Enhancement of High-Intensity Focused Ultrasound Heating by Short-Pulse Generated Cavitation
Abstract
:1. Introduction
2. Trigger HIFU Sequence
3. High-Speed Observation of Cavitation Bubbles Induced by Trigger HIFU Exposure
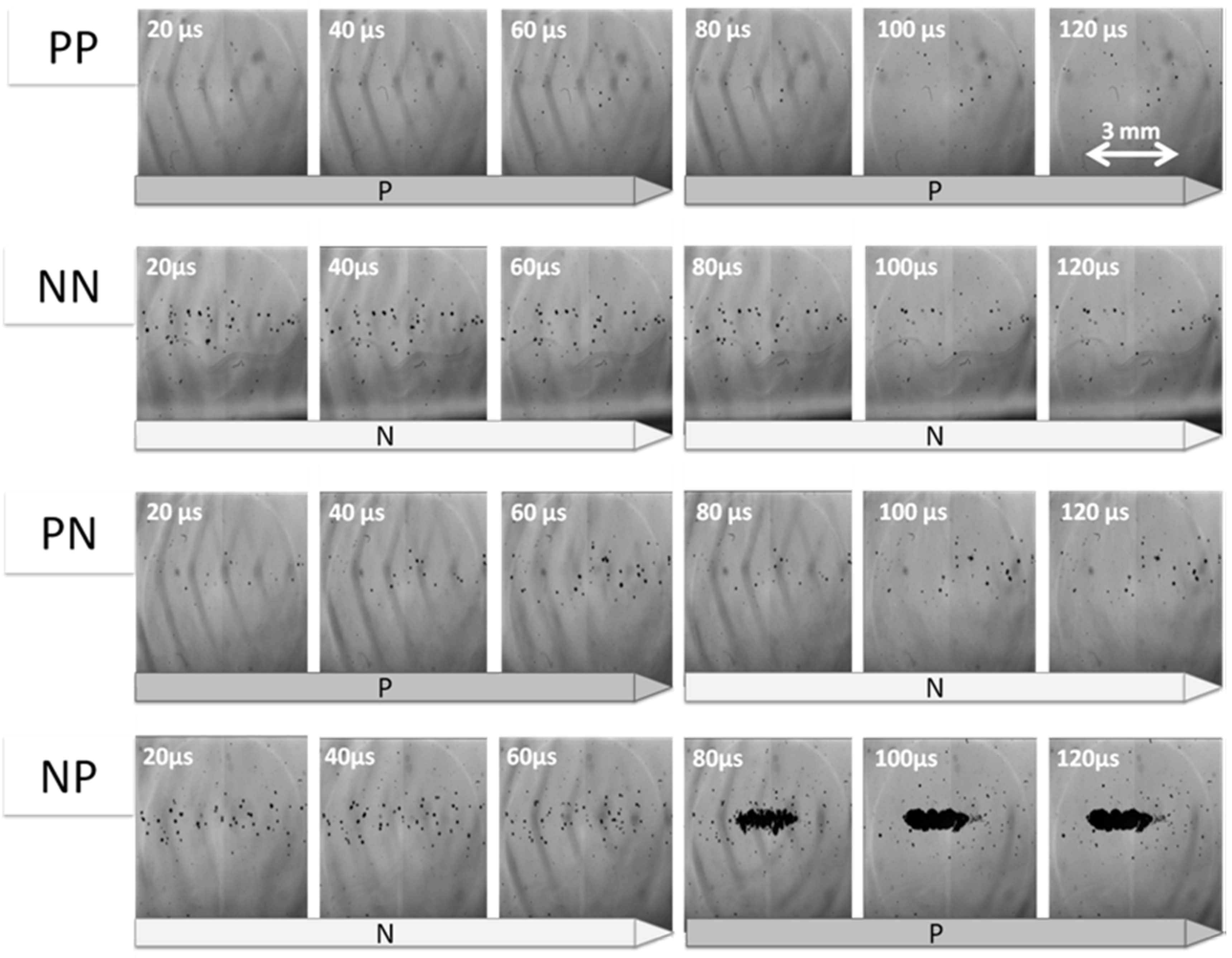
3.1. Cloud Cavitaion Formation by Dual-Frequency Ultrasound Exposure
