceli, S.A.; Jiang, H. Miniature endoscope for multimodal imaging. ACS Photonics 2017, 4, 174–180. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, X.; Qian, W.; Yang, H.; Yang, L.; Jiang, H. Targeted Molecular Imaging of Pancreatic Cancer with a Miniature Endoscope. Appl. Sci. 2017, 7, 1241. https://doi.org/10.3390/app7121241
Dai X, Qian W, Yang H, Yang L, Jiang H. Targeted Molecular Imaging of Pancreatic Cancer with a Miniature Endoscope. Applied Sciences. 2017; 7(12):1241. https://doi.org/10.3390/app7121241
Chicago/Turabian StyleDai, Xianjin, Weiping Qian, Hao Yang, Lily Yang, and Huabei Jiang. 2017. "Targeted Molecular Imaging of Pancreatic Cancer with a Miniature Endoscope" Applied Sciences 7, no. 12: 1241. https://doi.org/10.3390/app7121241




