Synthesis and Properties of Shape Memory Poly(γ-Benzyl-l-Glutamate)-b-Poly(Propylene Glycol)-b-Poly(γ-Benzyl-l-Glutamate)
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
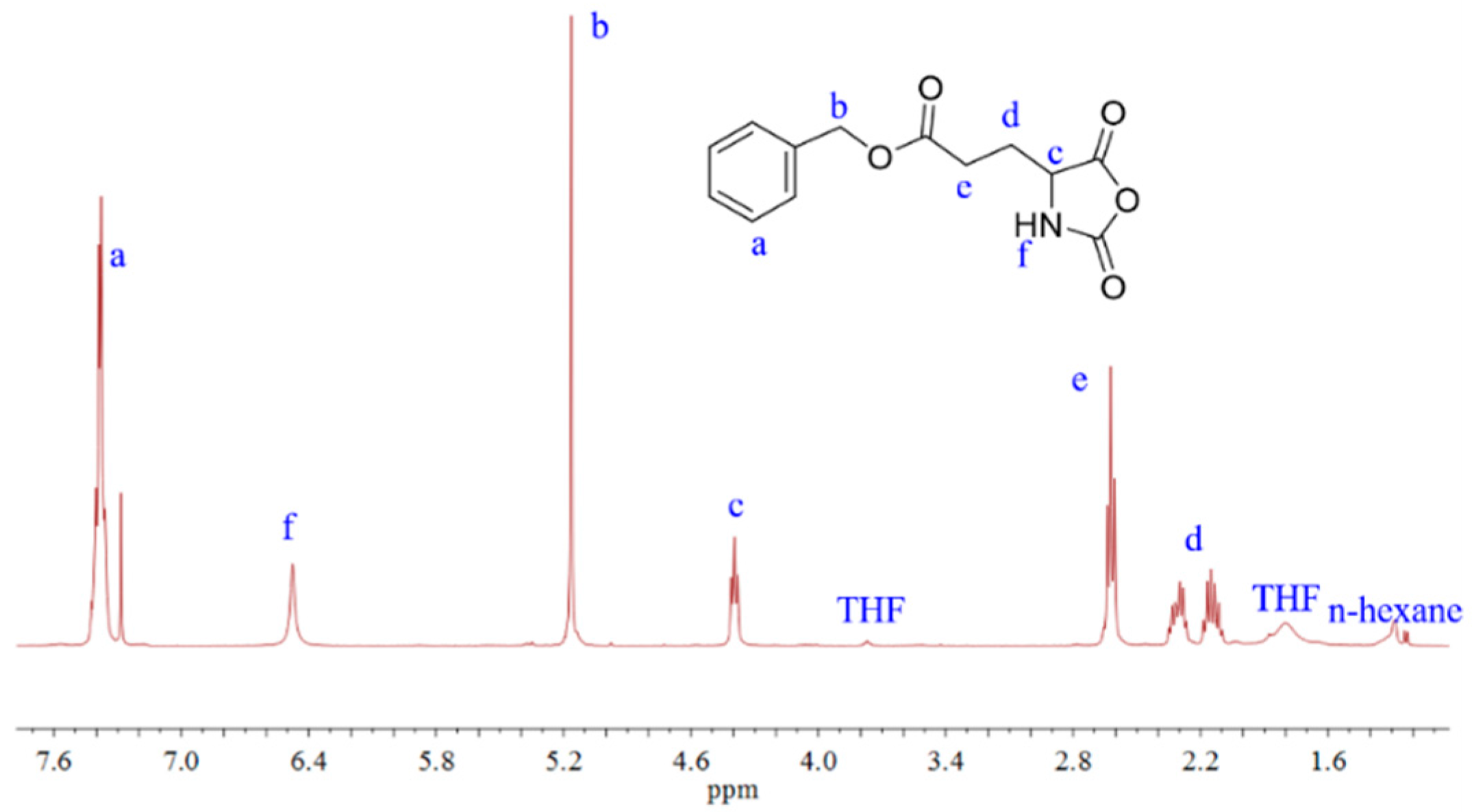
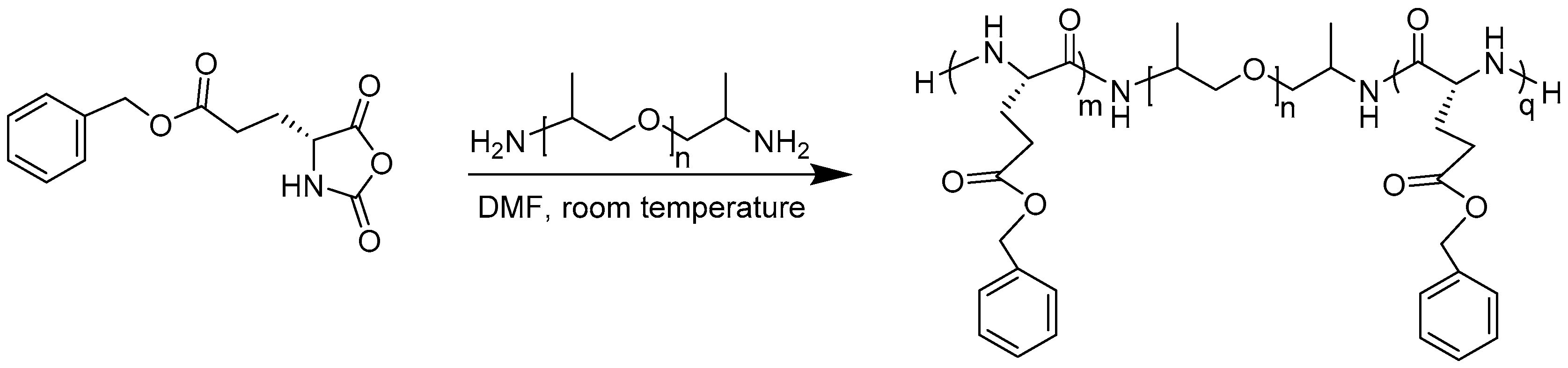
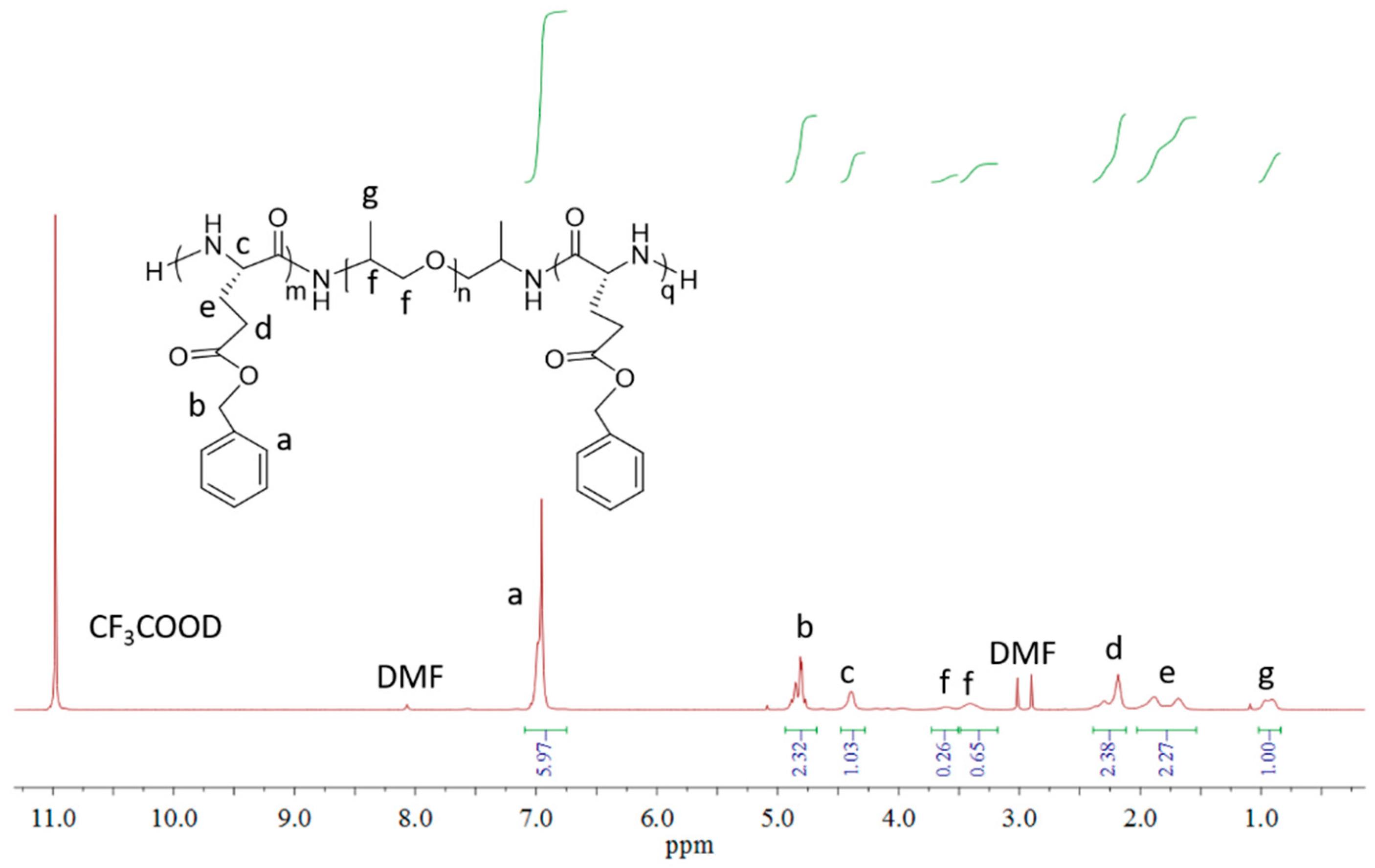
2.2. Preparation of PBLG-PPG-PBLG and Film
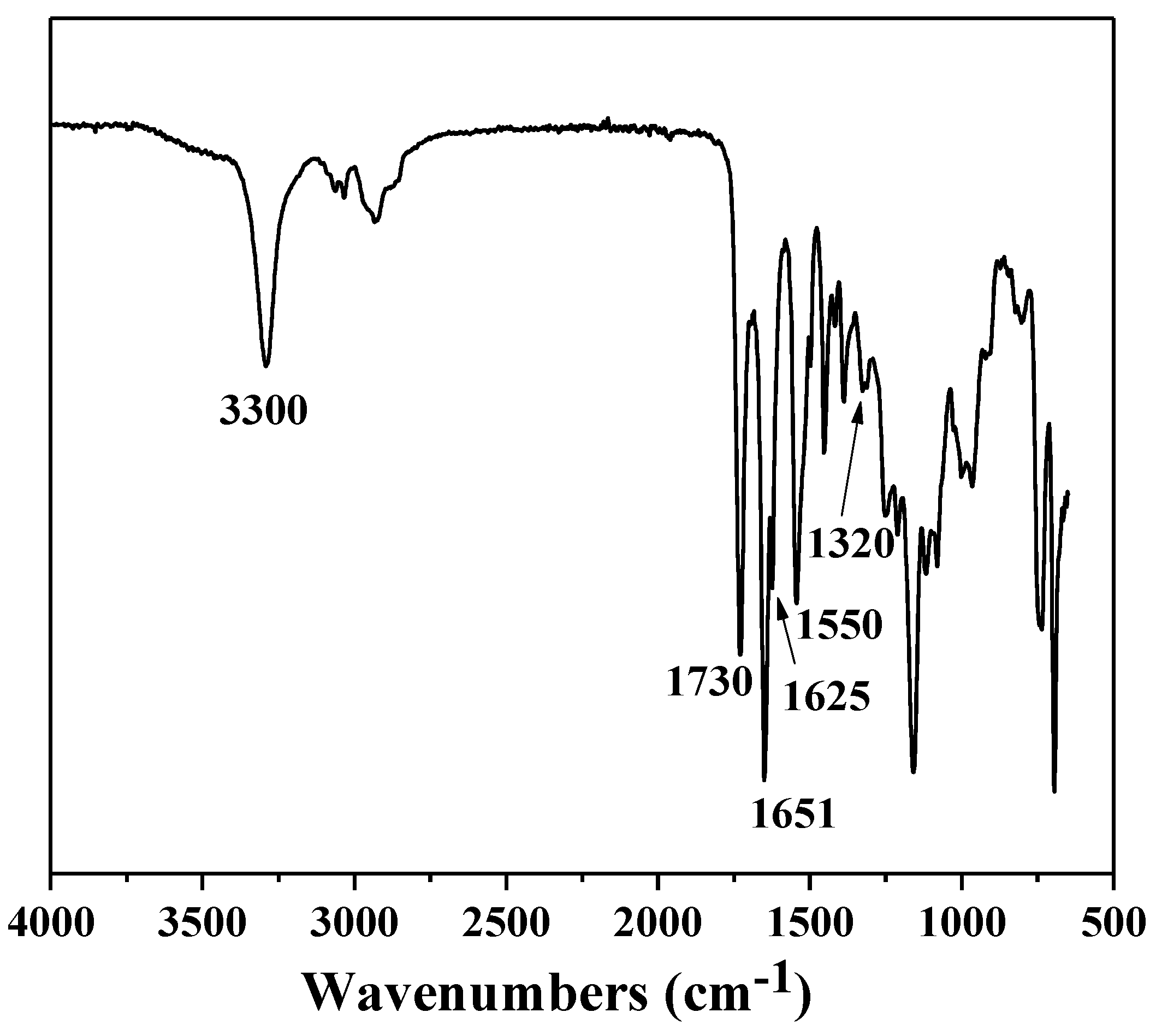
2.3. Characterization
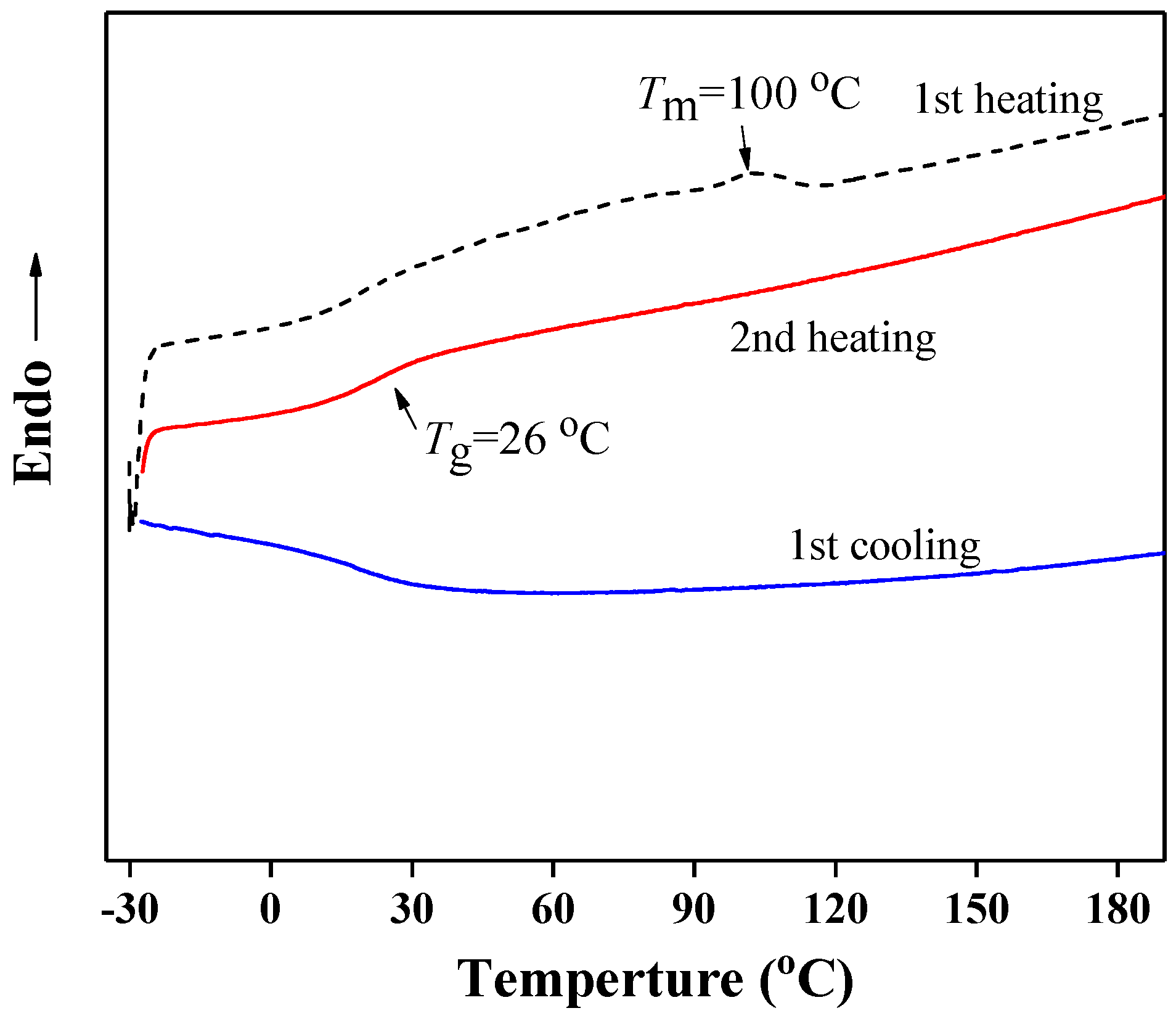
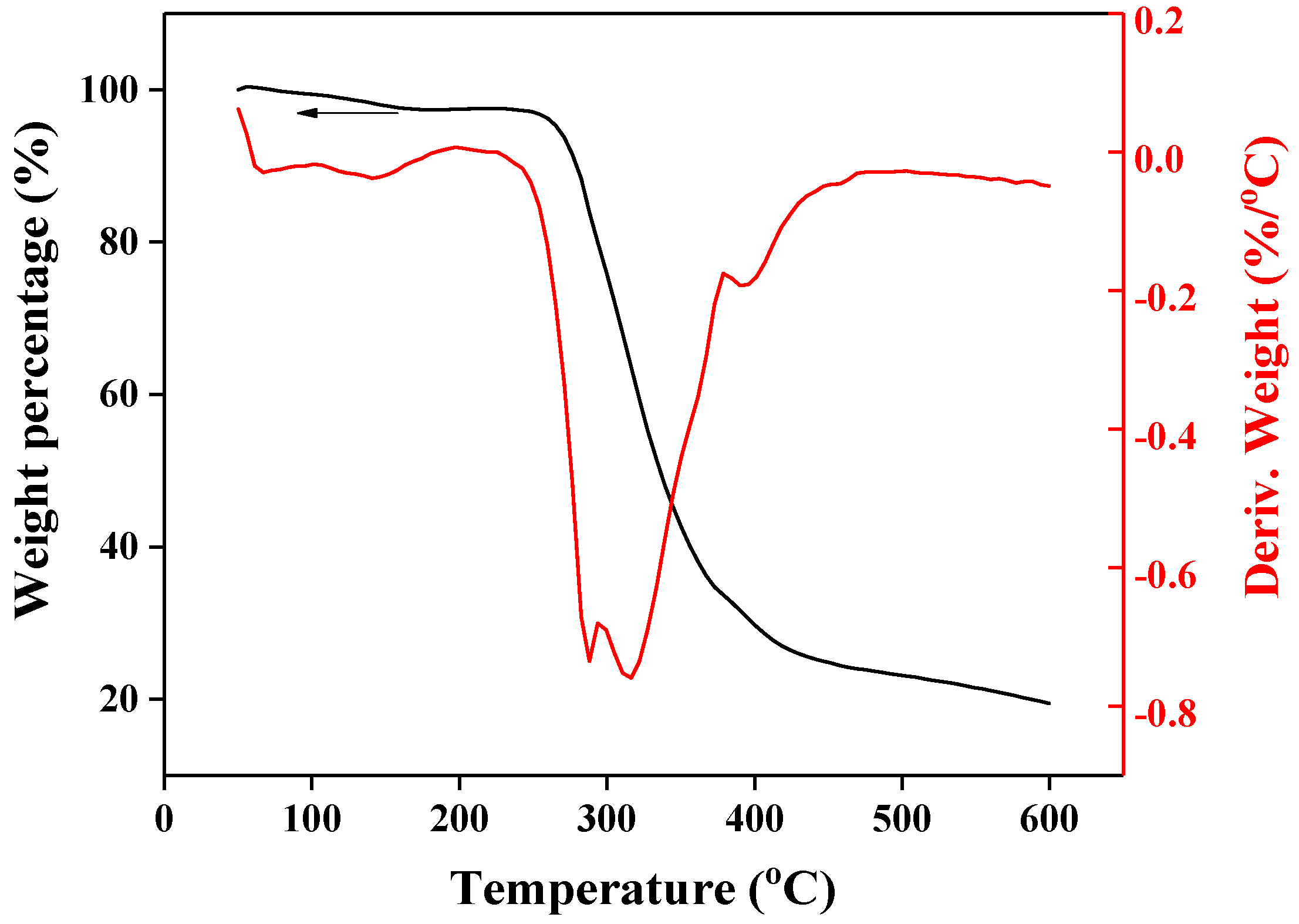
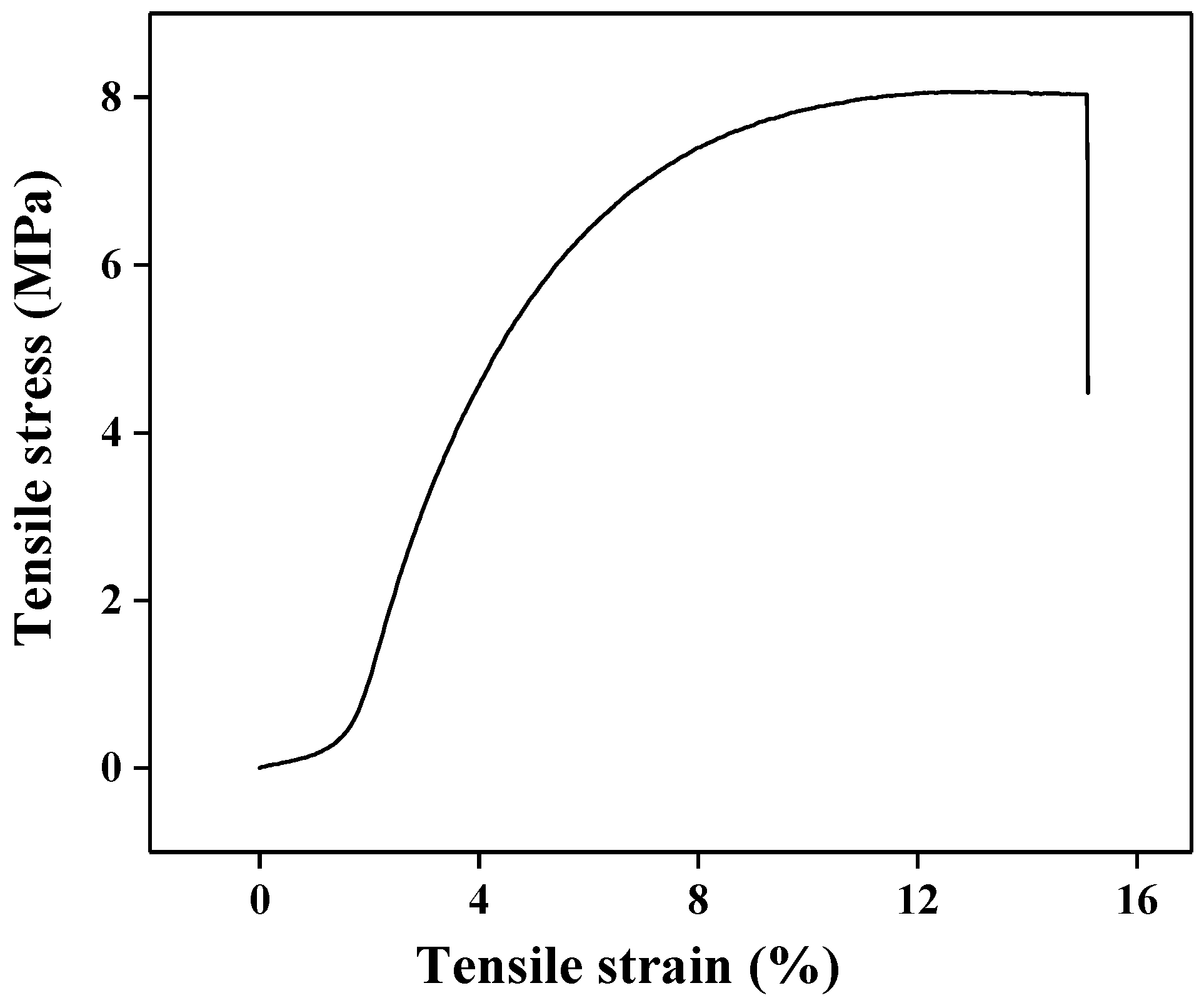
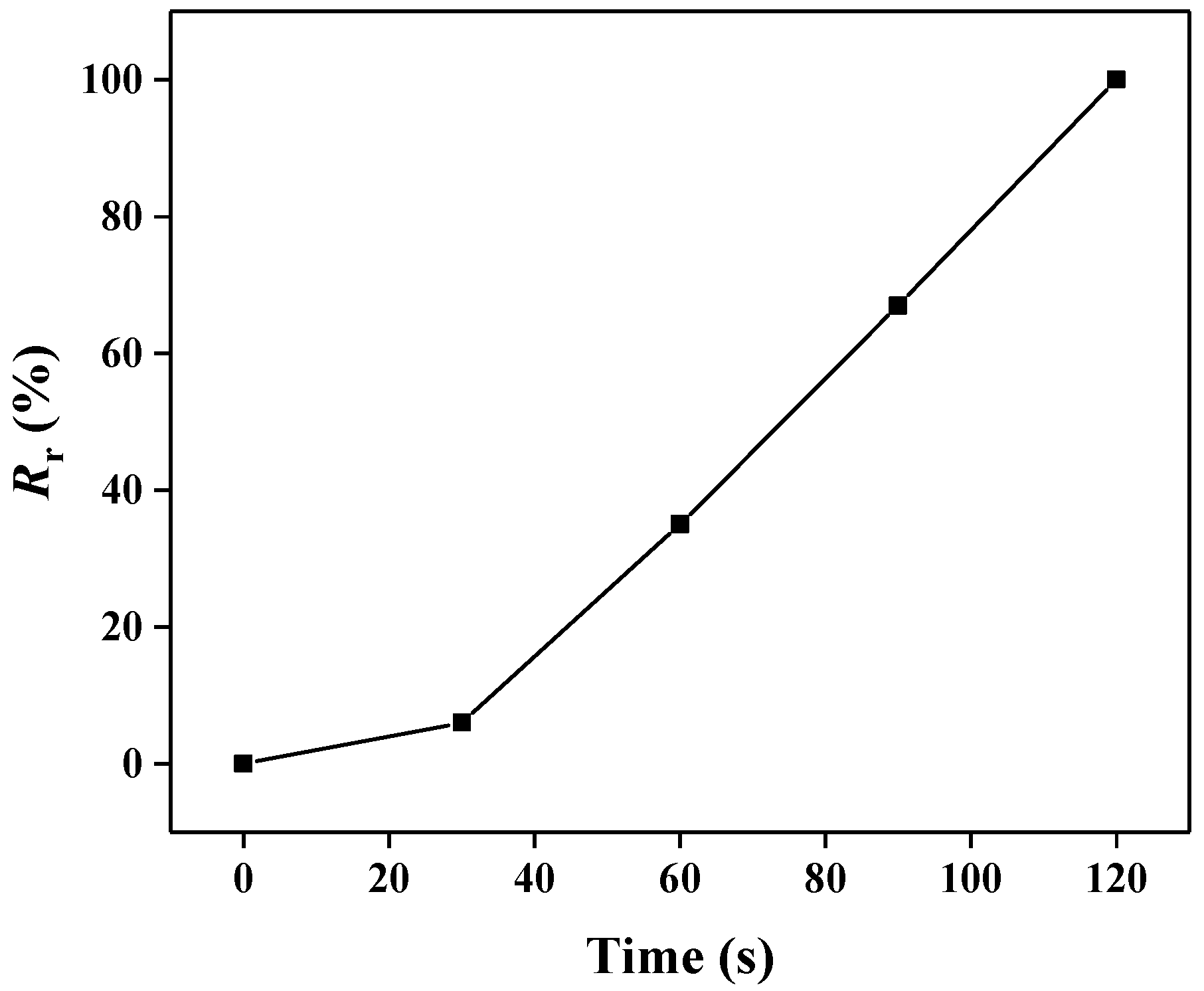
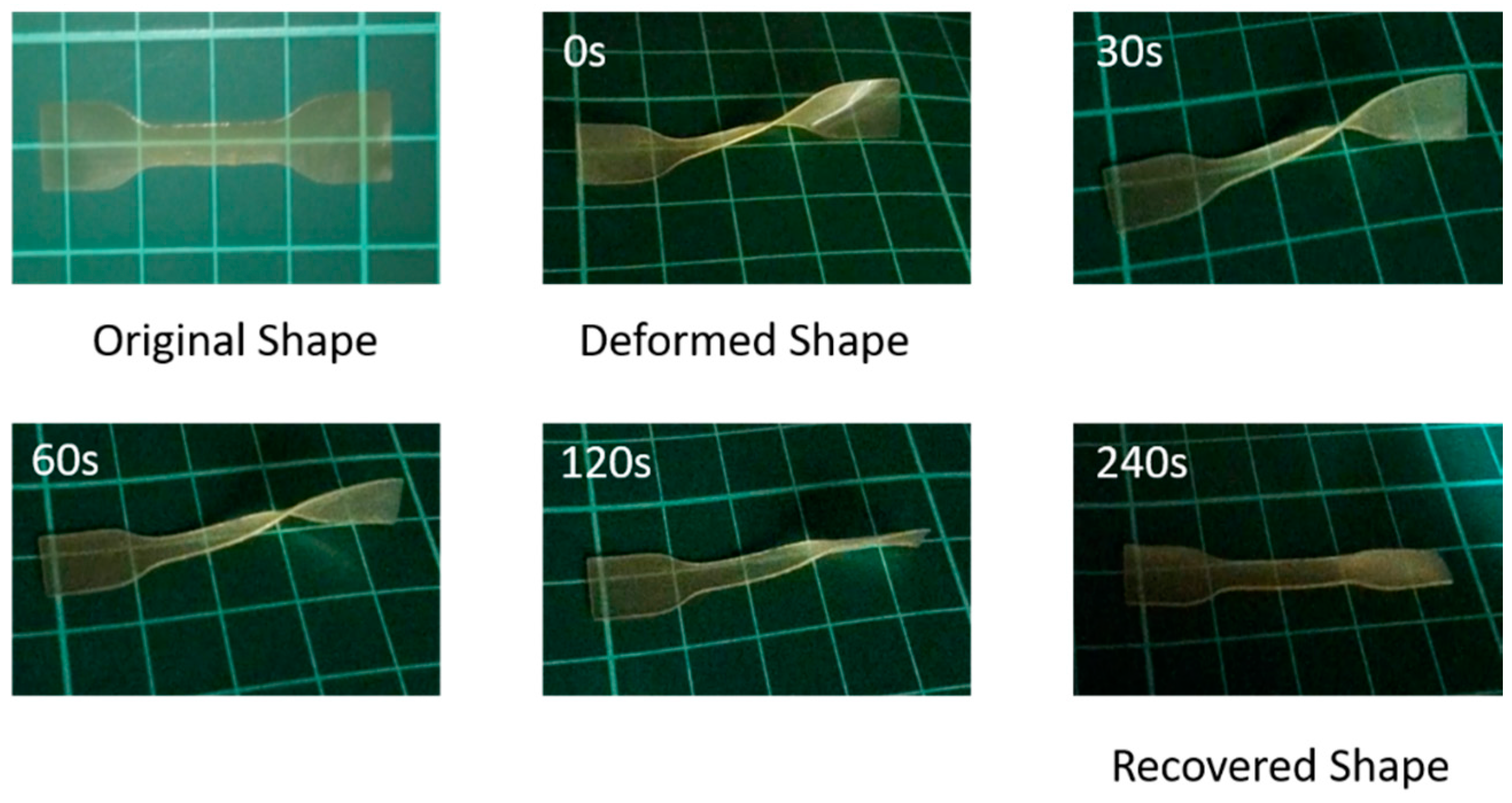
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xie, R.; Hu, J.; Ng, F.; Tan, L.; Qin, T.; Zhang, M.; Guo, X. High performance shape memory foams with isocyanate-modified hydroxyapatite nanoparticles for minimally invasive bone regeneration. Ceram. Int. 2017, 43, 4794–4802. [Google Scholar] [CrossRef]