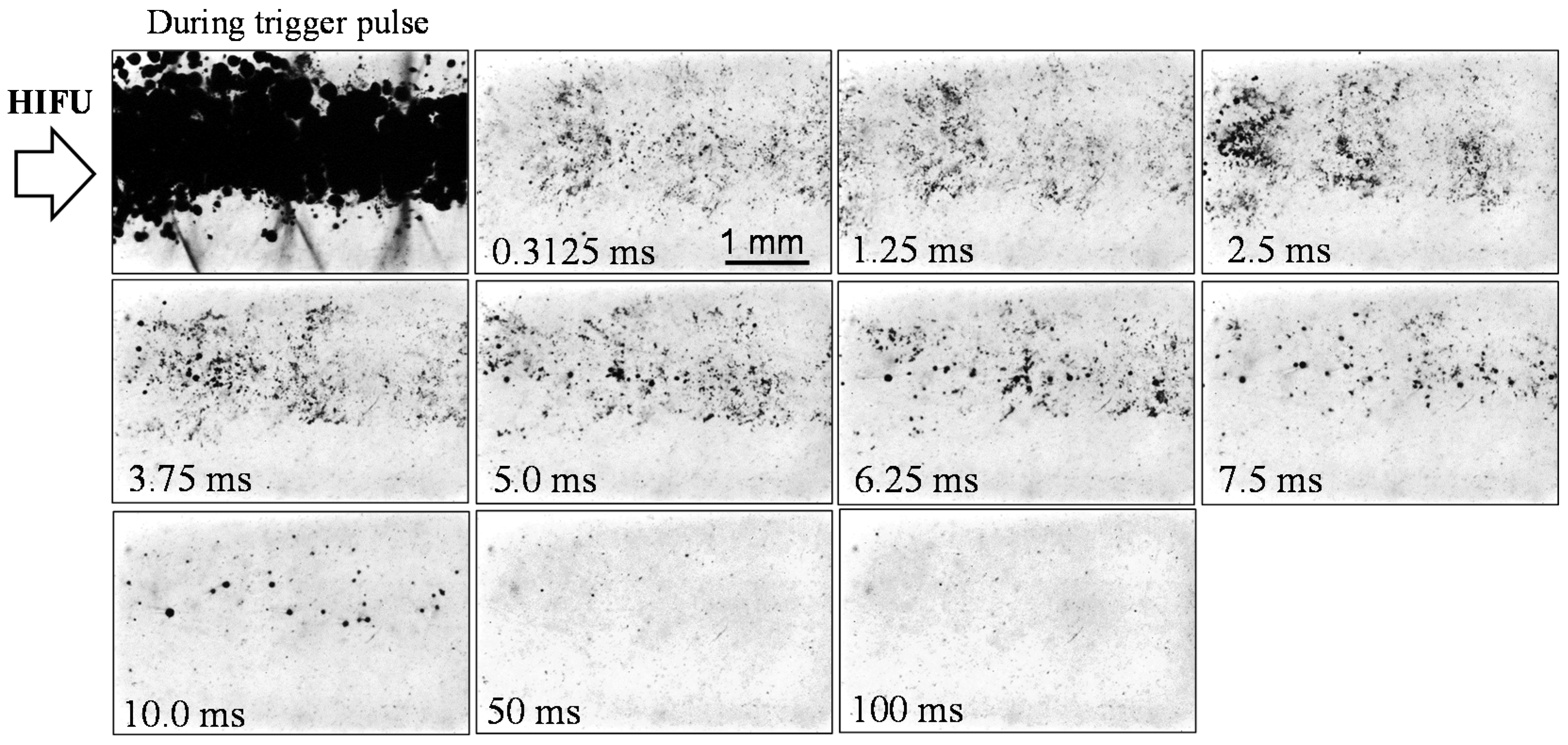
3.2. Lifetime of Cavitaion during and after HIFU Exposure
