Aqueous-Organic Phase Transfer of Gold and Silver Nanoparticles Using Thiol-Modified Oleic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
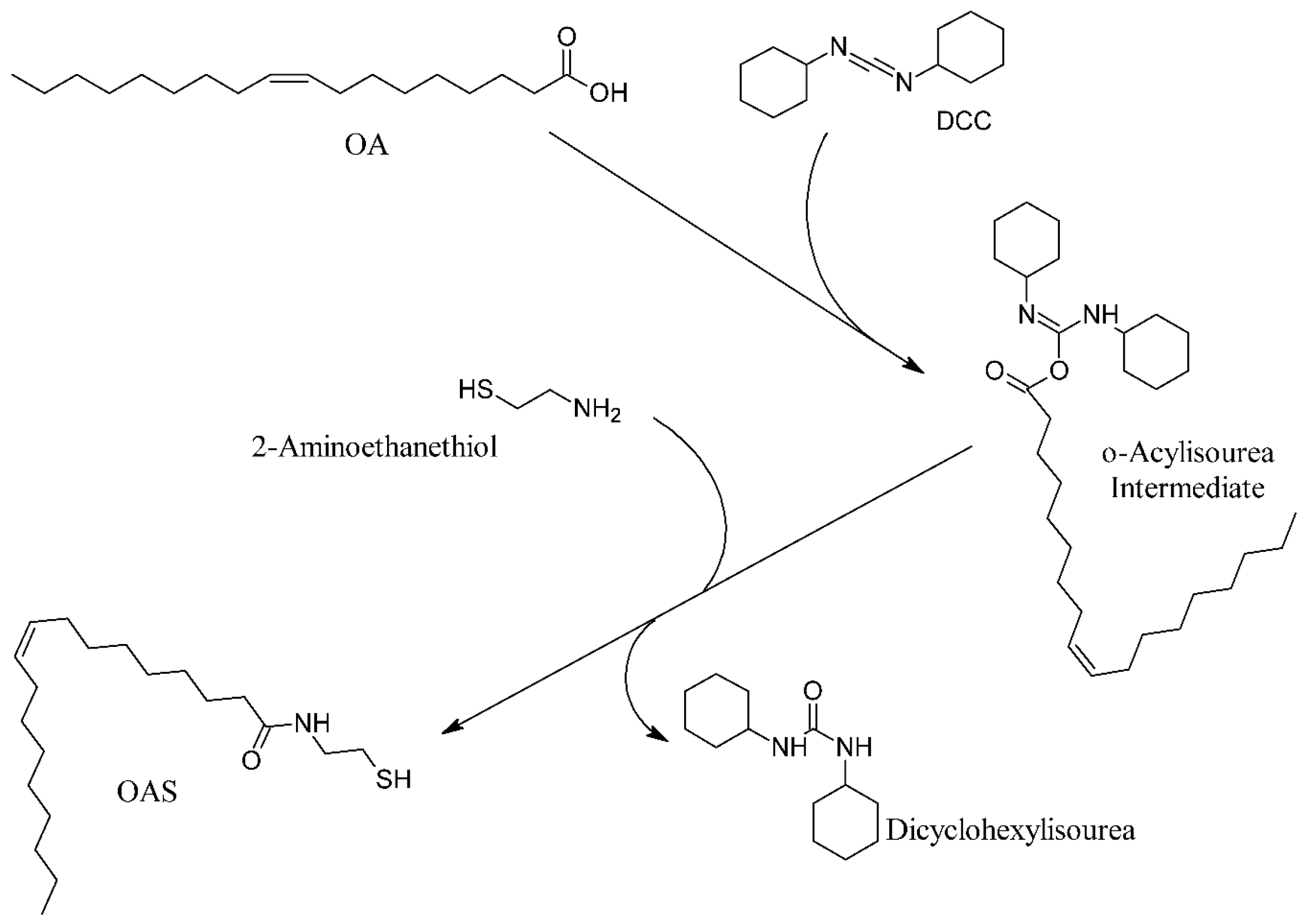
2.2. Preparation of OAS
2.3. Preparation of GNP and SNP
2.4. Aqueous-Organic Phase Transfer of GNP and SNP
2.5. Characterization
