Simultaneous Extraction, Enrichment and Removal of Dyes from Aqueous Solutions Using a Magnetic Aqueous Micellar Two-Phase System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Magnetic Nanoparticles
2.2.1. Preparation of Fe3O4@SiO2
2.2.2. Preparation of Fe3O4@NH2and Fe3O4@SiO2-NH2
2.3. Magnetic Solid-Phase Extraction
2.4. Aqueous Micellar Two-Phase Extraction
2.5. Magnetic Aqueous Micellar Two-Phase Extraction
2.6. Quantitative Determination of Dyes
2.7. Statistical Analysis
3. Results and Discussion
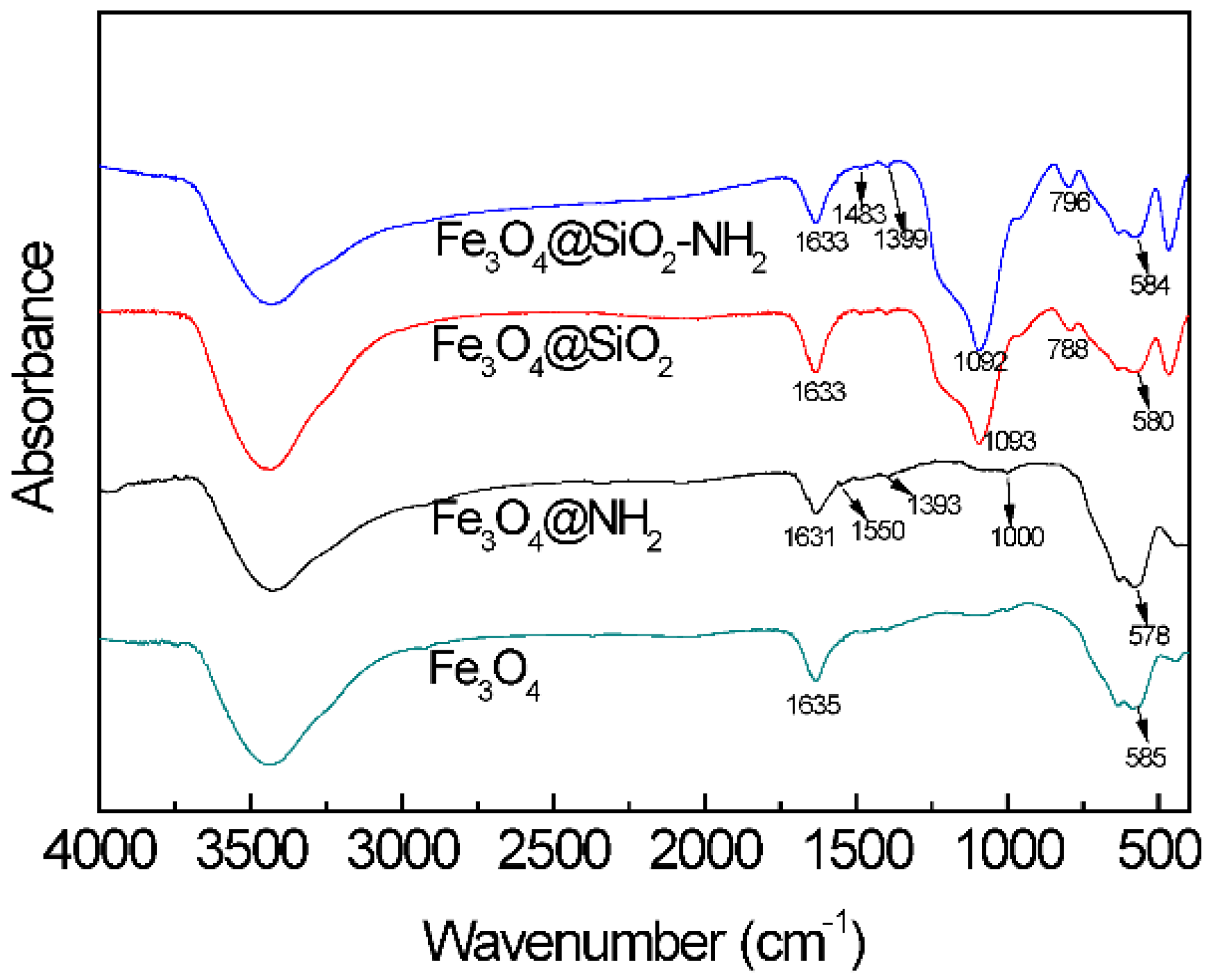
3.1. Characterization of the Magnetic Nanoparticles by Fourier-Transform Infrared Spectroscopy
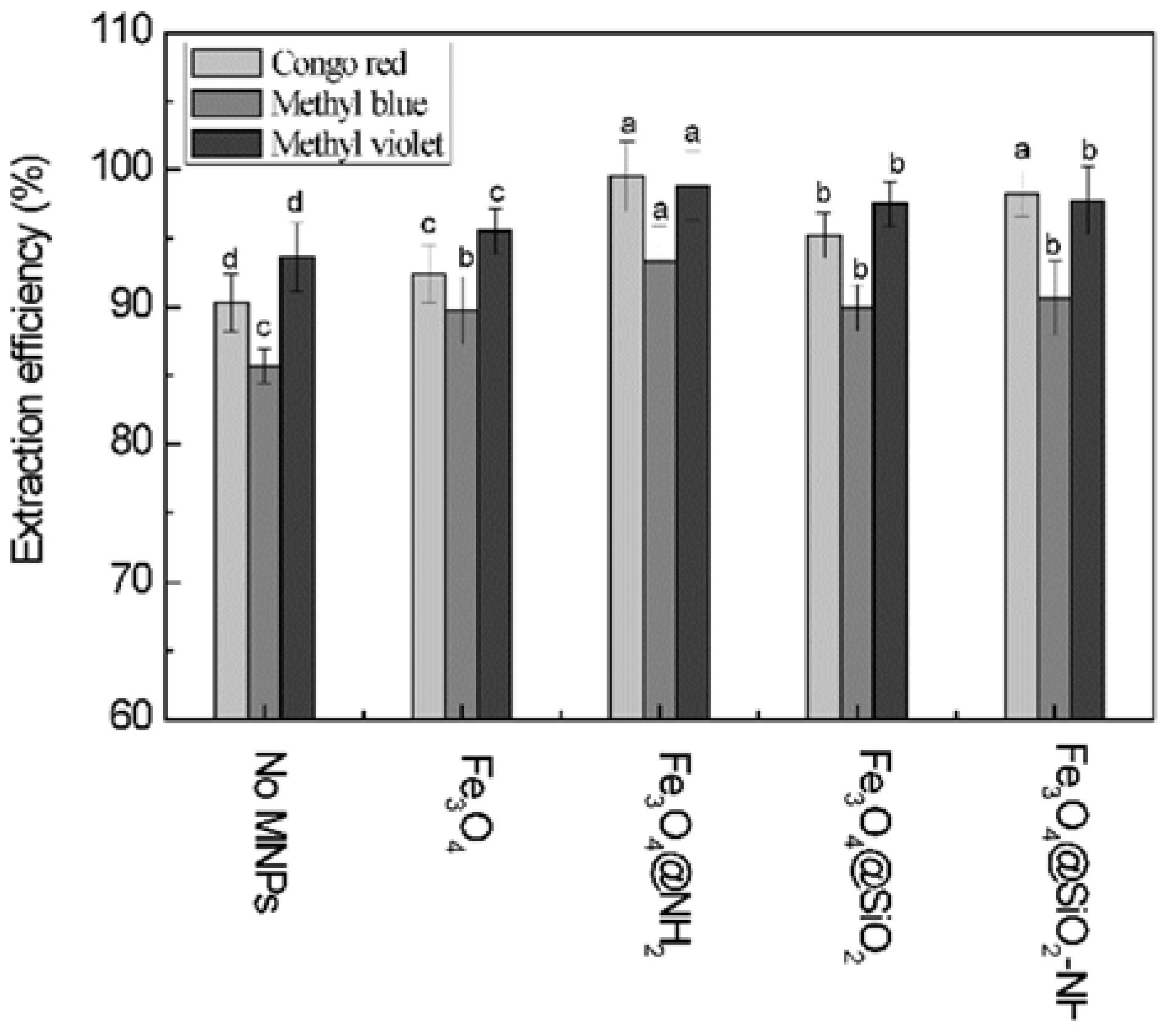
3.2. Selection of the Optimal Magnetic Nanoparticles
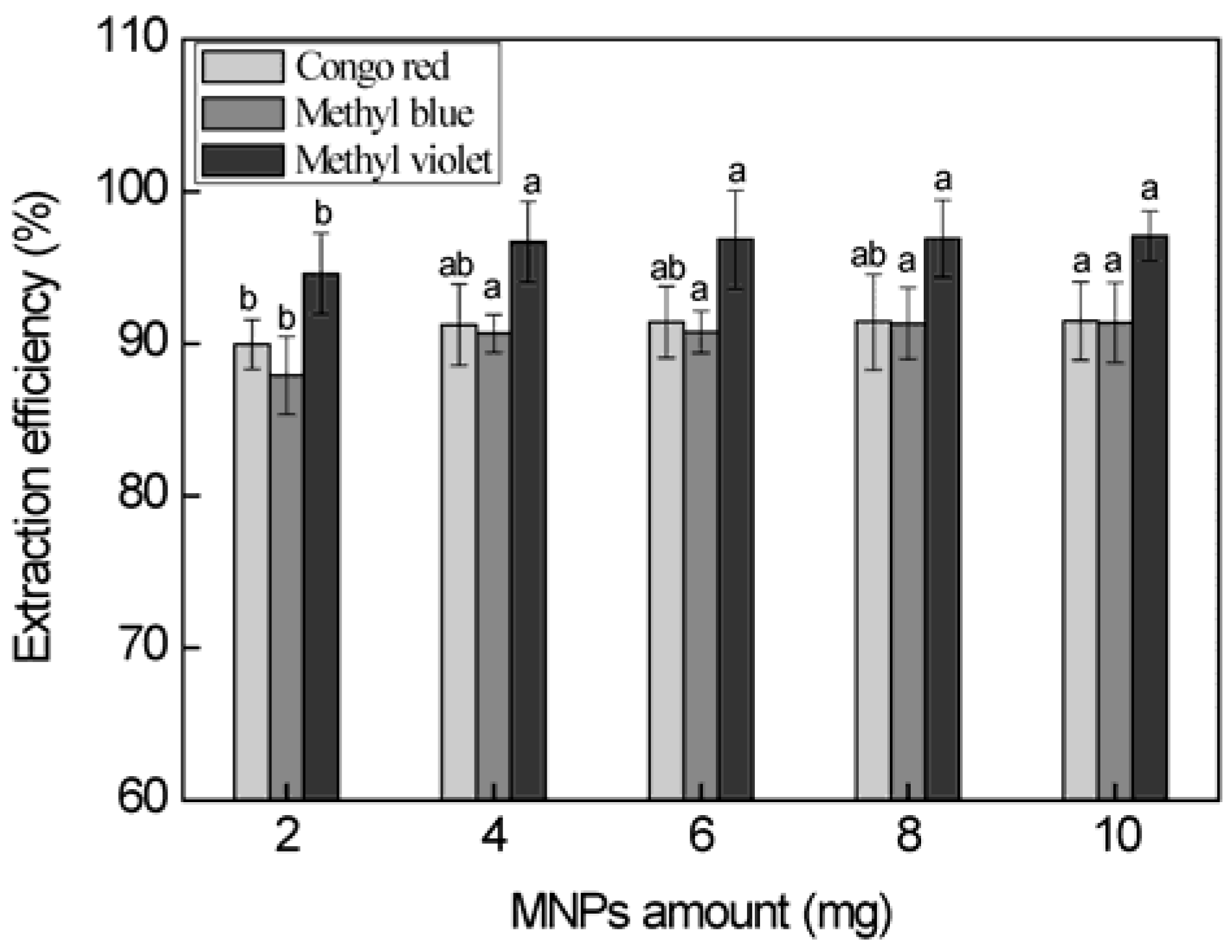
3.3. Effect of the Magnetic Nanoparticles Amount
3.4. Effect of the TX-114 Concentration
3.5. Effect of the Vibration Time
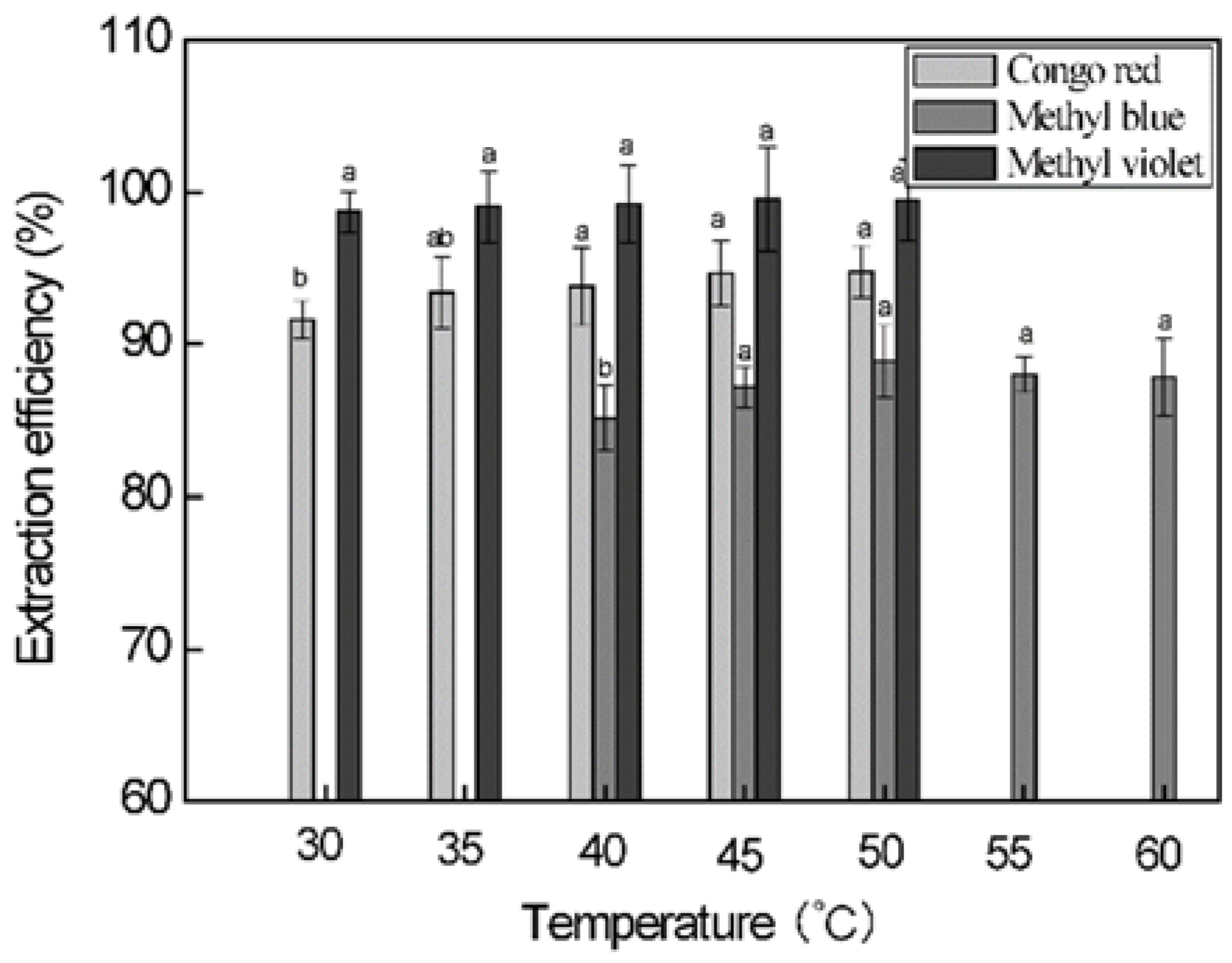
3.6. Effect of Extraction Temperature
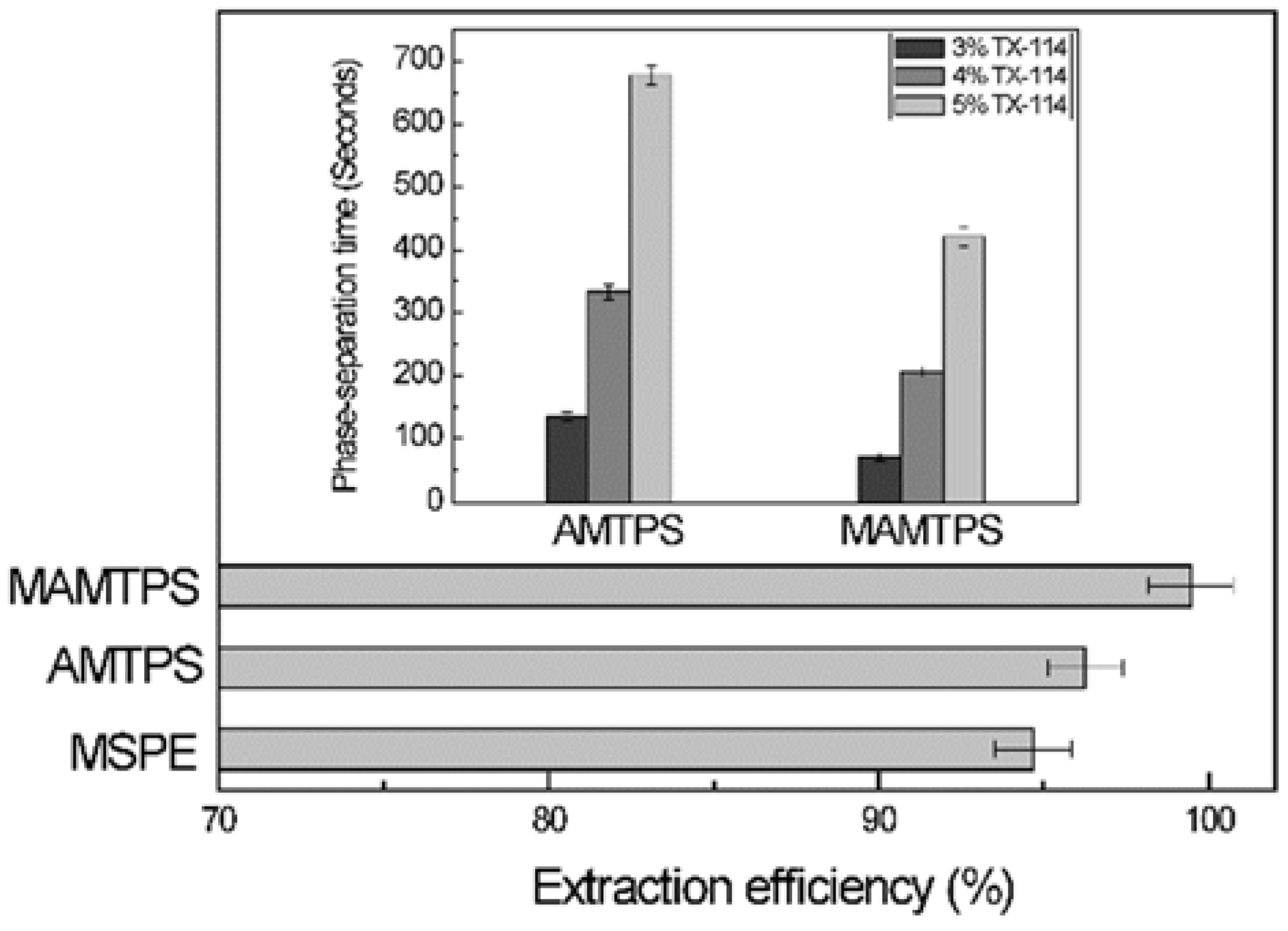
3.7. Comparison of Magnetic Aqueous Micellar Two-Phase System to Magnetic Solid Phase Extraction and Aqueous Micellar Two-Phase System
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Albertsson, P.A. Partition of Cell Particles and Macromolecules; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Tan, T.W.; Huo, Q.; Ling, Q. Purification of glycyrrhizin from Glycyrrhiza uralensis Fisch with ethanol/phosphate aqueous two-phase system. Biotechnol. Lett. 2002, 24, 1417–1420. [Google Scholar]
