Determination of Boron, Phosphorus, and Molybdenum Content in Biosludge Samples by Microwave Plasma Atomic Emission Spectrometry (MP-AES)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Reagents and Materials
2.3. Sample Preparation
3. Experimental Section
4. Results and Discussion
4.1. Accuracy, Precision, and Detection Limits
4.2. MP-AES Spectra of B, P, and Mo
4.3. Calibrations
4.4. Effect of Different Digestion Methods of USEPA 3050, 3051, and 3051A on Analytical Results
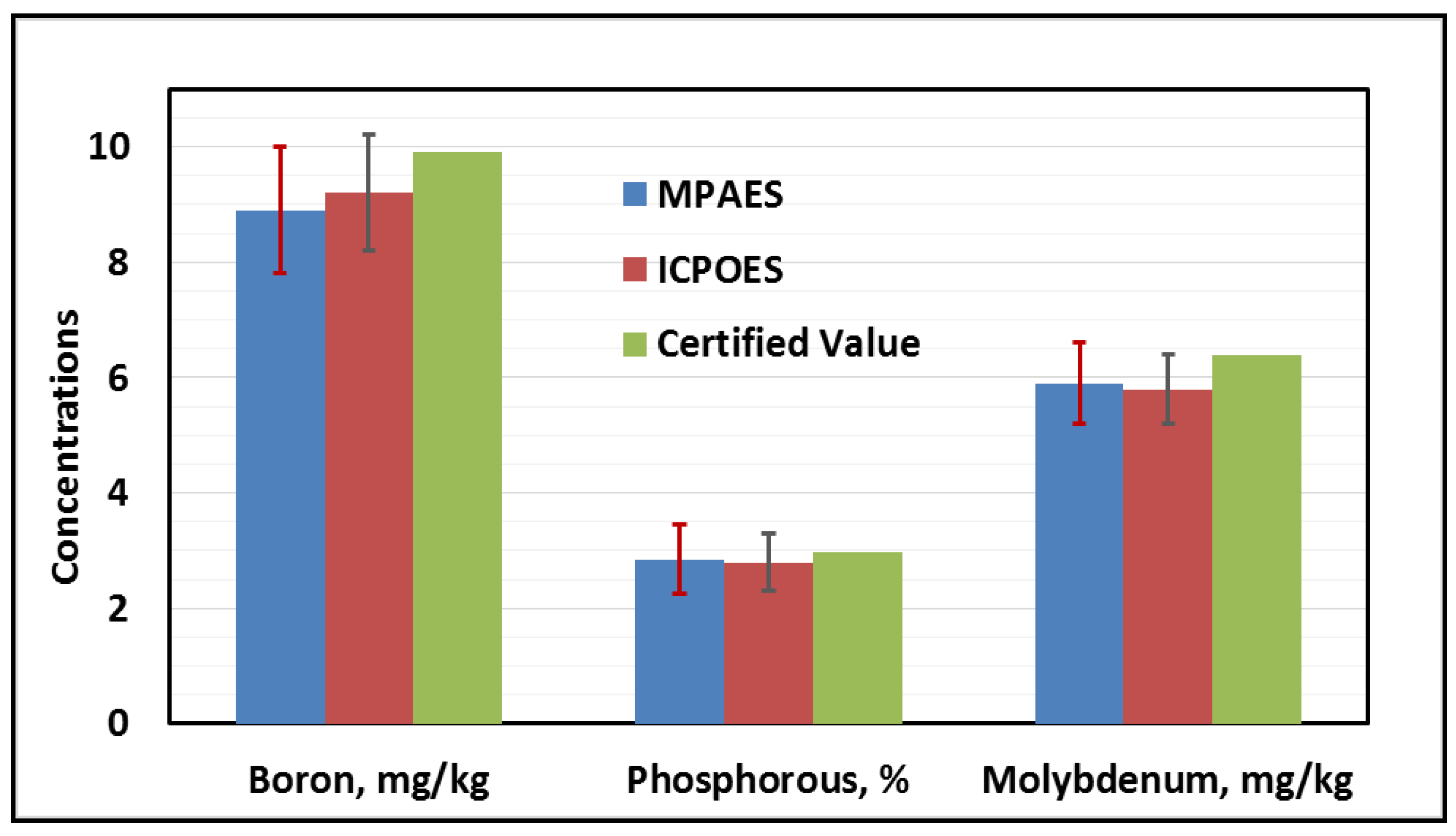
4.5. Comparison of the Analytical Performance of MP-AES, ICP-OES, and FAAS
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sankaram, A. Soil fertility management for reconciling sustainability with productivity. J. Indian Soc. Soil Sci. 1996, 44, 593–600. [Google Scholar]
- Reddy, D.D.A.; Subbarao, A.; Rupa, T.R. Effects of continuous use of cattle manure and fertilizer phosphorus on crop yields and soil organic phosphorus in a vertisol. Bioresour. Technol. 2000, 75, 113–118. [Google Scholar] [CrossRef]
- Matthews, P. Agricultural and other land uses. In Sludge into Biosolids: Processing, Disposal and Utilization; Spinosa, L., Vesilind, P.A., Eds.; IWA Publishing: London, UK, 2001; pp. 41–73. [Google Scholar]
- Okuno, N. Production of usable materials. In Sludge into Biosolids: Processing, Disposal, Utilization; Spinosa, L., Vesilind, P.A., Eds.; IWA Publishing: London, UK, 2001; pp. 147–163. [Google Scholar]
- Tay, J.H.; Show, K.Y. Utilization of municipal wastewater sludge as building and construction materials. Resour. Conserv. Recycl. 1992, 6, 191–204. [Google Scholar] [CrossRef]
- Wen-Tien, T. An Analysis of waste management policies on utilizing biosludge as material Resources in Taiwan. Sustainability 2012, 4, 1879–1887. [Google Scholar]
- Vesilind, P.A.; Spinosa, L. Production and regulations. In Sludge into Biosolids: Processing, Disposal, Utilization; Spinosa, L., Vesilind, P.A., Eds.; IWA Publishing: London, UK, 2001; pp. 3–18. [Google Scholar]
- Sarica, D.Y.; Ertas, N. Flow injection Analysis for Boron Determination by Using Methyl Borate Generation and Flame Atomic Emission Spectrometry. Turk. J. Chem. 2001, 25, 305–310. [Google Scholar]
- Farooq, M.; Wahid, A.; Siddique, K.H.M. Micronutrient application through seed treatments—A review. J. Soil Sci. Plant Nutr. 2012, 12, 125–142. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Kate, L.G.; Joanne, N.B.; Phillips, T.; Tyerman, D. The role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lasztity, A.; Viczian, M.; Israel, Y.; Barnes, R.M. Inductively coupled plasma spectrometry in the study of childhood soil ingestion. J. Anal. Atom. Spectrom. 1989, 4, 727–735. [Google Scholar] [CrossRef]
