Nanostructured Ceramic Photocatalytic Membrane Modified with a Polymer Template for Textile Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Fabrication
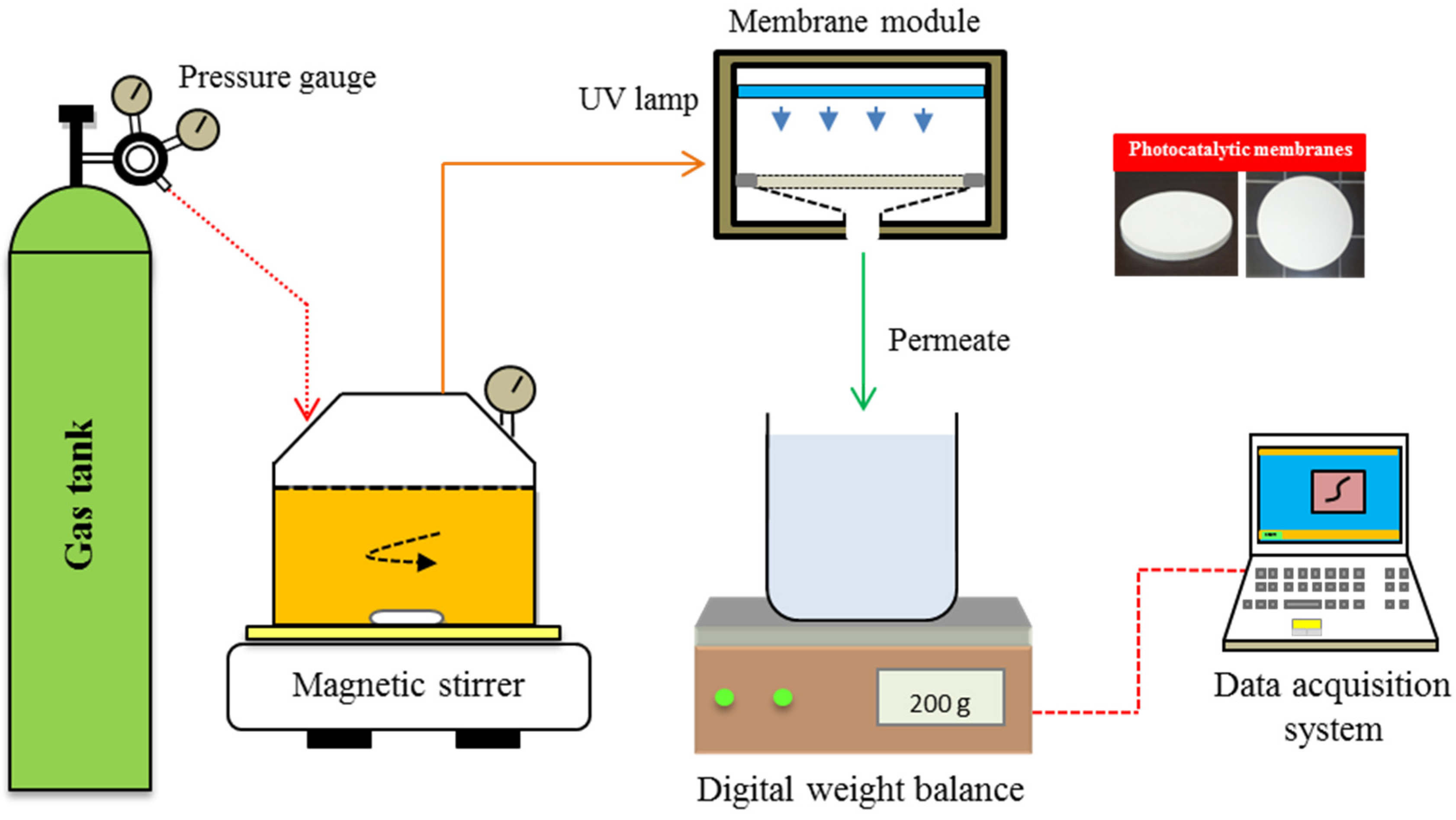
2.3. Experimental Set-Up
2.4. Photocatalytic Performance of the Membranes
2.5. Analytical Methods for Membrane Characterization
3. Results and Discussion
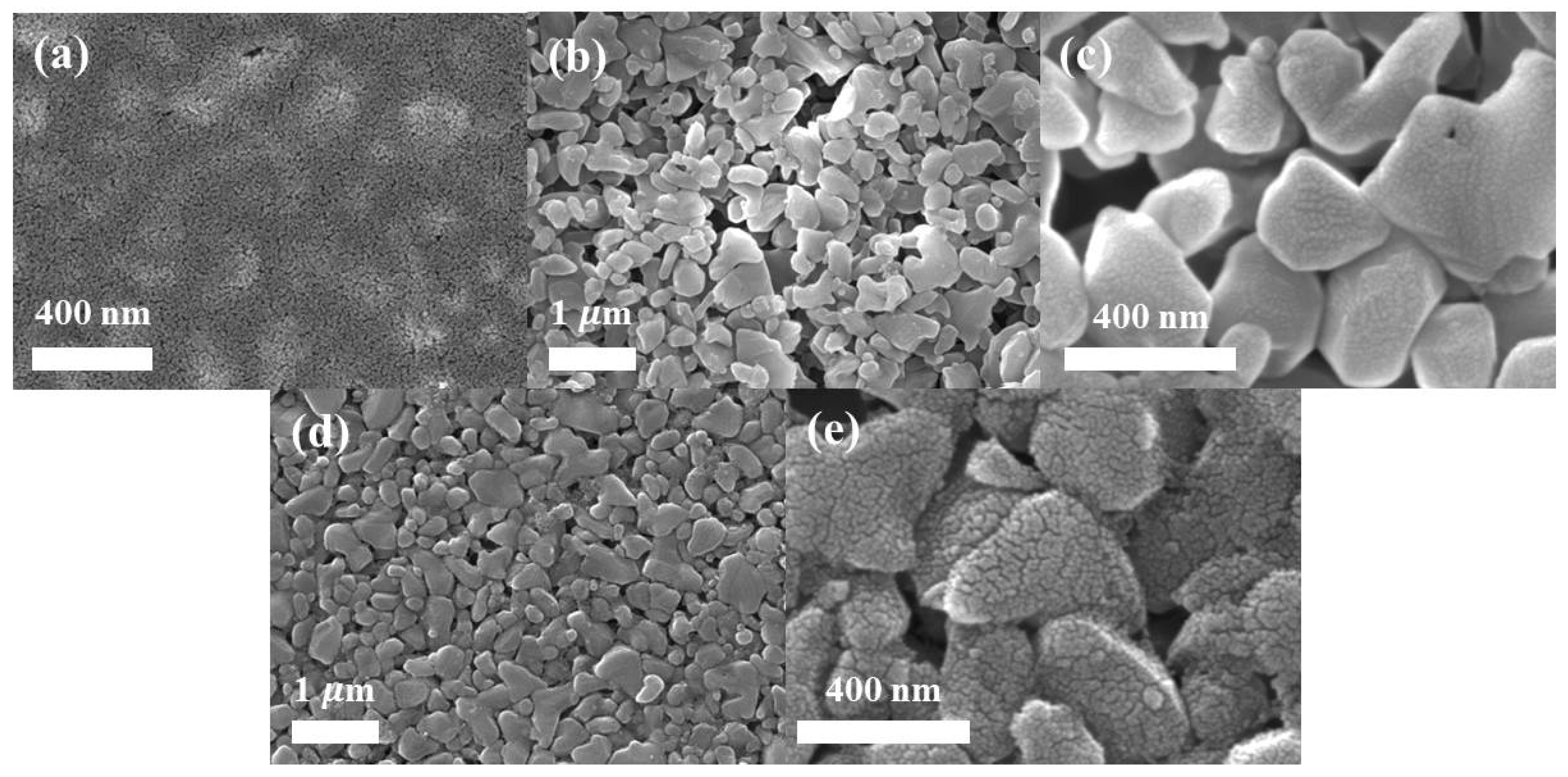
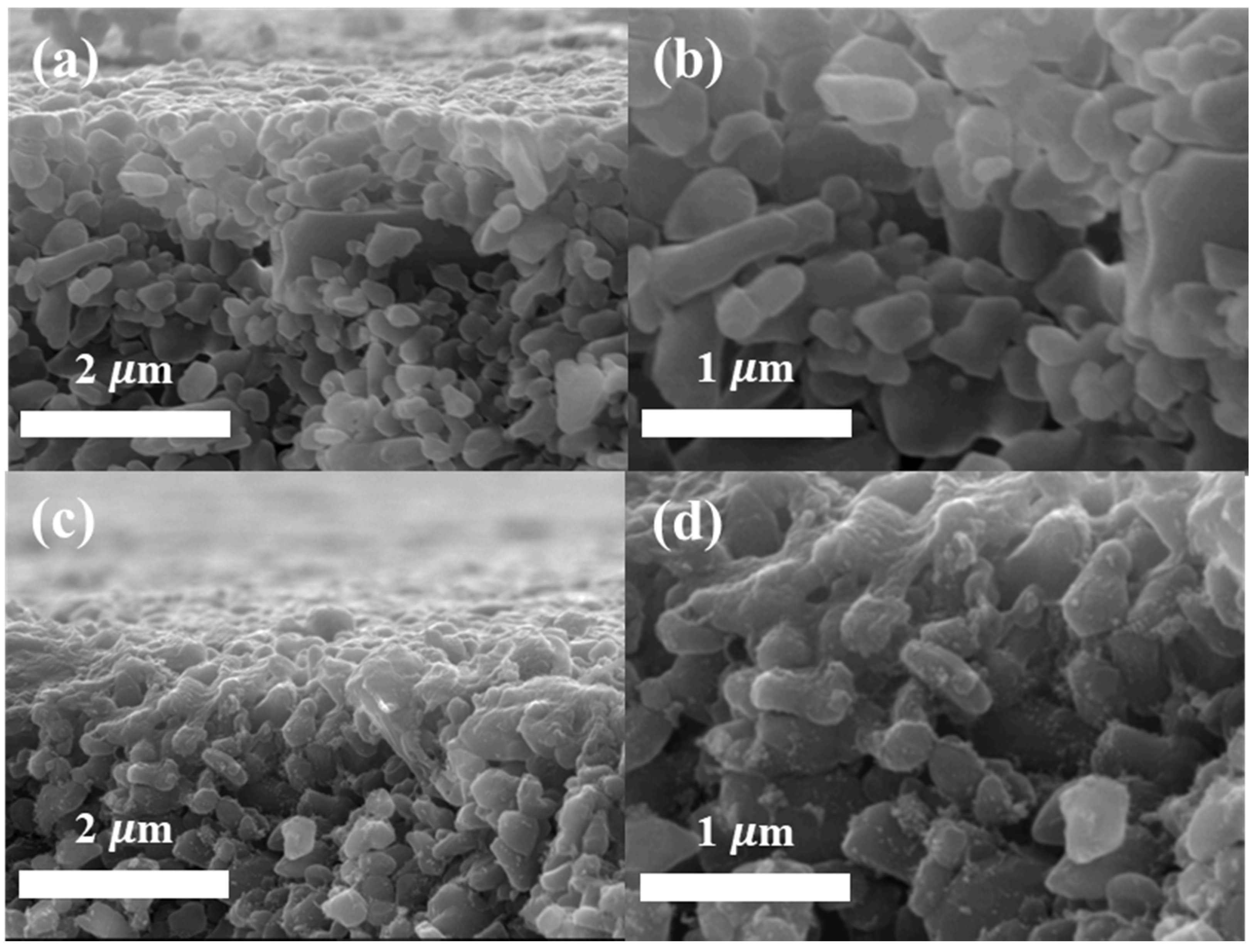
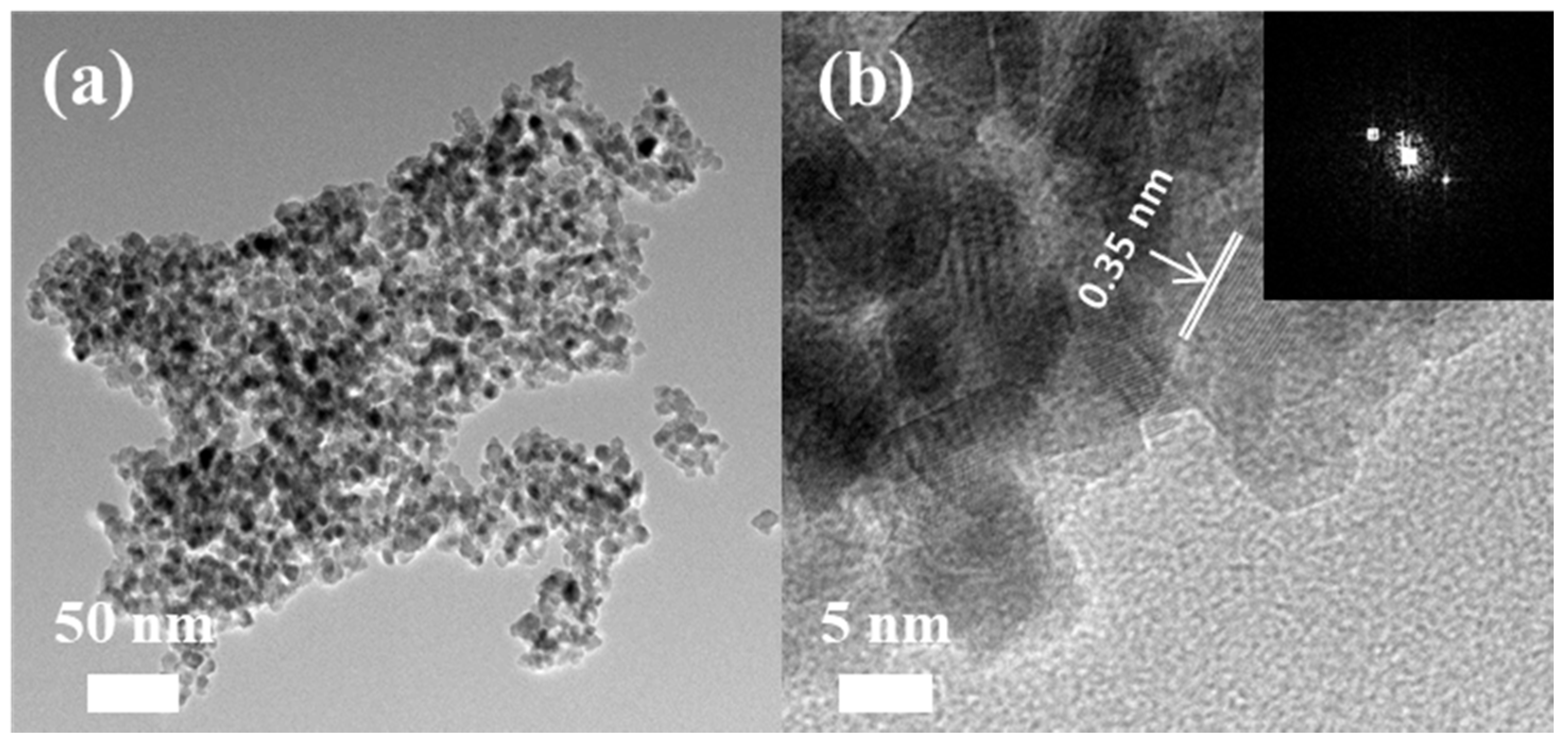
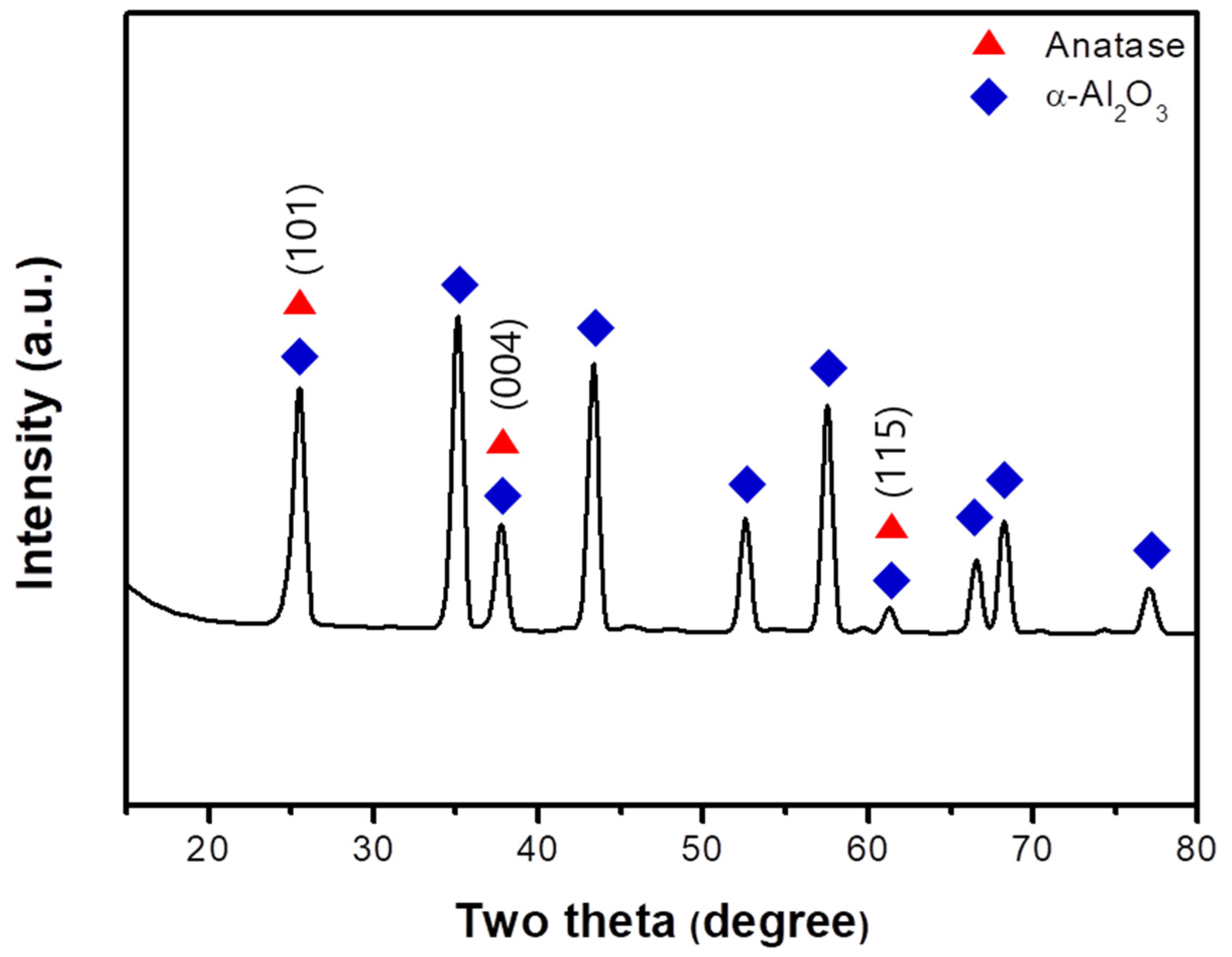
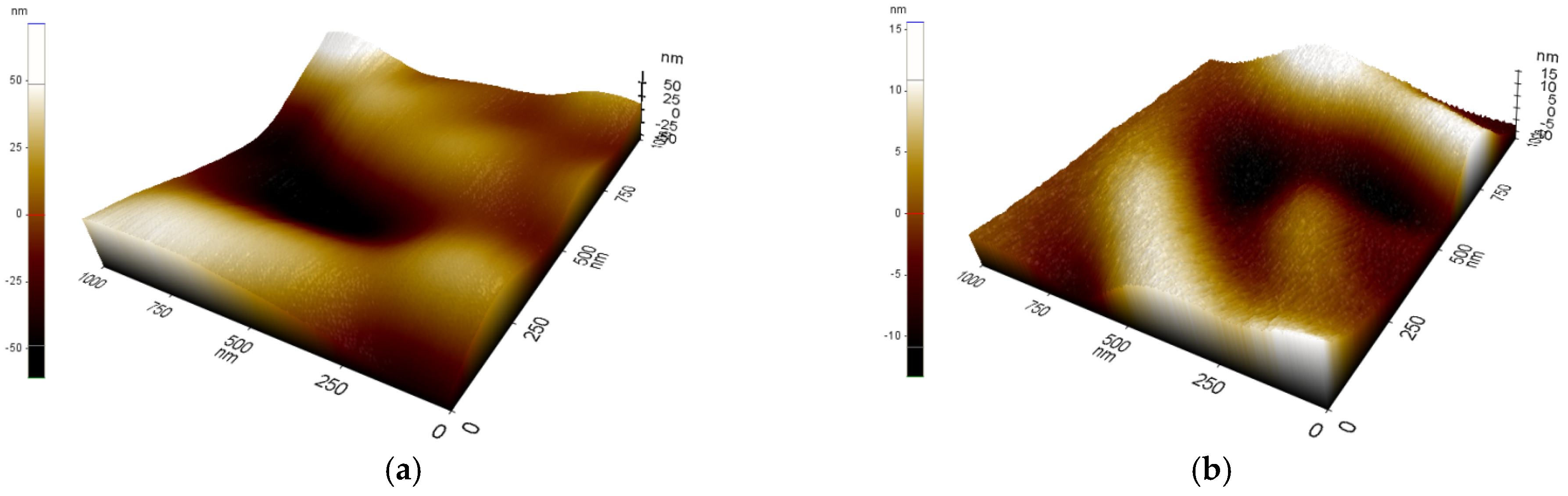
3.1. Membrane Morphology
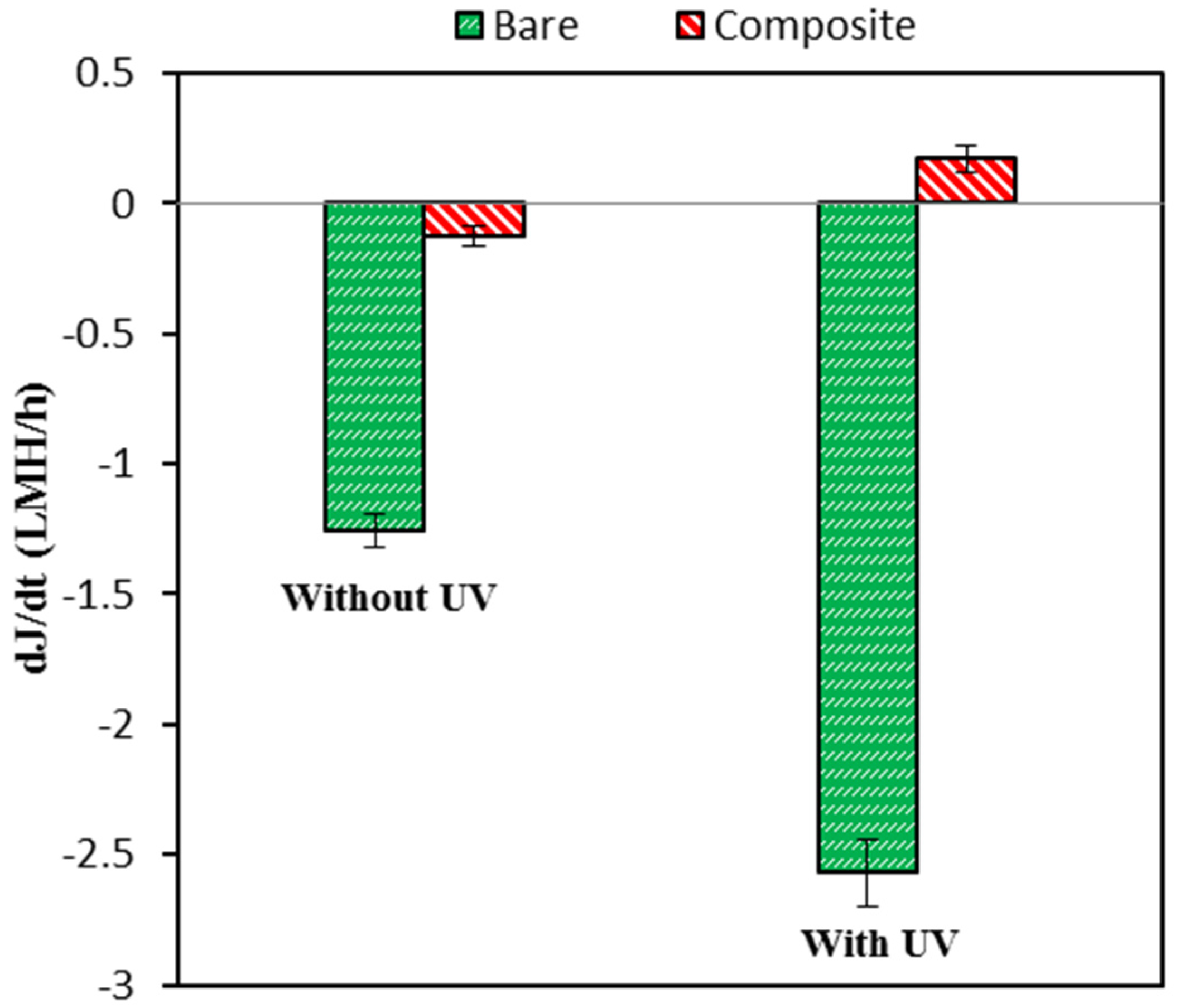
3.2. Pure Water Permeability and Antifouling Properties
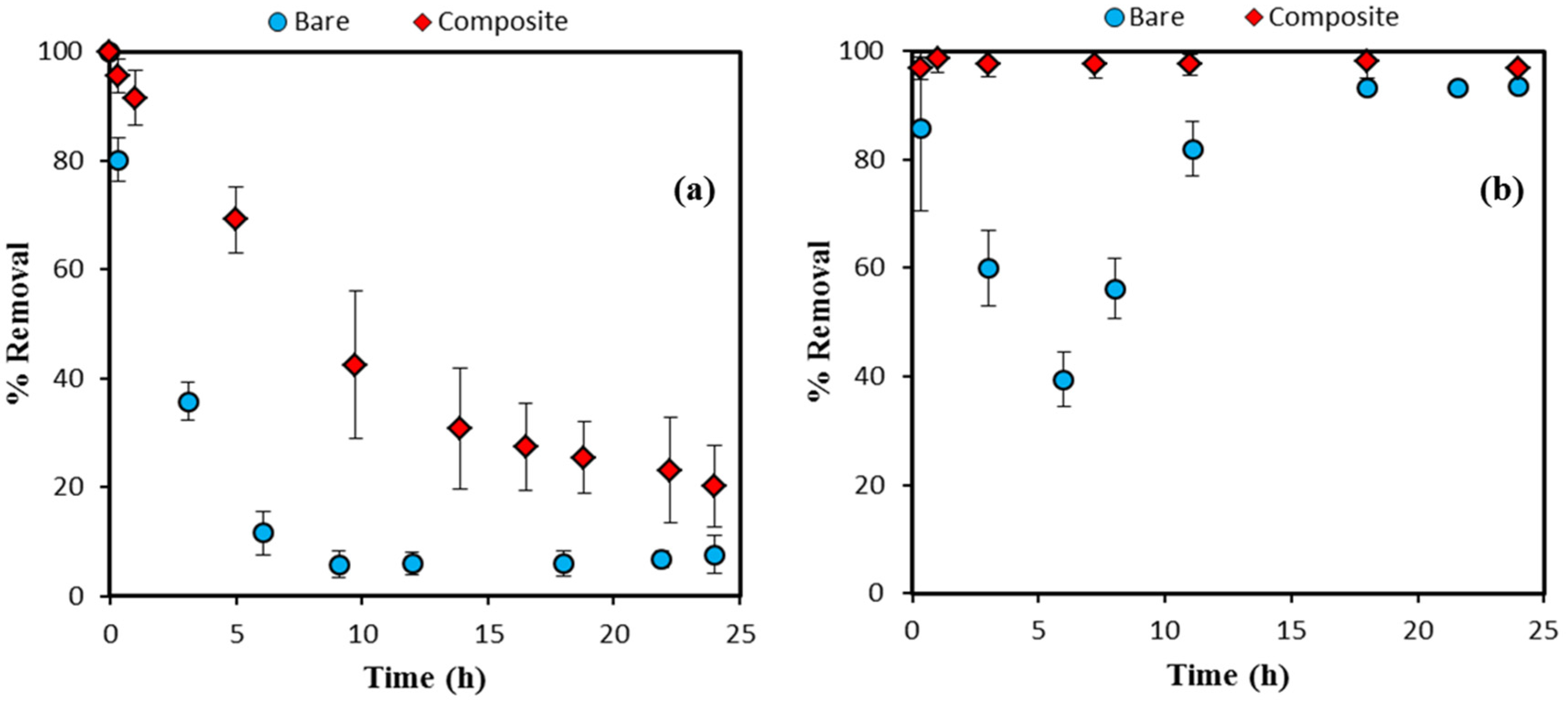
3.3. Organic Dye Removal Efficiency
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, G.; Han, K.; Ye, H.; Zhu, C.; Gao, Y.; Liu, Y.; Zhou, Y. Graphene oxide/triethanolamine modified titanate nanowires as photocatalytic membrane for water treatment. Chem. Eng. J. 2017, 320, 74–80. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Yu, H.; Chang, H.; Quan, X.; Chen, S. CNTs–TiO2/Al2O3 composite membrane with a photocatalytic function: Fabrication and energetic performance in water treatment. Sep. Purif. Technol. 2013, 116, 360–365. [Google Scholar] [CrossRef]
- Aslam, M.; McCarty, P.L.; Shin, C.; Bae, J.; Kim, J. Low energy single-staged anaerobic fluidized bed ceramic membrane bioreactor (AFCMR) for wastewater treatment. Bioresour. Technol. 2017, 240, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-C.; Chen, C.-H.; Chen, Y.-R.; Wu, Y.-H.; Lu, S.-C.; Hu, F.-C.; Li, C.-L.; Tung, K.-L. Increasing the performance of vacuum membrane distillation using micro-structured hydrophobic aluminum hollow fiber membranes. Appl. Sci. 2017, 7, 357. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Ahmad, R.; Kim, J.K.; Kim, J.H.; Kim, J. Effect of polymer template on structure and membrane fouling of TiO2/Al2O3 composite membranes for wastewater treatment. J. Ind. Eng. Chem. 2017, 57, 55–63. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Y.; Quan, X.; Fan, X.; Zhao, H. Performing a microfiltration integrated with photocatalysis using an Ag-TiO2/HAP/Al2O3 composite membrane for water treatment: Evaluating effectiveness for humic acid removal and anti-fouling properties. Water Res. 2010, 44, 6104–6114. [Google Scholar] [CrossRef] [PubMed]
- Goei, R.; Lim, T.-T. Asymmetric TiO2 hybrid photocatalytic ceramic membrane with porosity gradient: Effect of structure directing agent on the resulting membranes architecture and performances. Ceram. Int. 2014, 40, 6747–6757. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic systems as an advanced environmental remediation: Recent developments, limitations and new avenues for applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Kwon, S.; Fan, M.; Cooper, A.T.; Yang, H. Photocatalytic applications of micro- and nano-TiO2 in environmental engineering. Crit. Rev. Env. Sci. Technol. 