- Nikas, Y.; Liu, C.; Srivastava, T.; Abbott, N.; Blankschtein, D. Protein partitioning in two-phase aqueous nonionic micellar solutions. Macromolecules 1992, 25, 4797–4806. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the aqueous miscibility of ionic liquids: Aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J. Am. Chem. Soc. 2003, 125, 6632–6633. [Google Scholar] [CrossRef] [PubMed]
- Altunay, N.; Gurkan, R. A new cloud point extraction procedure for determination of inorganic antimony species in beverages and biological samples by flame atomic absorption spectrometry. Food Chem. 2015, 175, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, J.B.; Xu, X.Y.; Cheng, Z.H.; Zeng, P.; Wang, F.Q.; Zhang, Q. Application of mixed cloud point extraction for the analysis of six flavonoids in Apocynum venetum leaf samples by high performance liquid chromatography. J. Pharm.Biomed. 2015, 107, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H.; Han, J.; Wang, L.; Ni, L.; Wang, T.; Tang, X. A novel cyclic non-ligand dual-cloud point extraction for the preconcentration of Cadmium(II) through pH regulation in food and environmental matrices. New J. Chem. 2015, 39, 9116–9123. [Google Scholar] [CrossRef]
- Norseyrihan, M.; Noorashikin, M.; Adibah, M.; Yusoff, F. Cloud point extraction of methylphenol in water samples with low viscosity of non-ionic surfactant Sylgard 309 coupled with high-performance liquid chromatography. Sep. Sci. Technol. 2016, 51, 2386–2393. [Google Scholar] [CrossRef]
- Youcef, M.H.; Benabdallah, T.; Reffas, H. Cloud point extraction studies on recovery of Nickel (II) from highly saline sulfate medium using salicylideneaniline mono-schiff base chelating extractant. Sep. Purif. Technol. 2015, 149, 146–155. [Google Scholar] [CrossRef]
- Madej, K.; Sekiewicz, A.; Kalenik, T.K.; Piekoszewski, W. Cloud-point extraction followed by high pressure liquid chromatography with UV spectrophotometric detection for determination of permethrin in urine samples. Anal. Methods UK 2015, 7, 7758–7764. [Google Scholar] [CrossRef]
- Heidarizadi, E.; Tabaraki, R. Simultaneous spectrophotometric determination of synthetic dyes in food samples after cloud point extraction using multiple response optimizations. Talanta 2016, 148, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, J.; Liu, Y.; Wang, L.; Ni, L.; Tang, X. Recyclable non-ligand dual cloud point extraction method for determination of lead in food samples. Food Chem. 2016, 190, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.P.F.; Barros Neto, E.L.; Moura, M.C.P.A.; Castro Dantas, T.N.; Dantas Neto, A.A.; Oliveira, H.N.M. Removal of reactive Blue 19 using nonionic surfactant in cloud point extraction. Sep. Purif. Technol. 2014, 138, 71–76. [Google Scholar] [CrossRef]
