1. Introduction
In the last decade, powder-based additive manufacturing (AM) techniques, such as selective laser melting (SLM), and their applications have evolved significantly. Thus, more and more efforts have been made to develop novel specialized powders, especially in the field of metal matrix composite powders. In general, micrometer-sized ceramic particles are integrated into a metal matrix with the aim of enhancing mechanical properties such as the Young’s modulus and strength, while they often severely degrade the plasticity and machinability of the matrix. The ductility and toughness of such metal matrix composites (MMCs) can be maintained or even improved with a simultaneous increase in strength by reducing the particle size to the nanometer range [
1,
2], hence the so-called nanocomposites. However, to homogeneously distribute nanoparticles in the metal matrix is still a challenging task [
3,
4]. For example, during powder metallurgy processing, only a low volume fraction of nanoparticles can be dispersed well in a metal matrix under optimized conditions. However, when the volume fraction of nanoparticles exceeds 2 vol. %, the dispersion is worse even after high-energy ball milling for a long time. The nanoparticles tend to agglomerate along the grain boundaries [
4].
Titanium diboride (TiB
2) is an attractive candidate as a reinforcement in the Al matrix since it exhibits a high melting point (3173 K), high modulus (565 GPa), high hardness (2500 HV), and good thermal stability. One of the advantages of these particles is that they have a well-documented crystallographic orientation relationship [
5,
6] which provides high coherency, thus (1) acting as a nucleus during the solidification of Al [
5]; and (2) lowering the solid-particle interfacial energy to improve particle engulfment during solidification [
6]. Besides, previous works have proved that the rapid solidification process and the decrease of the particle size can improve the particle engulfment during solidification [
7,
8,
9,
10].
Here we show that a uniform distribution of a high fraction of TiB
2 nanoparticles in Al-based metal matrix powders was achieved by gas atomization solidification processing through the combined effects of coherency among the metal-diboride interface, supercooling and a nanoscale particle size. The resulting Al-based composite powders exhibited a fine grain structure, with uniformly dispersed TiB
2 nanoparticles, and thereby are promising candidates for nanocomposite synthesis. Since one of the major challenges in the laser-based additive manufacturing (AM) field (e.g., selective laser melting) is the severe limitation of powder materials with acceptable laser processability [
11,
12,
13], the introduction of pre-embedded nanometer-sized TiB