- Gu, L.; Cui, B.; Wu, Q.-Y.; Yu, H. Bio-based polyurethanes with shape memory behavior at body temperature: Effect of different chain extenders. RSC Adv. 2016, 6, 17888–17895. [Google Scholar] [CrossRef]
- Shi, S.; Wu, Q.-Y.; Gu, L.; Zhang, K.; Yu, H. Bio-based (co)polylactide-urethane networks with shape memory behavior at body temperature. RSC Adv. 2016, 6, 79268–79274. [Google Scholar] [CrossRef]
- Chen, C.; Hu, J.; Huang, H.; Zhu, Y.; Qin, T. Design of a Smart Nerve Conduit Based on a Shape-Memory Polymer. Adv. Mater. Technol. 2016, 1, 1600015. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, J.; Zhang, C.; Han, J.; Wang, Y.; Kumar, B. A facile approach to fabricate a UV/heat dual-responsive triple shape memory polymer. J. Mater. Chem. A 2015, 3, 97–100. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, J.; Huang, H.; Li, J.; Zhu, Y.; Tang, B.; Han, J.; Li, L. Memory chromic polyurethane with tetraphenylethylene. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 104–110. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, J.; Han, J.; Zhu, Y.; Huang, H.; Li, J.; Tang, B. Two-way shape memory polymer with “switch–spring” composition by interpenetrating polymer network. J. Mater. Chem. A 2014, 2, 18816–18822. [Google Scholar] [CrossRef]
- Xiao, X.; Qiu, X.; Kong, D.; Zhang, W.; Liu, Y.; Leng, J. Optically transparent high temperature shape memory polymers. Soft Matter 2016, 12, 2894–2900. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qin, H.; Mather, P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543–1558. [Google Scholar] [CrossRef]
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape memory polymers: Past, present and future developments. Prog. Polym. Sci. 2015, 49–50, 3–33. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Kéki, S. Biodegradable polyester-based shape memory polymers: Concepts of (supra)molecular architecturing. Express Polym. Lett. 2014, 8, 397–412. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Hu, J.; Yeung, K. Effect of soft segment crystallization and hard segment physical crosslink on shape memory function in antibacterial segmented polyurethane ionomers. Acta Biomater. 2009, 5, 3346–3357. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, J.L.; Yeung, K.W.; Liu, Y.Q.; Liem, H.M. Influence of ionic groups on the crystallization and melting behavior of segmented polyurethane ionomers. J. Appl. Polym. Sci. 2006, 100, 4603–4613. [Google Scholar] [CrossRef]
- Cui, B.; Wu, Q.Y.; Gu, L.; Shen, L.; Yu, H.B. High performance bio-based polyurethane elastomers: Effect of different soft and hard segments. Chin. J. Polym. Sci. 2016, 34, 901–909. [Google Scholar] [CrossRef]
- Hu, J.; Chen, S. A review of actively moving polymers in textile applications. J. Mater. Chem. 2010, 20, 3346–3355. [Google Scholar] [CrossRef]
- Huang, H.; Hu, J.; Zhu, Y. Shape-Memory Biopolymers Based on β-Sheet Structures of Polyalanine Segments Inspired by Spider Silks. Macromol. Biosci. 2013, 13, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Deming, T.J. Synthetic polypeptides for biomedical applications. Prog. Polym. Sci. 2007, 32, 858–875. [Google Scholar] [CrossRef]
- Song, Z.; Han, Z.; Lv, S.; Chen, C.; Chen, L.; Yin, L.; Cheng, J. Synthetic polypeptides: From polymer design to supramolecular assembly and biomedical application. Chem. Soc. Rev. 2017, 46, 6570–6599. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Joo, M.K.; Sohn, Y.S.; Jeong, B. Significance of secondary structure in nanostructure formation and thermosensitivity of polypeptide block copolymers. Soft Matter 2008, 4, 2383–2387. [Google Scholar] [CrossRef]
- Lavilla, C.; Byrne, M.; Heise, A. Block-Sequence-Specific Polypeptides from α-Amino Acid N-Carboxyanhydrides: Synthesis and Influence on Polypeptide Properties. Macromolecules 2016, 49, 2942–2947. [Google Scholar] [CrossRef]