2008, 38, 197–226. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, M.; Mi, B. Membrane surface modification with TiO2–graphene oxide for enhanced photocatalytic performance. J. Membr. Sci. 2014, 455, 349–356. [Google Scholar] [CrossRef]
- Romanos, G.E.; Athanasekou, C.P.; Katsaros, F.K.; Kanellopoulos, N.K.; Dionysiou, D.D.; Likodimos, V.; Falaras, P. Double-side active TiO2-modified nanofiltration membranes in continuous flow photocatalytic reactors for effective water purification. J. Hazard. Mater. 2012, 211–212, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Athanasekou, C.P.; Romanos, G.E.; Katsaros, F.K.; Kordatos, K.; Likodimos, V.; Falaras, P. Very efficient composite titania membranes in hybrid ultrafiltration/photocatalysis water treatment processes. J. Membr. Sci. 2012, 392, 192–203. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Morales-Torres, S.; Likodimos, V.; Romanos, G.E.; Pastrana-Martinez, L.M.; Falaras, P.; Dionysiou, D.D.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T. Prototype composite membranes of partially reduced graphene oxide/TiO2 for photocatalytic ultrafiltration water treatment under visible light. Appl. Catal. B Environ. 2014, 158, 361–372. [Google Scholar] [CrossRef]
- Ahmad, R.; Kim, J.K.; Kim, J.H.; Kim, J. Well-organized, mesoporous nanocrystalline TiO2 on alumina membranes with hierarchical architecture: Antifouling and photocatalytic activities. Catal. Today 2017, 282, 2–12. [Google Scholar] [CrossRef]
- Goei, R.; Dong, Z.; Lim, T.-T. High-permeability pluronic-based TiO2 hybrid photocatalytic membrane with hierarchical porosity: Fabrication, characterizations and performances. Chem. Eng. J. 2013, 228, 1030–1039. [Google Scholar] [CrossRef]
- Choi, H.; Stathatos, E.; Dionysiou, D.D. Sol–gel preparation of mesoporous photocatalytic TiO2 films and TiO2/Al2O3 composite membranes for environmental applications. Appl. Catal. B Environ. 2006, 63, 60–67. [Google Scholar] [CrossRef]