- Tan, Z.J.; Li, F.F.; Xing, J.M. Cloud point extraction of aloe anthraquinones based on non-ionic surfactant aqueous two-phase system. Nat. Prod. Res. 2012, 26, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; He, M.; Peng, H.; Hu, B. Dithizone modified magnetic nanoparticles for fast and selective solid phase extraction of trace elements in environmental and biological samples prior to their determination by ICP-OES. Talanta 2012, 88, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Wierucka, M.; Biziuk, M. Application of magnetic nanoparticles for magnetic solid-phase extraction in preparing biological, environmental and food samples. TrAC-Trend Anal. Chem. 2014, 59, 50–58. [Google Scholar] [CrossRef]
- Gao, Q.; Luo, D.; Bai, M.; Chen, Z.-W.; Feng, Y.-Q. Rapid determination of estrogens in milk samples based on magnetite nanoparticles/polypyrrole magnetic solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 8543–8549. [Google Scholar] [CrossRef] [PubMed]
- Dhadge, V.L.; Rosa, S.A.S.L.; Azevedo, A.; Aires-Barros, R.; Roque, A.C.A. Magnetic aqueous two phase fishing: A hybrid process technology for antibody purification. J. Chromatogr. A 2014, 1339, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Fischer, I.; Hsu, C.