- He, X.; Fan, J.; Wooley, K.L. Stimuli-Triggered Sol-Gel Transitions of Polypeptides Derived from -alpha-Amino Acid N-Carboxyanhydride (NCA) Polymerizations. Chem. Asian J. 2016, 11, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Ma, Y.; Shen, Y.; Fu, W.; Li, Z. Oxidation-Responsive OEGylated Poly-l-cysteine and Solution Properties Studies. Biomacromolecules 2014, 15, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Heise, A. Stimuli responsive synthetic polypeptides derived from N-carboxyanhydride (NCA) polymerisation. Chem. Soc. Rev. 2013, 42, 7373–7390. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Shen, Y.; Fu, W.; Li, Z. Thermoresponsive Oligo(ethylene glycol) Functionalized Poly-l-cysteine. Macromolecules 2013, 46, 3753–3760. [Google Scholar] [CrossRef]
- Peterson, G.I.; Dobrynin, A.V.; Becker, M.L. Alpha-Amino Acid-Based Poly(Ester urea)s as Multishape Memory Polymers for Biomedical Applications. ACS Macro Lett. 2016, 5, 1176–1179. [Google Scholar] [CrossRef]
- Guo, A.-R.; Yang, W.-X.; Yang, F.; Yu, R.; Wu, Y.-X. Well-Defined Poly(γ-benzyl-l-glutamate)-g-Polytetrahydrofuran: Synthesis, Characterization, and Properties. Macromolecules 2014, 47, 5450–5461. [Google Scholar] [CrossRef]
- Lee, N.H.; Frank, C.W. Surface-Initiated Vapor Polymerization of Various α-Amino Acids. Langmuir 2003, 19, 1295–1303. [Google Scholar] [CrossRef]
- Kotharangannagari, V.K.; Sánchez-Ferrer, A.; Ruokolainen, J.; Mezzenga, R. Thermoreversible Gel–Sol Behavior of Rod–Coil–Rod Peptide-Based Triblock Copolymers. Macromolecules 2012, 45, 1982–1990. [Google Scholar] [CrossRef]
- Yang, W.-X.; Wang, L.-L.; Zhu, H.; Xu, R.-W.; Wu, Y.-X. Synthesis of poly(glutamic acid-co-aspartic acid) VIA combination of N-carboxyanhydride ring opening polymerization with debenzylation. Chin. J. Polym. Sci. 2013, 31, 1706–1716. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, A.; Mezzenga, R. Secondary Structure-Induced Micro- and Macrophase Separation in Rod-Coil Polypeptide Diblock, Triblock, and Star-Block Copolymers. Macromolecules 2010, 43, 1093–1100. [Google Scholar] [CrossRef]