2 into the metal matrix (e.g., Al-Cu-Mg in this study) would help to expand the powder materials’ palette for AM due to the higher laser absorptivity of TiB
2 compared to the Al matrix [
14]. Furthermore, since the nanometer-sized TiB
2 particles are embedded into spherical, micrometer-sized composite powders obtained by gas atomization, the powder flowability is not jeopardized.
2. Experimental Procedures
In a previous study, the nanometer sized TiB
2 reinforced Al composites were synthesized via an in-situ reaction process. The size of the in-situ synthesized particles ranged from 20 to 500 nm, but with a predominant number of nanometer sized particles (less than 100 nm) [
15]. In the present study, Pure Al was melted at 900 °C with electrical resistance furnace under the protection of an argon atmosphere. The mixed salts of K
2TiF
6 and KBF
4 according to an atomic ratio of Ti/2B were preheated at 250 °C for 2 h and were introduced into the molten aluminum in 15 min. Mechanical stirring of 600 rpm was carried out and heating was maintained at 900 °C for 30 min to allow the in situ TiB
2 particulates to form in the matrix. The reaction slag was skimmed from the surface of the melt. Mg and Al-10Cu master alloys were subsequently added into the melt and homogenized for 10 min. Afterwards, the composite powders were produced by conventional gas atomization. Atomization of the Al-Cu-Mg composite melt was carried out in a confined nozzle atomizer. A schematic diagram of a gas atomization unit and the facility used in the work are shown in
Figure 1a,b, respectively. The capacity of the facility used in this experiment is 25 kg melt for a single charging. Prior to melting and atomization, both the melting and the cooling chambers were evacuated to 10
−2 Pa several times, each time being back filled with nitrogen. During heating of the alloy, its temperature is acquired by means of a thermocouple in the melt. The atomization temperature is 800 °C with a gas pressure of 2.8 MPa. The atomized powder was allowed to cool down to room temperature in the nitrogen gas atmosphere of the atomizer. Afterward, the powders were collected in air. The chemical composition of the composite powders was 3.8 wt. % Cu, 1.3 wt. % Mg and 7.6 wt. % TiB
2 particles with Al balance (a prototype of a 2024 Al alloy with TiB
2 addition), measured by inductively coupled plasma atomic emission spectroscopy analysis (ICP-AES). The powder size distribution was measured by Mastersizer 2000 analyzer. In order to study its microstructure, the as-synthesized powder was sieved into four different size ranges: 63–75 μm (group A); 45–53 μm (group B); 10–26 μm (group C); ≤10 μm (group D). The microstructure of the different gas atomized TiB
2/Al composite powder fractions as well as the distribution of TiB