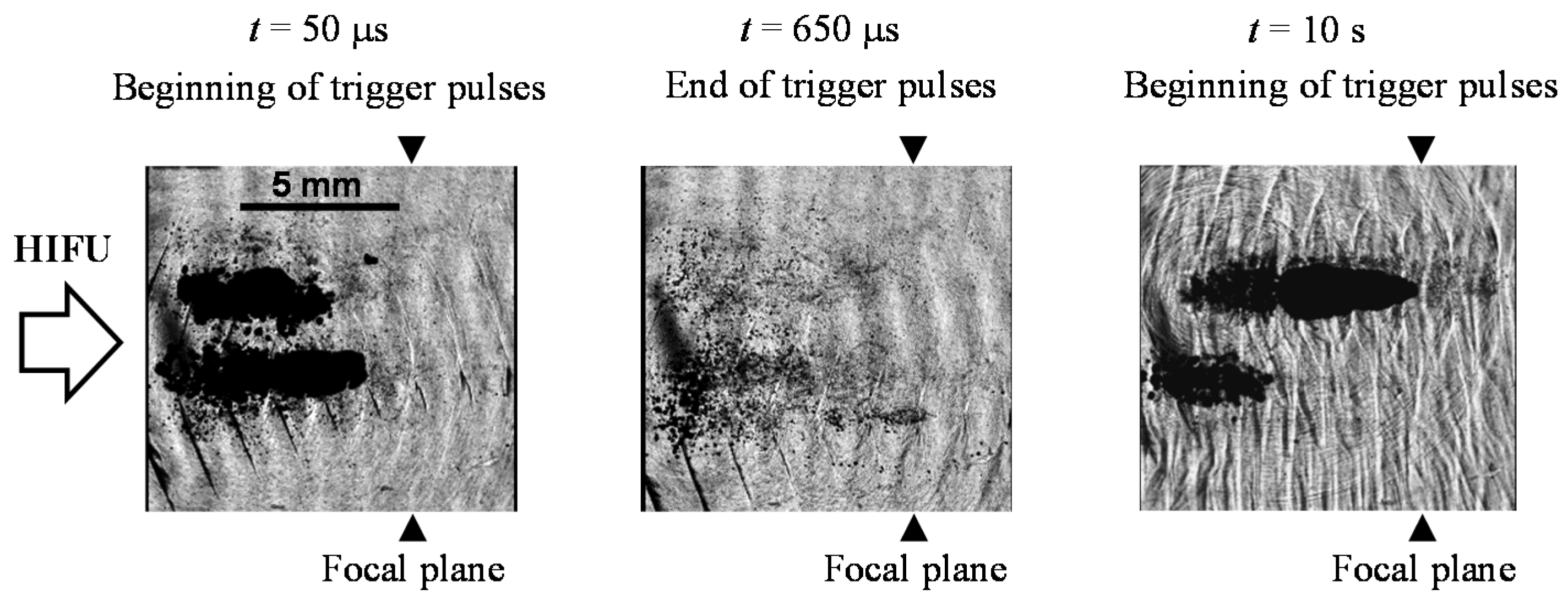
3.3. Cavitation Generated in Multiple Focal Spots
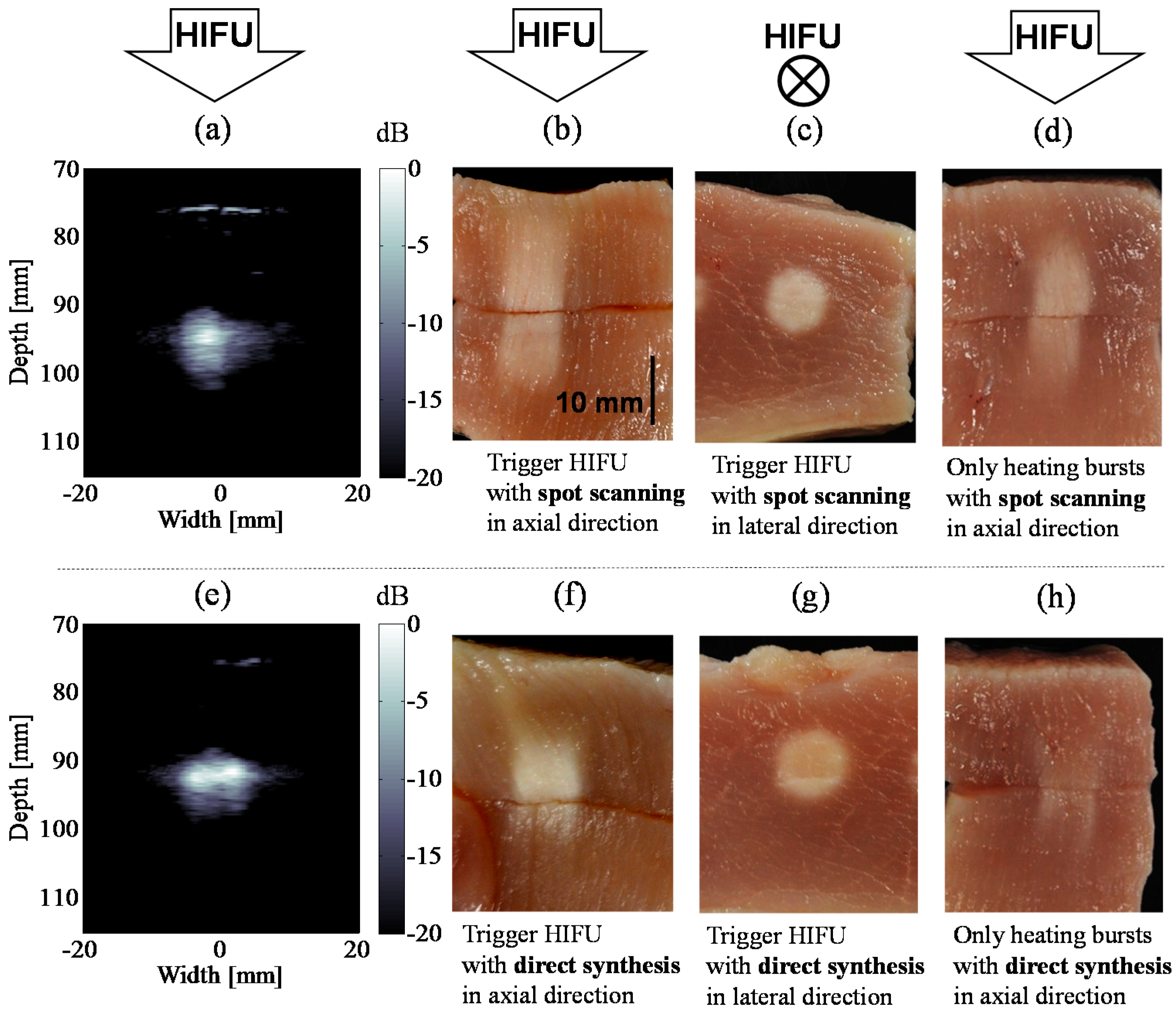