- Balaram, V. Recent trends in the instrumental analysis of rare earth elements in geological and Industrial Materials. Trends Anal. Chem. 1996, 15, 475–486. [Google Scholar] [CrossRef]
- Bader, N.R.; Zimmermann, B. Sample preparation for atomic spectroscopic analysis: An overview. Adv. Appl. Sci. Res. 2012, 3, 1733–1737. [Google Scholar]
- Oliveira, S.R.; Jose, A.G.N.; Joaquim, A.N.; Bradley, T.J. Determination of macro and micronutrients in plant leaves by high-resolution continuum source flame atomic absorption spectrometry combining instrumental and sample preparation strategies. Spectrochim. Acta B At. Spectrosc. 2010, 65, 316–320. [Google Scholar] [CrossRef]
- Balaram, V.; Anjaiah, K.V.; Reddy, M.R.P. A comparative study on the trace and rare earth element analysis of an Indian Polymetallic Nodule Reference Sample by Inductively Coupled Plasma Atomic Emission Spectrometry and Inductively Coupled Plasma Mass Spectrometry. Analyst. 1995, 120, 1401–1406. [Google Scholar] [CrossRef]
- Boss, C.B.; Fredeen, K.J. Concepts, Instrumentation and Techniques in Inductively Coupled Plasma Optical Emission Spectrometry; Perkin-Elmer Corporation: Waltham, MA, USA, 1997; p. 125. [Google Scholar]
- Hammer, M.R. A magnetically excited microwave plasma source for atomic emission spectroscopy with performance approaching that of the inductively coupled plasma. Spectrochim. Acta B 2008, 63, 456–464. [Google Scholar] [CrossRef]
- Balaram, V.; Dharmendra, V.; Roy, P.; Taylor, C.; Kar, P.; Kumar Raju, A.; Krishnaiah, A. Determination of Precious Metals in Rocks and Ores by Microwave Plasma-Atomic Emission Spectrometry (MP-AES) for geochemical prospecting. Curr. Sci. 2013, 104, 1207–1215. [Google Scholar]
- Balaram, V.; Dharmendra, V.; Roy, P.; Taylor, C.; Kamala, C.T.; Satyanarayanan, M.; Kar, P.; Subramanyam, K.S.V.; Kumar Raju, A.; Krishnaiah, A. Analysis of geochemical samples by microwave plasma-AES. At. Spectrosc. 2014, 35, 65–78. [Google Scholar]