3. Results
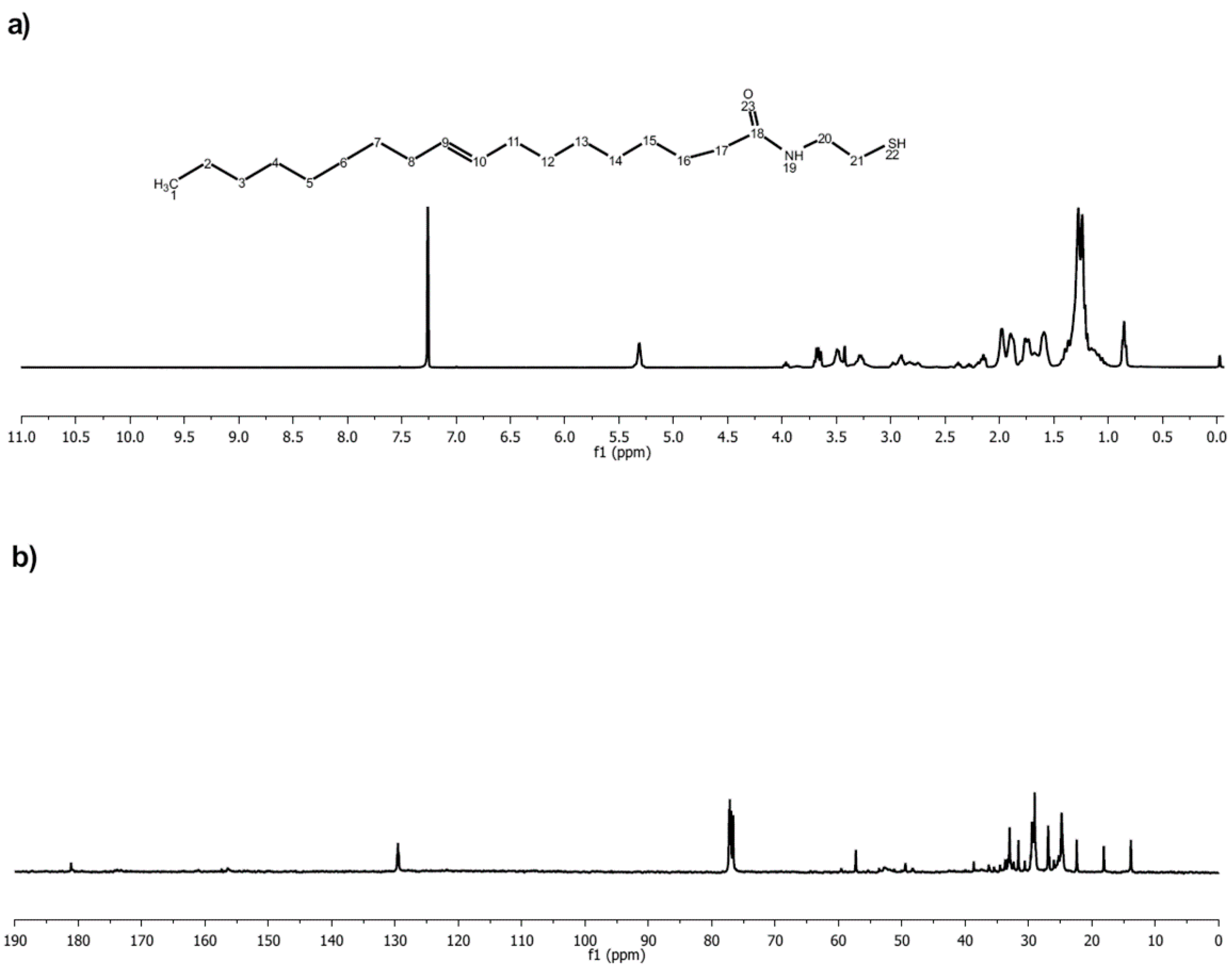
3.1. Preparation of Thiol Terminated Oleic Acid (OAS)
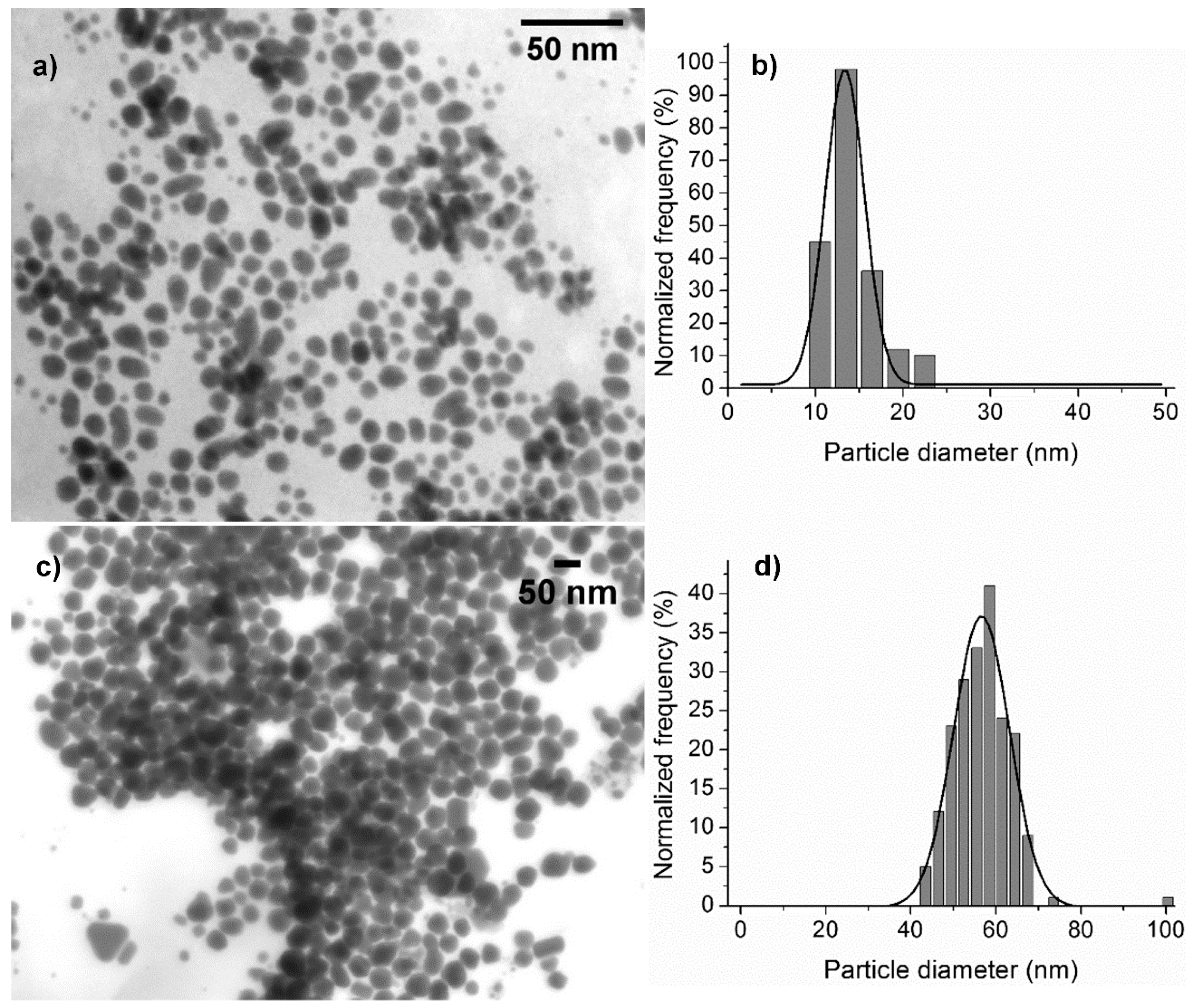