4. Temperature Increase and Coagulation Region by Trigger HIFU Exposure
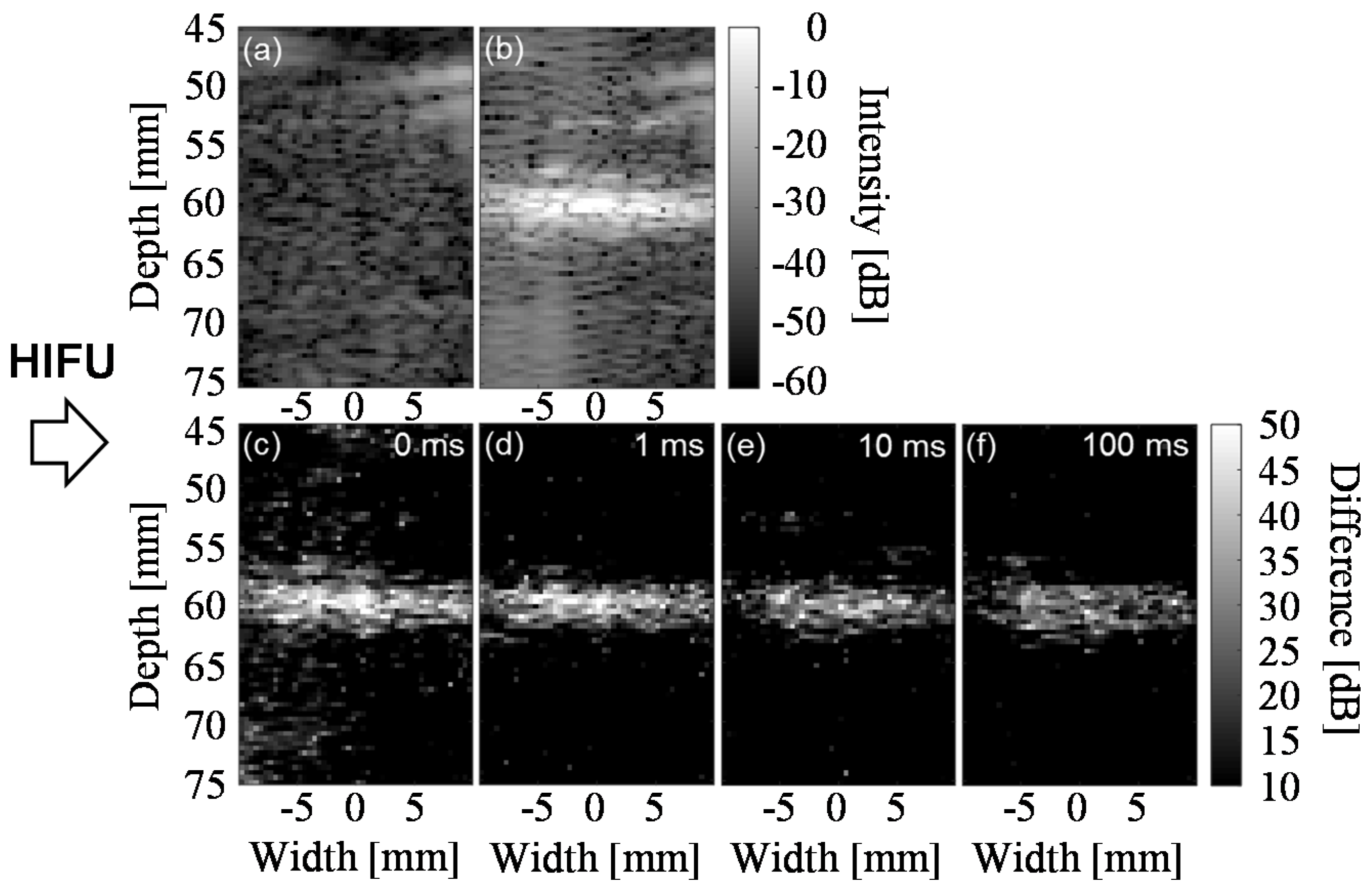