2 particles was investigated by scanning electron microscopy (SEM), energy dispersive X-ray (EDX) and electron backscattered scattering detection (EBSD). EBSD samples were prepared by Focused Ion beam (FIB) in order to detect both the TiB
2 and Al phase. The crystal structure was characterized by synchrotron radiation X-ray diffraction at the beamline BL14B1 of the Shanghai Synchrotron Radiation Facility (SSRF) using a diffractometer of negligible instrumental broadening (less than 0.001°), equipped with a double crystal monochromator and a position sensitive point detector. The wavelength of the X-ray used was 0.124 nm.
Diffuse reflectance spectroscopy (DRS) was measured from 200–2500 nm using an UV-Visible-NIR Lambda 950 Perkin Elmer spectrometer equipped with a 150 mm diameter integrating sphere coated with Spectralon with 1 nm spectral resolution. A Spectralon reference was used to measure the 100% reflectance and internal attenuators were used to determine 0% reflectance in order to remove background and noise. The samples were placed in a quartz cuvette, sealed, and mounted on a Teflon sample holder for the DRS measurement. The reflectance spectra were subsequently converted to Kubelka-Munk (K-M) to calculate the absorption spectra of the powder. This conversion is performed by the device software, using the K-M equation: , where R is reflectivity.
3. Results and Discussion
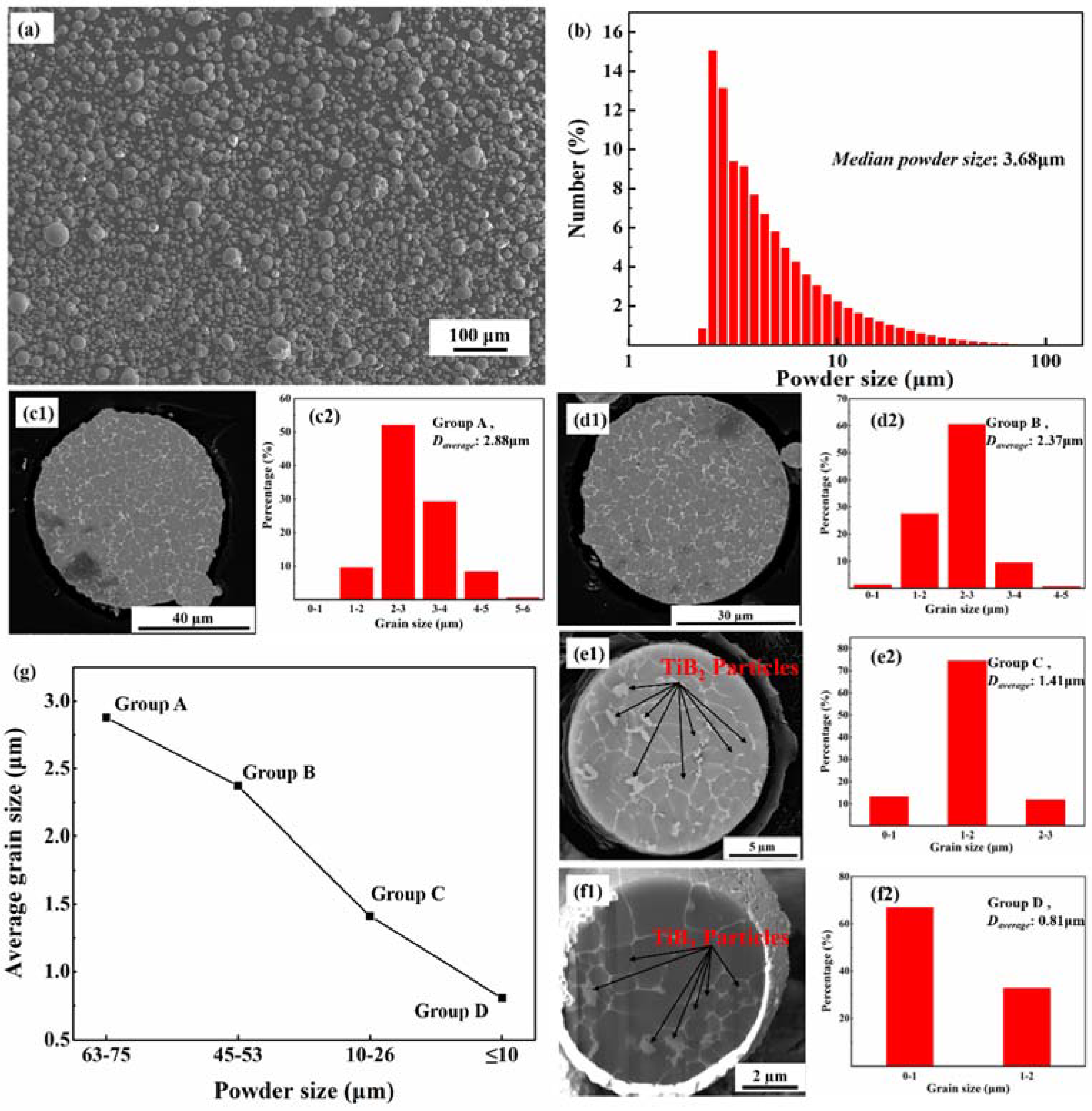
As shown in
Figure 2a, the largest Al–3.8Cu–1.3Mg composite powder particles are around 70 μm. All the powders have a spherical morphology.
Figure 2b shows the size distribution of the composite powders measured by laser diffraction and the average powder size is 3.68 μm. The atomized composite powders exhibited a typical rapid solidification microstructure with a fine equiaxed grain structure, as shown in
Figure 2(c1–f1). The grain size of the different powder fractions was measured from SEM images (more than 1000 grains were measured for each fraction), and the statistical results are illustrated in
Figure 2(c2–f2). The equiaxed grain structure had a median grain size of 2.88 μm in fraction A, and then decreased with the decrease of the powder size to 0.81 μm in group D.
Figure 2g shows the variation of the average grain size versus the powder size. The grain size decreased as the powder size decreased. Hong et al. [
10,
16] proved that the average grain size depends linearly on the powder size and is proportional to the cooling rate of the powders. The fine structure of the powders benefits from the high rate of solidification of the gas atomization process, in which the crystallization process has been suppressed due to the large under-cooling. TiB
2 particles distributed both inside the α-Al grains and along the grain boundaries, as indicated by the arrows in
Figure 2(e1,f1).