-C.; Gartner, M.; Muller, C.; Overton, T.W.; Thomas, O.R.; Franzreb, M. Continuous protein purification using functionalized magnetic nanoparticles in aqueous micellar two-phase systems. J. Chromatogr. A. 2013, 1305, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Gai, Q.; Qu, F.; Zhang, T.; Zhang, Y. Integration of carboxyl modified magnetic particles and aqueous two-phase extraction for selective separation of proteins. Talanta 2011, 85, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Paulus, A.; Morhardt, C.; Lehle, N.; Franzreb, M. Recovery of chymotrypsin using magnetic particles and aqueous micellar two-phase systems: Influence of non-ionic surfactants on enzyme activity. J. Mol. Catal. B. Enzym. 2014, 110, 165–170. [Google Scholar] [CrossRef]
- Zhang, J.M.; Zhai, S.-R.; Zhai, B.; An, Q.D.; Tian, G. Crucial factors affecting the physicochemical properties of sol-gel produced Fe3O4@ SiO2-NH2 core-shell nanomaterials. J. Sol-Gel Sci. Technol. 2012, 64, 347–357. [Google Scholar] [CrossRef]
- Xie, G.; Xi, P.; Liu, H.; Chen, F.; Huang, L.; Shi, Y.; Hou, F.; Zeng, Z.; Shao, C.; Wang, J. A facile chemical method to produce superparamagnetic graphene oxide-Fe3O4 hybrid composite and its application in the removal of dyes from aqueous solution. J. Mater. Chem. 2012, 22, 1033–1039. [Google Scholar] [CrossRef]
- Zhang, S.; Du, B.; Li, H.; Xin, X.; Ma, H.; Wu, D.; Yan, L.; Wei, Q. Metal ions-based immunosensor for simultaneous determination of estradiol and diethylstilbestrol. Biosens. Bioelectron. 2014, 52, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Huo, Y.; Yang, N.; Yang, X.; Hao, T. Determination of cobalt in water by thermal lens spectrometry with cloud point extraction. Anal. Lett. 2015, 48, 2096–2106. [Google Scholar] [CrossRef]