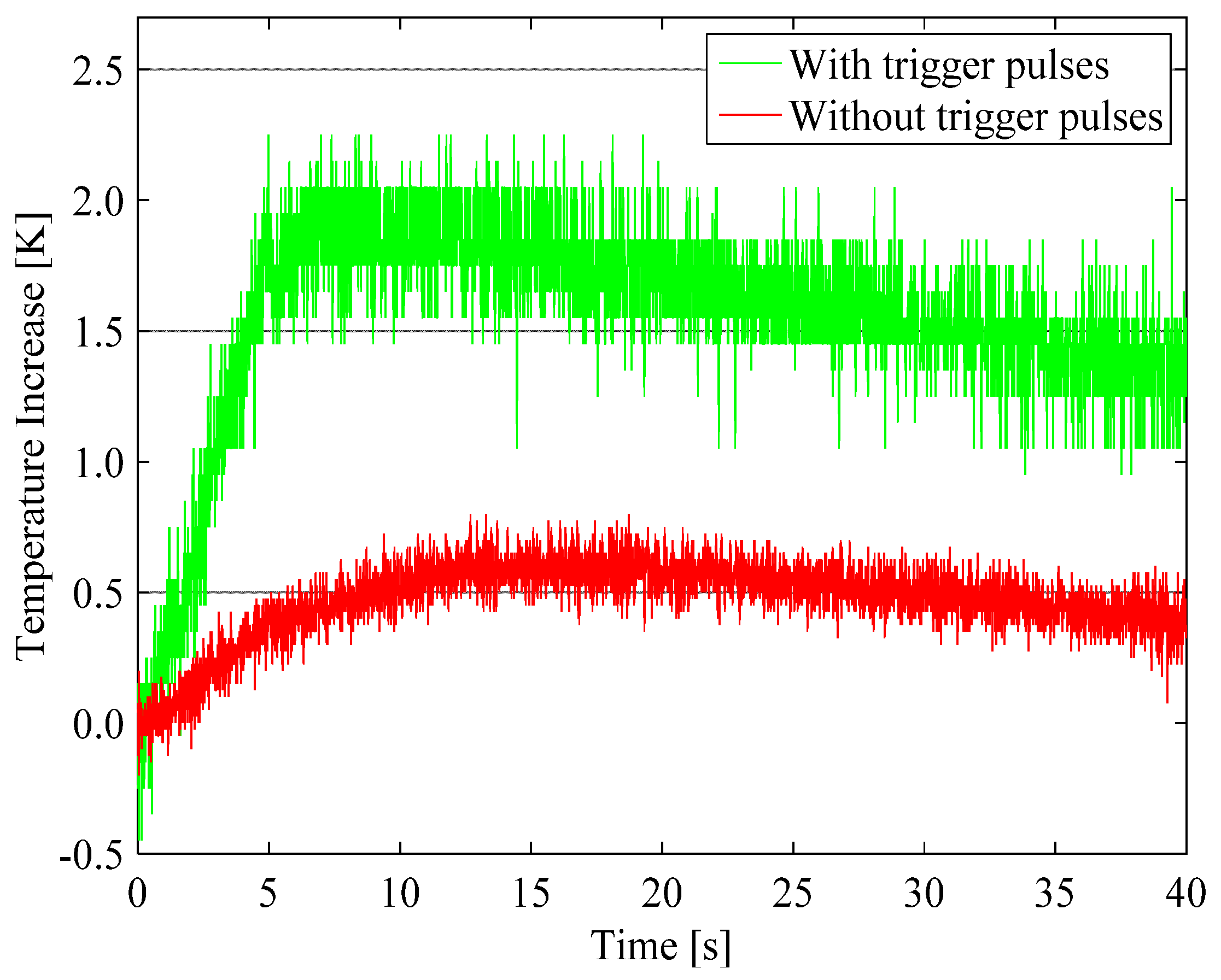
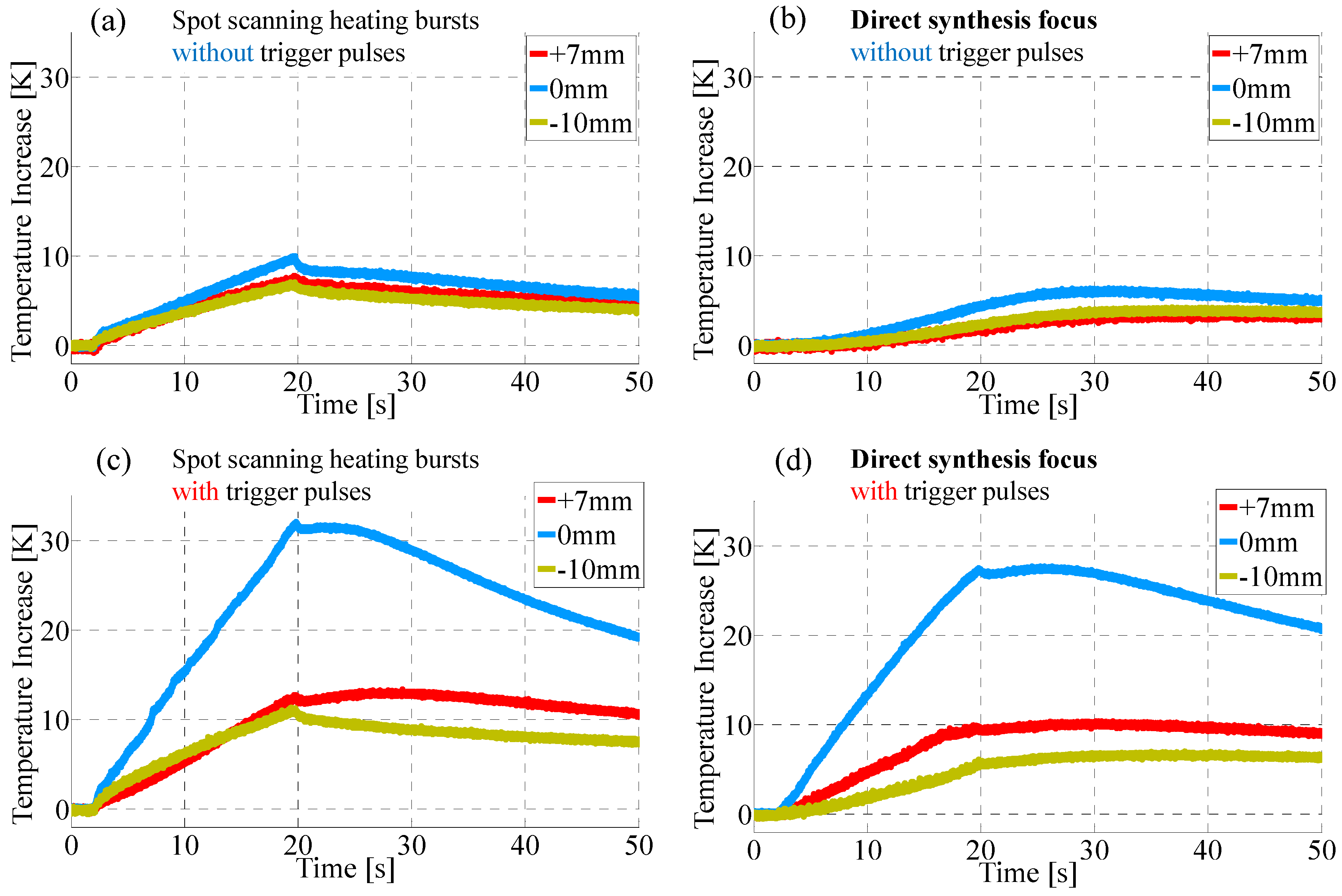
4.1. Enhancement of Temperature