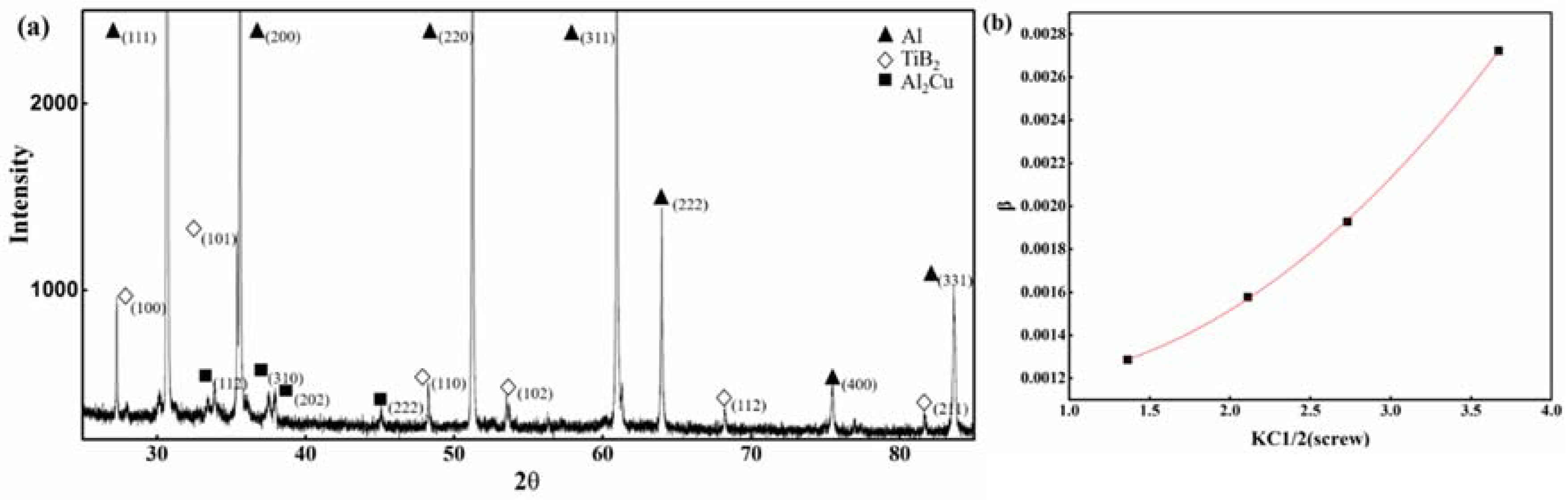
Figure 3a shows a synchrotron X-ray diffraction pattern of TiB
2-reinforced Al–3.8Cu–1.3Mg composite powders. The diffraction peaks of Al, TiB
2 and Al
2Cu were detected correspondingly, as shown in
Figure 3a. From the diffraction pattern, the calculated value (7.1 wt. %) of the TiB
2 mass fraction was obtained through the reference intensity ratio (RIR) method [
17], which is in agreement with the ICP result.
Figure 3b shows the modified Williamson-Hall plot [
18] obtained from the diffraction peaks of aluminum. The intercept of the plot indicates that the average grain size was 731.7 nm while the weighted average grain size calculated from the raw data of
Figure 2b,g was 870 nm. The difference is due to the fact that synchrotron X-ray diffraction is more sensitive to low-angle grain boundaries, while the SEM can only show relatively high-angle grain boundaries. It suggests small-angle grain boundaries exist in the composite powders, which was later observed in the EBSD.
It is pointed out in [
19] that along with the decreasing powder size, the rapid solidification microstructure can change from dendritic grains to cellular and even to equiaxed grains. In a recent study, Zheng et al. [
14] investigated atomized Al–Cu–Mg (grade 2024) alloy powders, which exhibit a large dendrite structure. In the current study, the composite powders had a much finer equiaxed grain structure (
Figure 2(c1–f1)). The modification of the grain structure was due to the effect of TiB
2 nanoparticles on the solidification process, since TiB
2 particles can significantly improve the crystal nucleation rate of α-Al to refine the grains due to the interfacial effect reported in [
5,
20].