- Kamala, C.T.; Balaram, V.; Dharmendra, V.; Satyanarayanan, M.; Subramanyam, K.S.V.; Krishnaiah, A. Application of microwave plasma atomic emission spectrometry (MP-AES) for environmental monitoring of industrially contaminated sites in Hyderabad city. Environ. Monit. Assess. 2014, 186, 7097–7113. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Simmons, P.; Shrader, D.; Herrman, T.J.; Dai, S.Y. Microwave plasma-atomic emission spectroscopy as a tool for the determination of copper, iron, manganese and zinc in animal feed and fertilizer. Talanta 2013, 112, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Donati, G.L.; Amais, R.S.; Schiavo, D.; Nobrega, J.A.N. Determination of Cr, Ni, Pb and V in gasoline and ethanol fuel by microwave plasma optical emission spectrometry. J. Anal. At. Spectrom. 2013, 28, 755. [Google Scholar] [CrossRef]
- Nelson, J.; Gilleland, G.; Poirier, L.; Leong, D.; Hajdu, P.; Lopez Linares, F. Elemental analysis of crude oils using microwave plasma atomic emission spectrometry. Energy Fuels 2015, 29, 5587–5594. [Google Scholar] [CrossRef]
- Amais, R.S.; Donati, G.L.; Schiavo, D.; Nobrega, J.A. A simple dilute-and-shoot procedure for Si determination in diesel and biodiesel by microwave-induced plasma optical emission spectrometry. Microchem. J. 2013, 106, 318–322. [Google Scholar] [CrossRef]
- Barrientos, E.Y.; Wroberl, K.; Torres Guzman, J.C.; Corrales Escobosa, A.R.; Wrobel, K. Determinatio of SeMet and Se(IV) in biofortified yeast by ion-pair reversed phase liquid chromatography-hydride generation-microwave induced nitrogen plasma atomic emission spectrometry (HPLC-HG-MP-AES). J. Anal. At. Spectrom. 2016, 31, 203–211. [Google Scholar] [CrossRef]
- Khalid, U.; Sarfaraz, K.; Said, G.; Muhammad, U.; Niamatullah, Muhammad A.K.; Shad, K.K. Sewage Sludge: An Important biological resource for sustainable agriculture and its environmental implications. Am. J. Plant Sci. 2012, 3, 1708–1721. [Google Scholar]
- Balaram, V. Microwave dissolution techniques for the analysis of geological materials by ICP-MS. Curr. Sci. 1997, 73, 1019–1023. [Google Scholar]
- Association of Official Agricultural Chemists International. Association of Official Agricultural Chemists International. AOAC International method 985.01: Metals and other elements in plants and pet foods. In Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- United States Environmental Protection Agency. United States Environmental Protection Agency. USEPA-Test method for evaluating solid waste. In Laboratory Manual Physical/Chemical Methods, SW 846, 3rd ed.U.S. Government Printing Office: Washington, DC, USA, 1995; Volume 1A. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Method 3051A—Microwave Assisted Acid Digestion of Sediments, Sludges, Soils And Oils; United States Environmental Protection Agency: Washington, DC, USA, 1998.