- Wei, M.-J.; Guo, A.-R.; Wu, Y.-X. Microstructure and Micromorphology of Poly (γ-benzyl-l-glutamate)-g-(Polytetrahydrofuran-b-Polyisobutylene) Copolymer. Acta Polym. Sin. 2017, 3, 506–515. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Jiang, Y.; Hu, J. Synthesis and Properties of Shape Memory Poly(γ-Benzyl-l-Glutamate)-b-Poly(Propylene Glycol)-b-Poly(γ-Benzyl-l-Glutamate). Appl. Sci. 2017, 7, 1258. https://doi.org/10.3390/app7121258
Gu L, Jiang Y, Hu J. Synthesis and Properties of Shape Memory Poly(γ-Benzyl-l-Glutamate)-b-Poly(Propylene Glycol)-b-Poly(γ-Benzyl-l-Glutamate). Applied Sciences. 2017; 7(12):1258. https://doi.org/10.3390/app7121258
Chicago/Turabian StyleGu, Lin, Yuanzhang Jiang, and Jinlian Hu. 2017. "Synthesis and Properties of Shape Memory Poly(γ-Benzyl-l-Glutamate)-b-Poly(Propylene Glycol)-b-Poly(γ-Benzyl-l-Glutamate)" Applied Sciences 7, no. 12: 1258. https://doi.org/10.3390/app7121258