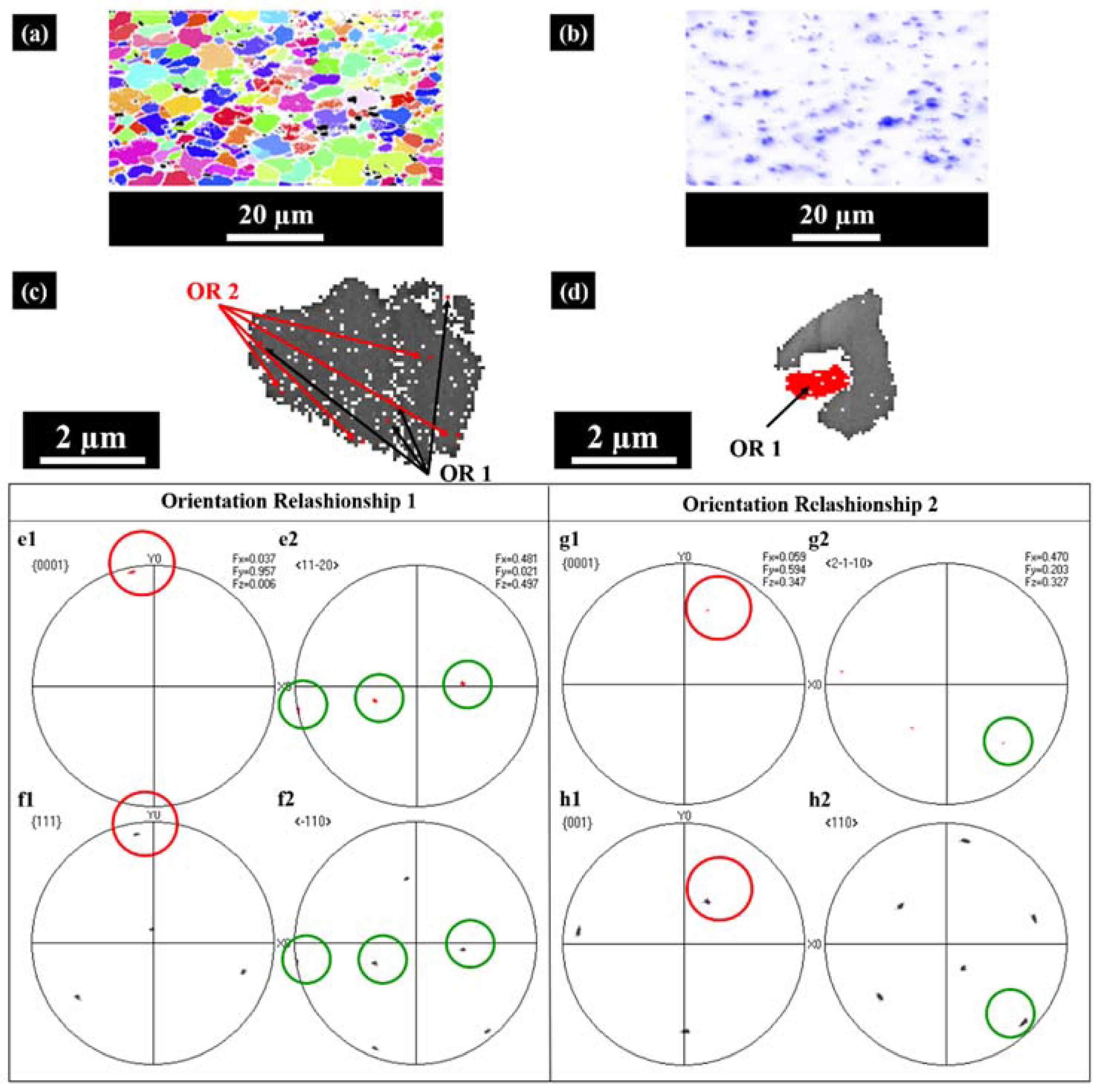
The powders with typical particle sizes of 50 and 10 μm, respectively, were analyzed by EBSD, applying a fine scan with a 0.1 μm step size.
Figure 4a shows an example of an EBSD IPF map of the 50 μm composite powder with TiB
2 phase particles in black contrast. It shows that the micrometer-sized equiaxed grains exhibited random orientations.
Figure 4b shows EDS mapping of the elemental distribution of titanium of the same powder, which evidences the homogeneous particle dispersion. In conventional solidification microstructures obtained by casting [
6,
21], the majority of TiB
2 particles are clustered at the grain boundaries among the equiaxed α-Al grains. In the case of the gas-atomized powders, however, SEM observation shows that TiB
2 particles (indicated by arrows) were distributed both within the grain and along the grain boundaries (as shown in
Figure 2(c1–f1) and
Figure 4). According to [
9,
22], the particles are engulfed when the moving front is above the critical velocity. The improved distribution of TiB
2 particles is obtained thanks to the high cooling rate, which promotes the velocity of the advancing solidification front. Furthermore, the transition between particle pushing and engulfment is mainly determined by the interfacial energies between the phases in the system. It has been proven that the TiB
2 particles and the α-Al grains tend to form a high-coherency orientation relationship between the two atomic structures to reduce the solid-particle interfacial energy [
6], which assists the engulfment to ensure the uniform distribution of the TiB
2 particles.