- Altunay, N.; Gurkan, R. Determination of low levels of Cd (II) in cosmetic products by spectrophotometry after separation/preconcentration with cloud point extraction. Anal. Methods UK 2016, 8, 2673–2683. [Google Scholar] [CrossRef]
- Peng, G.; He, Q.; Zhou, G.; Li, Y.; Su, X.; Liu, M.; Fan, L. Determination of heavy metals in water samples using dual-cloud point extraction coupled with inductively coupled plasma mass spectrometry. Anal. Methods UK 2015, 7, 6732–6739. [Google Scholar] [CrossRef]
- Ghaedi, M.; Shokrollahi, A.; Ahmadi, F.; Rajabi, H.R.; Soylak, M. Cloud point extraction for the determination of copper, nickel and cobalt ions in environmental samples by flame atomic absorption spectrometry. J. Hazard. Mater. 2008, 150, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Niazi, A.; Ghasemi, J.; Yazdanipour, A. Simultaneous spectrophotometric determination of nitroaniline isomers after cloud point extraction by using least-squares support vector machines. Spectrochim. Acta A. 2007, 68, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.S.; Hu, B.; Jiang, Z.C.; Li, M.F. Cloud point extraction for speciation of chromium in water samples by electrothermal atomic absorption spectrometry. Water Res. 2005, 39, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Wikstrom, P.; Flygare, S.; Grondalen, A.; Larsson, P.