- Chen, M.; Ma, L.Q. Comparison of four USEPA digestion methods for trace metal analysis using certified and Florida soils. J. Environ. Qual. 1998, 27, 1294–1300. [Google Scholar] [CrossRef]
| Agilent 4100 Microwave Plasma-Atomic Emission Spectrometer (MP-AES) with 4107 Nitrogen Generator | Plasma Conditions | |
| Plasma gas | Nitrogen (by using an Air Compressor and a Nitrogen Generator) | |
| Power of Magnetron out put | 1 kW | |
| Gas Flows | ||
| Plasma Gas flow-Nitrogen | 20 L/min | |
| Intermediate flow-Nitrogen | 1.1 L/min | |
| Pre-optics protection (POP) gas—Air | 25 L/min | |
| Nebulizer pressure | 140–240 kPa (Optimize for each group of elements) | |
| Nebulizer | OneNeb inert concentric for HF and high Total Dissolved Solid (TDS) solutions | |
| Spray chamber | Double pass glass cyclonic | |
| Solution uptake | 1.4 mL/min (pumped) | |
| Pump tubing | 1.02 mm i.d. PVC (Polyvinyl Chloride) | |
| Plasma Torch | Quartz torch | |
| Plasma Viewing | Axial | |
| Data Acquisition Parameters | ||
| Sample Uptake delay (s) | 8 | |
| Stabilization time (s) | 15 | |
| Rinse time (s) | 30 | |
| Read time (s) | 5 | |
| No. of replicates | 3 | |
| Background correction | Auto or FLIC (Fast Linear Interference Correction) | |
| Optical system | Czerny-Turner design Monochromator with 600 mm focal length and fixed entrance slit | |
| Detector | Back thinned solid state charge-coupled device (CCD) detector (532 × 128 pixels) | |
| Analytes (Wavelengths) | B (249.677 nm), P (213.618 nm) and Mo (379.825 nm) | |
| Stage | Power (W) | Ramp (min) | Temp. (°C) | Holding Time (min) | |
|---|---|---|---|---|---|
| Step | Max (w) | % | |||
| 1 | 800 | 70 | 15 | 180 | 10 |
| 2 | 800 | 80 | 15 | 190 | 10 |
| 3 | 800 | 100 | 10 | 220 | 5 |
| Elements | MP-AES | ICP-OES | FAAS |
|---|---|---|---|
| B | 249.677 | 249.677 | 249.8 |
| P | 213.618 | 213.618 | 213.6 |
| Mo | 379.825 | 379.825 | 313.3 |
| S. No. | Elements | Linearity Range (mg/L) | Slope | Y-Intercept | Correlation Coefficient (r2) | |||
|---|---|---|---|---|---|---|---|---|
| MP-AES | ICP-OES | MP-AES | ICP-OES | MP-AES | ICP-OES | |||
| 1 | B | 0.0–2.0 | 6962 | 9642 | 10.63 | 16.28 | 0.999 | 0.999 |
| 2 | P | 0.0–200.0 | 116 | 208.6 | 84.06 | 95.8 | 0.998 | 0.999 |
| 3 | Mo | 0.0–1.0 | 6075 | 7297 | 17.70 | 18.7 | 0.999 | 0.998 |