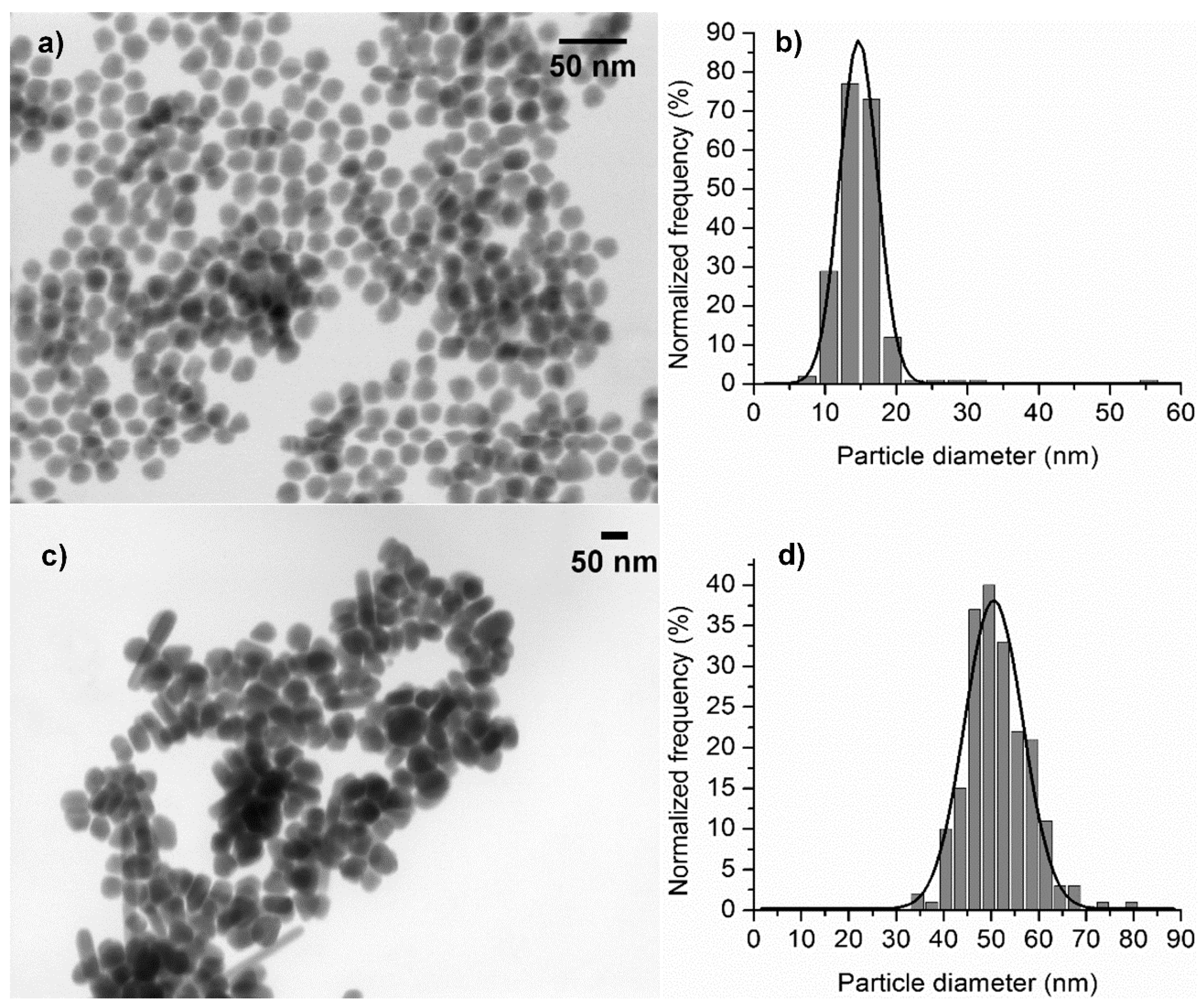
3.2. Synthesis of Gold and Silver Nanoparticles
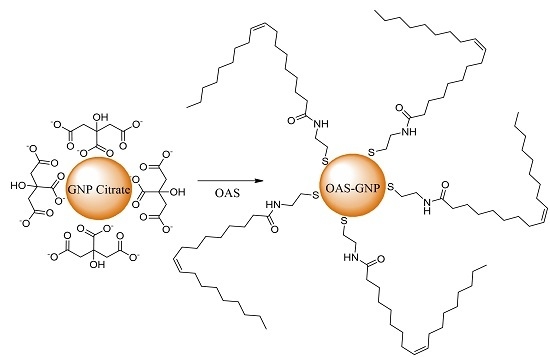
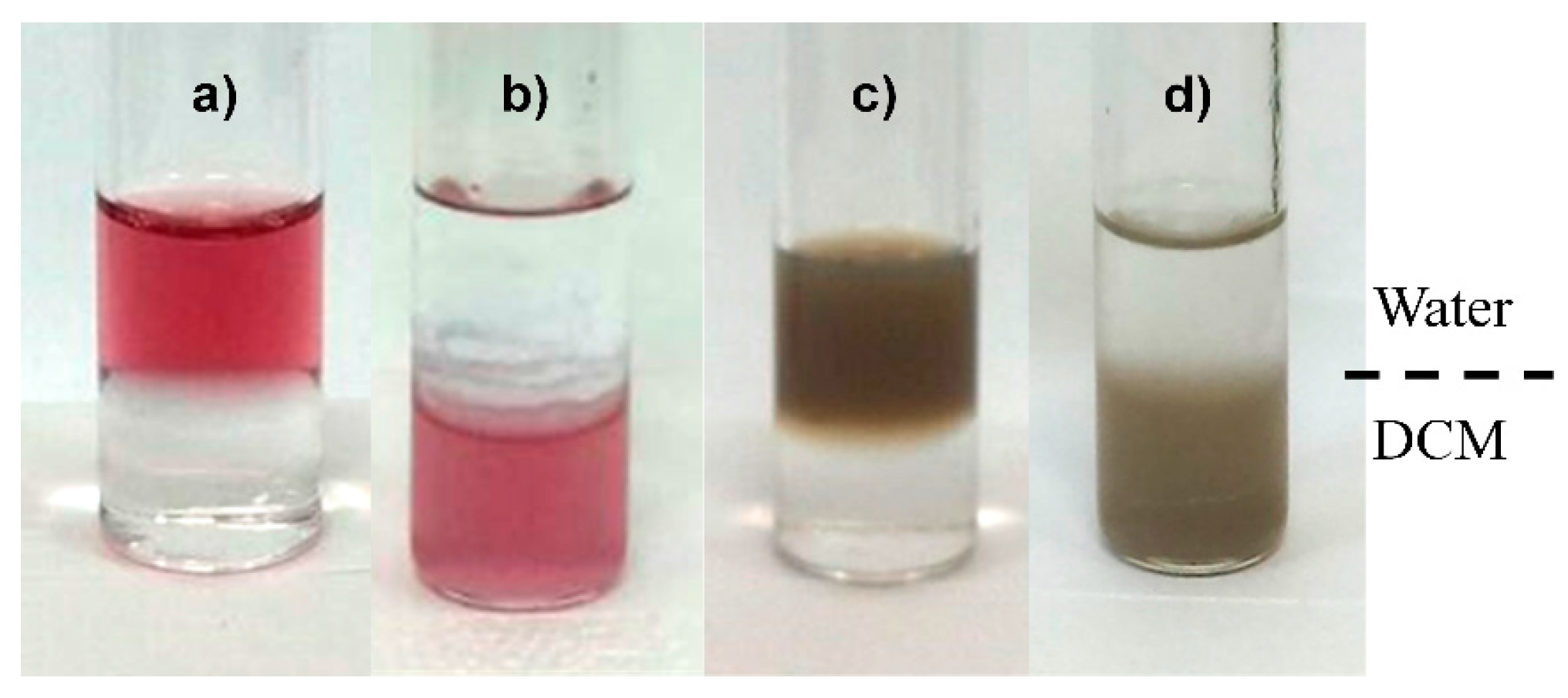