-O. Magnetic aqueous two-phase separation: A new technique to increase rate of phase-separation, using dextran-ferrofluid or larger iron oxide particles. Anal. Biochem. 1987, 167, 331–339. [Google Scholar] [CrossRef]


| Samples | Standard Curves | Detection Wavelength (nm) | Linear Range (mg/mL) | Correlation Coefficient (R2) |
|---|---|---|---|---|
| Congo red | Y = 13.459X + 0.0398 a | 498 | 0.01–0.16 | 0.9994 |
| Methyl blue | Y = 24.708X − 0.0282 a | 577 | 0.01–0.16 | 0.9993 |
| Methyl violet | Y = 35.356X − 0.0261 a | 570 | 0.005–0.08 | 1.0000 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Sun, D.; Wang, C.; Yang, Y.; Li, F.; Tan, Z. Simultaneous Extraction, Enrichment and Removal of Dyes from Aqueous Solutions Using a Magnetic Aqueous Micellar Two-Phase System. Appl. Sci. 2017, 7, 1257. https://doi.org/10.3390/app7121257
Wu S, Sun D, Wang C, Yang Y, Li F, Tan Z. Simultaneous Extraction, Enrichment and Removal of Dyes from Aqueous Solutions Using a Magnetic Aqueous Micellar Two-Phase System. Applied Sciences. 2017; 7(12):1257. https://doi.org/10.3390/app7121257
Chicago/Turabian StyleWu, Shuanggen, Danyu Sun, Chaoyun Wang, Yuanru Yang, Fenfang Li, and Zhijian Tan. 2017. "Simultaneous Extraction, Enrichment and Removal of Dyes from Aqueous Solutions Using a Magnetic Aqueous Micellar Two-Phase System" Applied Sciences 7, no. 12: 1257. https://doi.org/10.3390/app7121257