| Elements | Method 3050 | Method 3051 | Method 3051A | SRM-Sewage Sludge EnviroMAT (BE-1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration, (mg/kg) | Accuracy, % | Precision, % RSD | Concentration, (mg/kg) | Accuracy, % | Precision, % RSD | Bias 1 | Concentration, (mg/kg) | Accuracy, % | Precision, % RSD | Bias 2 | Consensus Value, (mg/kg) | Confidence Interval | |
| B | 8.9 ± 0.5 | 89.9 | 2.8 | 8.4 ± 0.6 | 84.8 | 2.9 | −5.6 | 9.2 ± 0.4 | 92.9 | 1.3 | 3.4 | (9.9) | - |
| P | 27,105.0 ± 45 | 90.9 | 3.4 | 27,505.0 ± 65 | 92.2 | 2.1 | 1.5 | 27,946.0 ± 70 | 93.7 | 0.8 | 3.1 | 29,826 | 27,906–31,746 |
| Mo | 5.9 ± 0.2 | 92.2 | 6.2 | 5.6 ± 0.4 | 87.5 | 2.6 | −5.1 | 5.8 ± 0.2 | 90.6 | 1.8 | −1.7 | 6.4 | 5.9–6.9 |
| MP-AES | ICP-OES | Deviation MP-AES vs. ICP-OES | p | ||||
|---|---|---|---|---|---|---|---|
| Sample | Elements | Concentration, (mg/kg) | Precision, % RSD | Concentration, (mg/kg) | Precision, % RSD | ||
| Biosludge-1 | B | 19.7 ± 1.2 | 2.4 | 18.5 ± 0.8 | 1.8 | 6.5 | 0.40 |
| P | 3513 ± 65 | 1.2 | 3548 ± 65 | 0.8 | −1.0 | 0.71 | |
| Mo | 7.7 ± 0.3 | 0.8 | 7.5 ± 0.2 | 0.6 | 2.7 | 0.06 | |
| Biosludge-2 | B | 17.1 ± 0.7 | 2.2 | 18 ± 0.7 | 2.4 | −5.0 | 0.30 |
| P | 3423 ± 55 | 0.6 | 3476 ± 60 | 0.8 | −1.5 | 0.42 | |
| Mo | 7.7 ± 0.5 | 1.2 | 7.7 ± 0.3 | 1 | 0.0 | 0.72 | |
| Biosludge-3 | B | 18.6 ± 0.8 | 2 | 18.2 ± 0.7 | 1.4 | 2.2 | 0.65 |
| P | 3489 ± 60 | 0.4 | 3450 ± 55 | 1.8 | 1.1 | 0.15 | |
| Mo | 7.1 ± 0.5 | 1 | 7.2 ± 0.2 | 1.6 | −1.4 | 0.92 | |
| Elements | MP-AES | ICP-OES | FAAS |
|---|---|---|---|
| B | 0.001 | 0.001 | 1 |
| P | 0.025 | 0.030 | 50 |
| Mo | 0.002 | 0.003 | 0.03 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vudagandla, S.; Siva Kumar, N.; Dharmendra, V.; Asif, M.; Balaram, V.; Zhengxu, H.; Zhen, Z. Determination of Boron, Phosphorus, and Molybdenum Content in Biosludge Samples by Microwave Plasma Atomic Emission Spectrometry (MP-AES). Appl. Sci. 2017, 7, 264. https://doi.org/10.3390/app7030264
Vudagandla S, Siva Kumar N, Dharmendra V, Asif M, Balaram V, Zhengxu H, Zhen Z. Determination of Boron, Phosphorus, and Molybdenum Content in Biosludge Samples by Microwave Plasma Atomic Emission Spectrometry (MP-AES). Applied Sciences. 2017; 7(3):264. https://doi.org/10.3390/app7030264
Chicago/Turabian StyleVudagandla, Sreenivasulu, Nadavala Siva Kumar, Vummiti Dharmendra, Mohammad Asif, Vysetti Balaram, Haung Zhengxu, and Zhou Zhen. 2017. "Determination of Boron, Phosphorus, and Molybdenum Content in Biosludge Samples by Microwave Plasma Atomic Emission Spectrometry (MP-AES)" Applied Sciences 7, no. 3: 264. https://doi.org/10.3390/app7030264