There are two commonly reported orientation relationships of the nucleation of α-Al on TiB
2 during solidification, noted as OR1 and OR2. OR1 is more commonly encountered according to previous studies [
6,
23].
Figure 4c,d give an example of small and large particles, respectively, in one grain. It was observed that most TiB
2 particles within one grain have two Euler angles which means that they have two orientation relationships with the surrounding aluminum. OR 1 and OR2 co-exist within one grain, as shown in
Figure 4c;
Figure 4d shows an example of the big particle with OR1.
Figure 4(e1,e2,f1,f2) present the {0001}<11-20> pole figures of certain engulfed TiB
2 particles and the {111}<−110> pole figures of the surrounding aluminum. The paralleled crystallographic planes and orientations are marked by red and green circles, respectively. The orientation relationship is consistent with OR1:
(0001)TiB2||(111)Al
[11-20] TiB2||[-110] Al
Figure 4(g1,g2,h1,h2) present the {0001} <2-1-10> pole figures of other engulfed TiB
2 particles and the {001}<110> pole figures of the surrounding aluminum. The orientation relationship matches with OR2:
(0001)TiB2||(001)Al
[2-1-10] TiB2||[110] Al
The statistical analyses of the orientation relationship of recorded TiB
2 particles inside grains and along grain boundaries are summarized in
Table 1 and
Table 2, respectively. Overall, the majority of particles inside the grains (
Table 1) form an OR1 relationship with Al as the [0001]
TiB2 direction is closest to the [111]
Al direction [
23]. Especially for large-sized TiB
2 particles, OR1 appears more frequently. According to the work by Sen and co-workers [
8], the faces of the TiB
2 particles in Al that provide the greatest contact area are the basal faces which form OR1. This enhances the engulfment of particles. It should be noted that three of 34 small TiB
2 particles of the 10 μm powder and four of 13 big particles of the 50 μm powder within the Al grains form neither OR1 nor OR2. This indicates that particle engulfment can take place without a coherent interface in the condition of fast-cooling. However, a well-documented crystallographic orientation relationship can lower the solid-particle interfacial energy to improve particle engulfment during solidification [
5]. Most TiB
2 particles segregated at grain boundaries (
Table 2) rarely develop any relationship with Al. It is proposed that such a phenomenon mainly results from the restriction of α-Al solid volume fractions (v
fs). At the beginning, when the v
fs is low, TiB
2 particles are relatively unconstrained and can reorient freely to the growing α-Al in order to reduce the interface energy of TiB
2 and α-Al σ
SP by forming OR1 or OR2. As v
fs increases, reorientation of TiB
2 becomes increasingly difficult due to the impingement by either neighboring TiB
2 particles or α-Al from multiple directions. Further, during the final stage of solidification, a high v
fs along with a high degree of particle-particle interaction will hinder particle motion. Only relatively unconstrained particles will be able to reorient and obtain OR1 or OR2 [
6,
24]. From the statistical analysis of the orientation relationship, it can be concluded that a uniform dispersion of TiB
2 particles is favored by a coherency interface, supercooling and a nanoscale particle size.