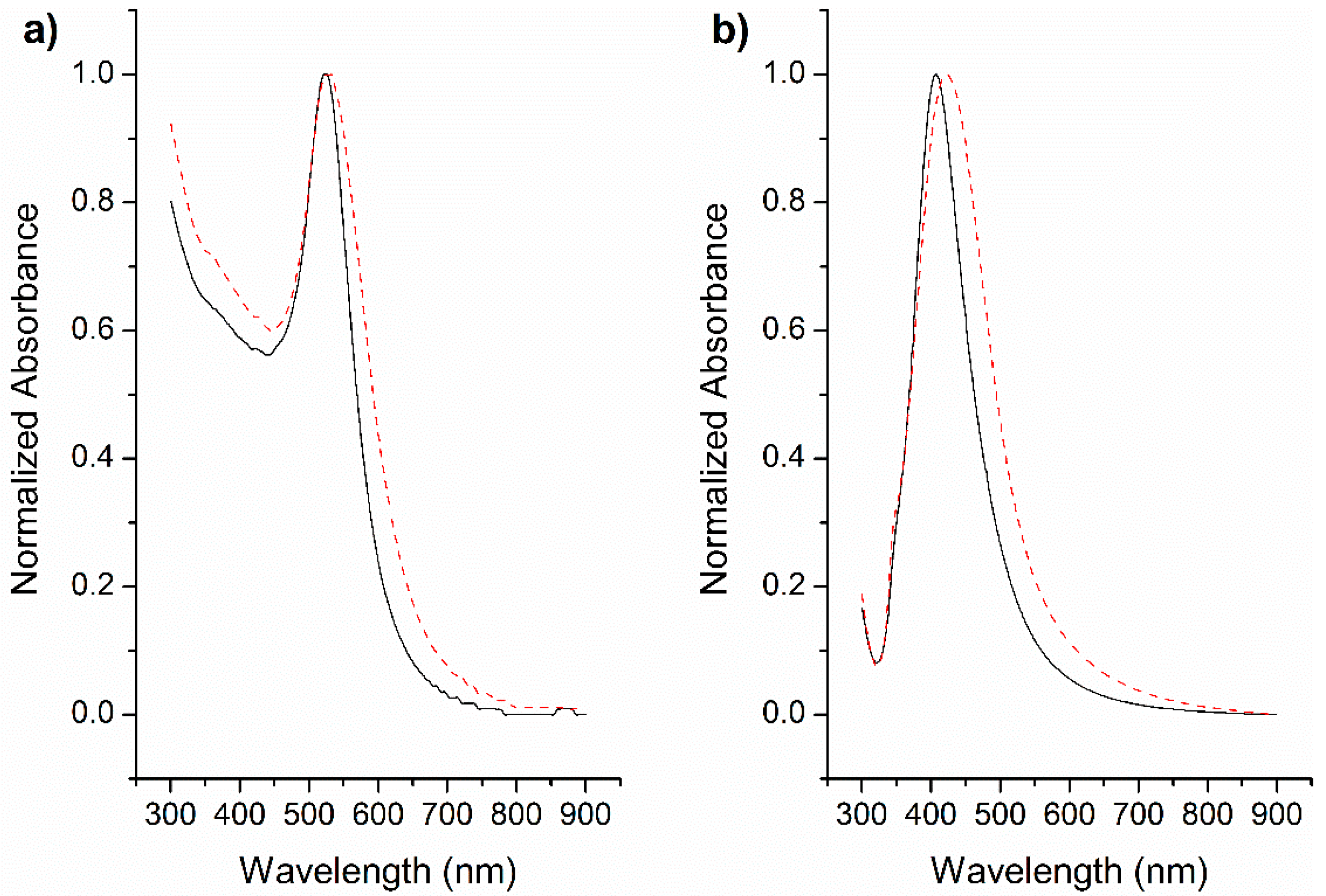
3.3. Aqueous-Organic Phase Transfer of GNP and SNP
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jingyue, Z.; Bernd, F. Synthesis of Gold Nanoparticles Via Chemical Reduction Methods. In Proceedings of the Nanocon, Brno, Czech Republic, 14–16 October, 2015; pp. 597–604.