- Zheng, M.-P.; Gu, M.-Y.; Jin, Y.-P.; Wang, H.-H.; Zu, P.-F.; Tao, P.; He, J.-B. Effects of PVP on structure of TiO2 prepared by the sol-gel process. Mater. Sci. Eng. B 2001, 87, 197–201. [Google Scholar] [CrossRef]
- Li, G.; Richter, C.P.; Milot, R.L.; Cai, L.; Schmuttenmaer, C.A.; Crabtree, R.H.; Brudvig, G.W.; Batista, V.S. Synergistic effect between anatase and rutile TiO2 nanoparticles in dye-sensitized solar cells. Dalton Trans. 2009, 45, 10078–10085. [Google Scholar] [CrossRef] [PubMed]
- Alem, A.; Sarpoolaky, H.; Keshmiri, M. Titania ultrafiltration membrane: Preparation, characterization and photocatalytic activity. J. Eur. Ceram. Soc. 2009, 29, 629–635. [Google Scholar] [CrossRef]
- Choi, H.; Stathatos, E.; Dionysiou, D.D. Photocatalytic TiO2 films and membranes for the development of efficient wastewater treatment and reuse systems. Desalination 2007, 202, 199–206. [Google Scholar] [CrossRef]
- Guo, B.; Pasco, E.V.; Xagoraraki, I.; Tarabara, V.V. Virus removal and inactivation in a hybrid microfiltration–UV process with a photocatalytic membrane. Sep. Purif. Technol. 2015, 149, 245–254. [Google Scholar] [CrossRef]
- Mendret, J.; Hatat-Fraile, M.; Rivallin, M.; Brosillon, S. Hydrophilic composite membranes for simultaneous separation and photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2013, 111, 9–19. [Google Scholar] [CrossRef]
- Ahmad, R.; Kim, J.K.; Kim, J.H.; Kim, J. In-situ TiO2 formation and performance on ceramic membranes in photocatalytic membrane reactor. Membr. J. 2017, 27, 328–335. [Google Scholar] [CrossRef]
- Kamel, D.; Sihem, A.; Halima, C.; Tahar, S. Decolourization process of an azoïque dye (congo red) by photochemical methods in homogeneous medium. Desalination 2009, 247, 412–422. [Google Scholar] [CrossRef]
- Zhao, K.; Feng, L.; Lin, H.; Fu, Y.; Lin, B.; Cui, W.; Li, S.; Wei, J. Adsorption and photocatalytic degradation of methyl orange imprinted composite membranes using TiO2/calcium alginate hydrogel as matrix. Catal. Today 2014, 236, 127–134. [Google Scholar] [CrossRef]

| Permeability (L m−2 h−1 bar−1) | ||
|---|---|---|
| ▭ | Bare Al2O3 Membrane | TiO2/Al2O3 Composite Membrane |
| Without UV | 65.2 | 9.9 |
| With UV | 71.5 | 14.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, R.; Kim, J.K.; Kim, J.H.; Kim, J. Nanostructured Ceramic Photocatalytic Membrane Modified with a Polymer Template for Textile Wastewater Treatment. Appl. Sci. 2017, 7, 1284. https://doi.org/10.3390/app7121284
Ahmad R, Kim JK, Kim JH, Kim J. Nanostructured Ceramic Photocatalytic Membrane Modified with a Polymer Template for Textile Wastewater Treatment. Applied Sciences. 2017; 7(12):1284. https://doi.org/10.3390/app7121284
Chicago/Turabian StyleAhmad, Rizwan, Jin Kyu Kim, Jong Hak Kim, and Jeonghwan Kim. 2017. "Nanostructured Ceramic Photocatalytic Membrane Modified with a Polymer Template for Textile Wastewater Treatment" Applied Sciences 7, no. 12: 1284. https://doi.org/10.3390/app7121284