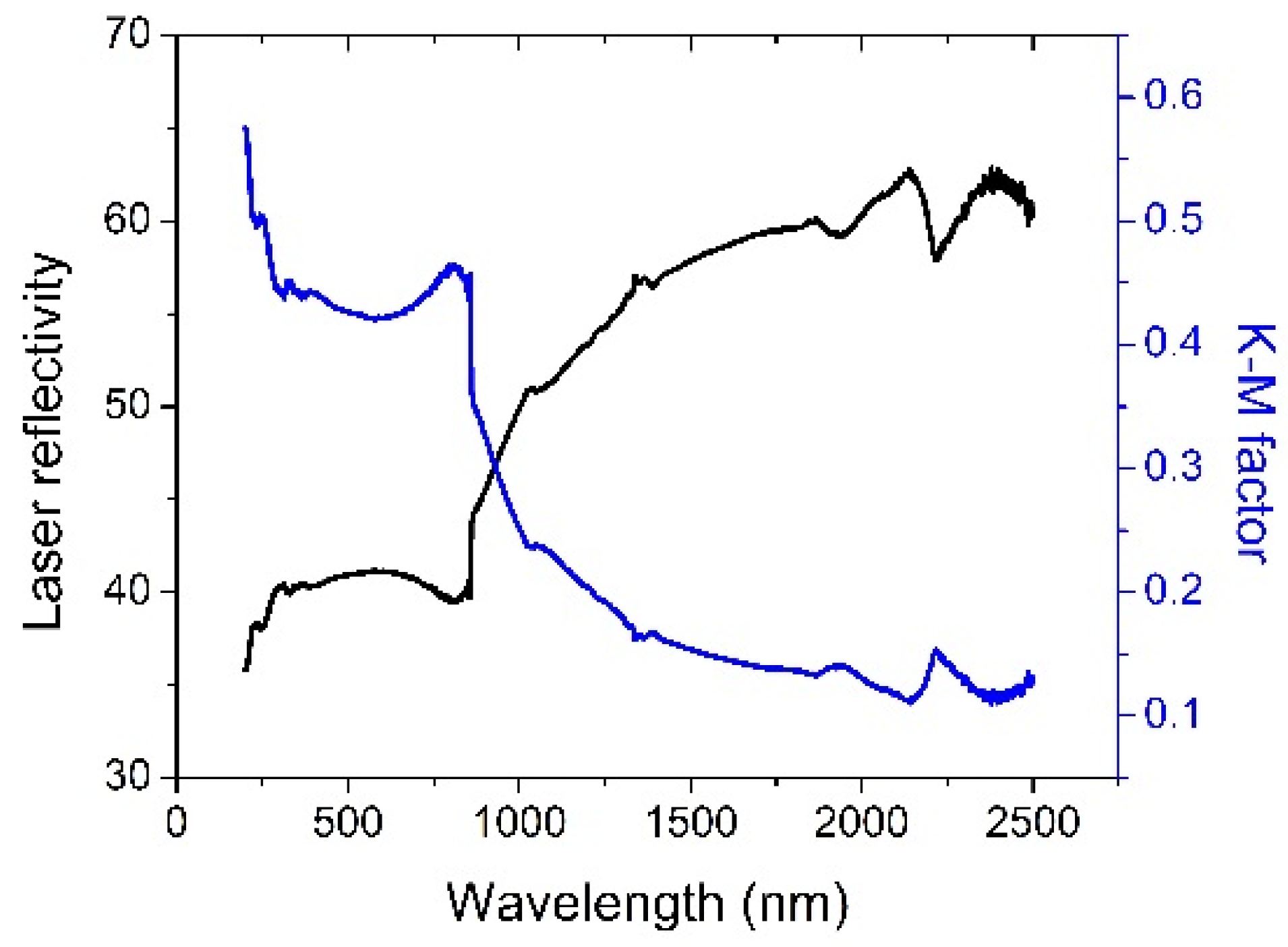
As shown in
Figure 5, the composite powder has a reflectivity of ~43% and a corresponding K-M absorption factor of ~0.37 at a wavelength of 1.06 µm, which is typically used for most SLM processes. The K-M absorption factor is comparable to most Al-Si alloy powders between 0.3–0.4. So the laser absorptivity increased significantly due to the addition of TiB
2.
The resulting Al-based composite powders with improved laser absorptivity provide promising candidates for nanocomposite synthesis via AM. The reasons for this are three-fold: (1) the composite powders with higher laser absorptivity will benefit the melt formation during SLM [
14]; (2) the introduced nano-sized TiB
2 was pre-embedded mainly into the powder and only a limited proportion was distributed on the powder surface, thus not imposing any negative effect on the flowability of the matrix Al-Cu powder; (3) the interfacial bonding between the nano-sized TiB
2 and the Al-Cu matrix was strong in the gas-atomized composite powder, which can help limit the interface de-bonding during rapid solidification in SLM.