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Nour, K.M.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Sastry, M.; Ahmad, A.; Khan, M.I.; Kumar, R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr. Sci. 2003, 85, 162–170. [Google Scholar]
- Zhang, Q.; Lee, J.Y.; Yang, J.; Boothroyd, C.; Zhang, J. Size and composition tunable Ag–Au alloy nanoparticles by replacement reactions. Nanotechnology 2007, 18, 245605. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H. Introduction. In Metal-Based Composite Nanomaterials; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–9. [Google Scholar]
- Wei, G.; Yang, Z.; Lee, C.; Yang, H.; Wang, C.R.C. Aqueous-Organic Phase Transfer of Gold Nanoparticles and Gold Nanorods Using an Ionic Liquid. J. Am. Chem. Soc. 2004, 126, 5036–5037. [Google Scholar] [CrossRef] [PubMed]
- Misra, T.K.; Chen, T.-S.; Liu, C.-Y. Phase transfer of gold nanoparticles from aqueous to organic solution containing resorcinarene. J. Colloid Interface Sci. 2006, 297, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Lista, M.; Liu, D.Z.; Mulvaney, P. Phase Transfer of Noble Metal Nanoparticles to Organic Solvents. Langmuir 2014, 30, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Lala, N.; Lalbegi, S.P.; Adyanthaya, S.D.; Sastry, M. Phase transfer of aqueous gold colloidal particles capped with inclusion complexes of cyclodextrin and alkanethiol molecules into chloroform. Langmuir 2002, 17, 3766–3768. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Ying, J.Y.; Lee, J.Y. Phase transfer and its applications in nanotechnology. Chem. Soc. Rev. 2011, 40, 1672–1696. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Phil. Trans. R. Soc. A 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Asfer, M.; Akhter, S.; Mohapatra, S.; Warsi, M.H.; Jain, N.; Mallick, N.; Jain, G.K.; Ali, A.; Panigrahi, P.K.; et al. Aqueous phase transfer of oleic acid coated iron oxide nanoparticles: Influence of solvents and surfactants on stability and pharmaceutical applications of ferrofluid. Magnetohydrodynamics 2013, 49, 339–343. [Google Scholar]
- Bala, T.; Swami, A.; Prasad, B.L.V; Sastry, M. Phase transfer of oleic acid capped NicoreAgshell nanoparticles assisted by the flexibility of oleic acid on the surface of silver. J. Colloid Interface Sci. 2005, 283, 422–431. [Google Scholar] [CrossRef] [PubMed]
- De La Presa, P.; Multigner, M.; De La Venta, J.; García, M.A.; Ruiz-González, M.L. Structural and magnetic characterization of oleic acid and oleylamine-capped gold nanoparticles. J. Appl. Phys. 2006, 100, 123915. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Prevo, B.G.; Esakoff, S.A.; Mikhailovsky, A.; Zasadzinski, J.A. Scalable routes to gold nanoshells with tunable sizes and response to near-infrared pulsed-laser irradiation. Small 2008, 4, 1183–1195. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; Wiley: Toronto, ON, Canada, 2004; Volume 8. [Google Scholar]
- Lee, D.H.; Condrate, R. A FTIR spectral characterization of thin film coatings of oleic acid on glasses: I. Coatings on glasses from ethyl alcohol. J. Mater. Sci. 1999, 34, 139–146. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Jain, P.K.; ElSayed, I.H.; El-Sayed, M.A. Au nanoparticles target cancer. Nano Today 2007, 2, 18–29. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Yaseen, A.I. B.; Park, J.; Eller, J.R.; Murphy, C.J. Facile phase transfer of gold nanoparticles from aqueous solution to organic solvents with thiolated poly(ethylene glycol). RSC Adv. 2014, 4, 52676–52679. [Google Scholar] [CrossRef]
- Wang, Y.; Wong, J.F.; Teng, X.; Lin, X.Z.; Yang, H. “Pulling” Nanoparticles into Water: Phase Transfer of Oleic Acid Stabilized Monodisperse Nanoparticles into Aqueous Solutions of α-Cyclodextrin. Nano Lett. 2003, 3, 1555–1559. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Millán, A.; Zavala-Rivera, P.; Esquivel, R.; Carrillo, R.; Alvarez-Ramos, E.; Moreno-Corral, R.; Guzmán-Zamudio, R.; Lucero-Acuña, A. Aqueous-Organic Phase Transfer of Gold and Silver Nanoparticles Using Thiol-Modified Oleic Acid. Appl. Sci. 2017, 7, 273. https://doi.org/10.3390/app7030273
López-Millán A, Zavala-Rivera P, Esquivel R, Carrillo R, Alvarez-Ramos E, Moreno-Corral R, Guzmán-Zamudio R, Lucero-Acuña A. Aqueous-Organic Phase Transfer of Gold and Silver Nanoparticles Using Thiol-Modified Oleic Acid. Applied Sciences. 2017; 7(3):273. https://doi.org/10.3390/app7030273
Chicago/Turabian StyleLópez-Millán, Alejandra, Paul Zavala-Rivera, Reynaldo Esquivel, Roberto Carrillo, Enrique Alvarez-Ramos, Ramón Moreno-Corral, Roberto Guzmán-Zamudio, and Armando Lucero-Acuña. 2017. "Aqueous-Organic Phase Transfer of Gold and Silver Nanoparticles Using Thiol-Modified Oleic Acid" Applied Sciences 7, no. 3: 273. https://doi.org/10.3390/app7030273