Increase by Trigger Pulses
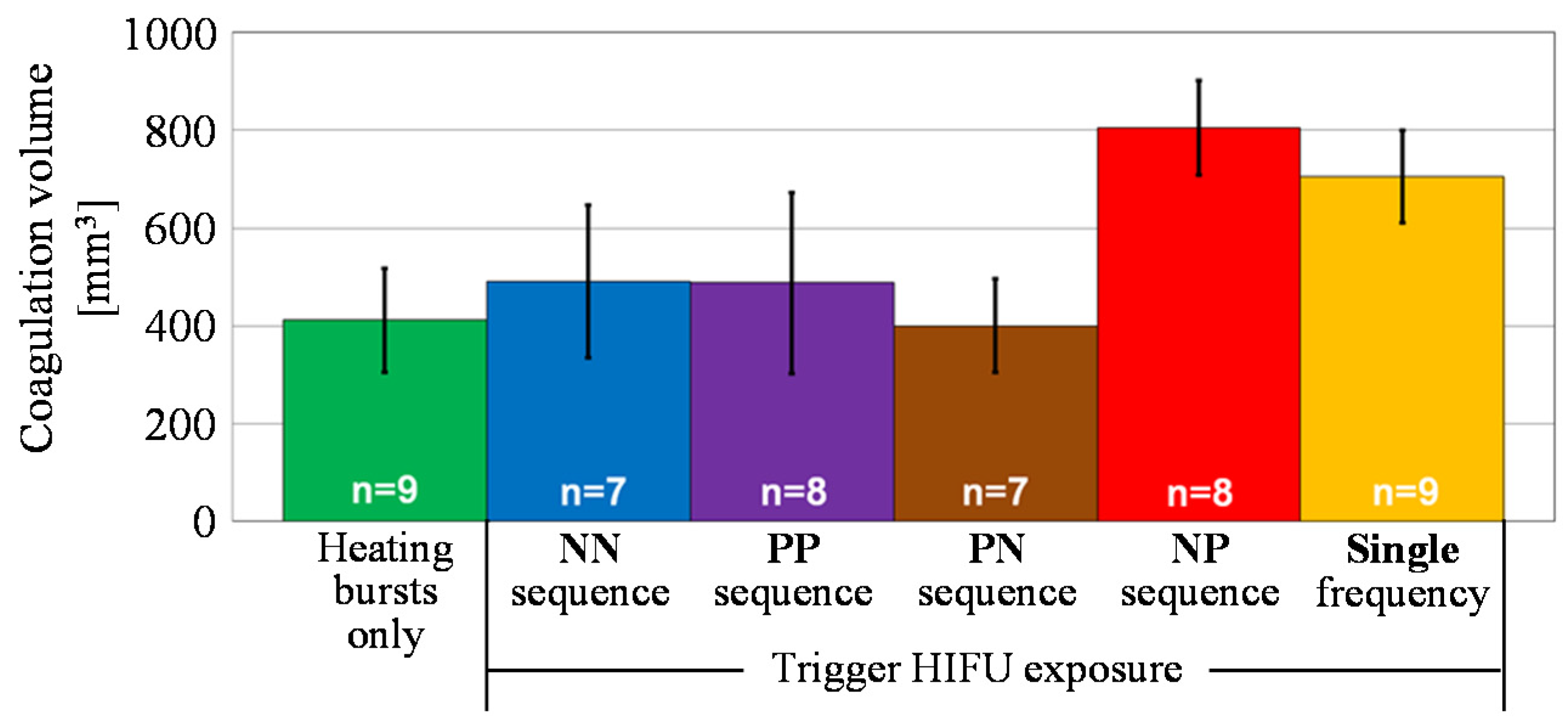
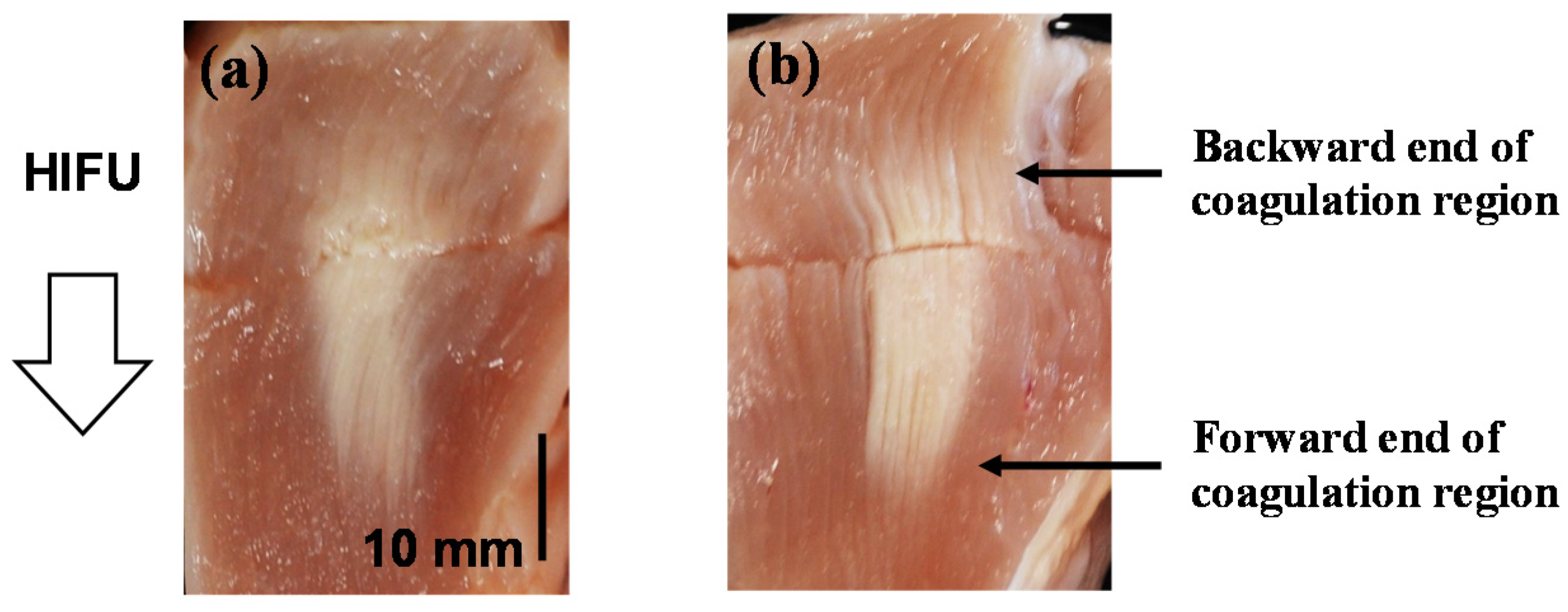
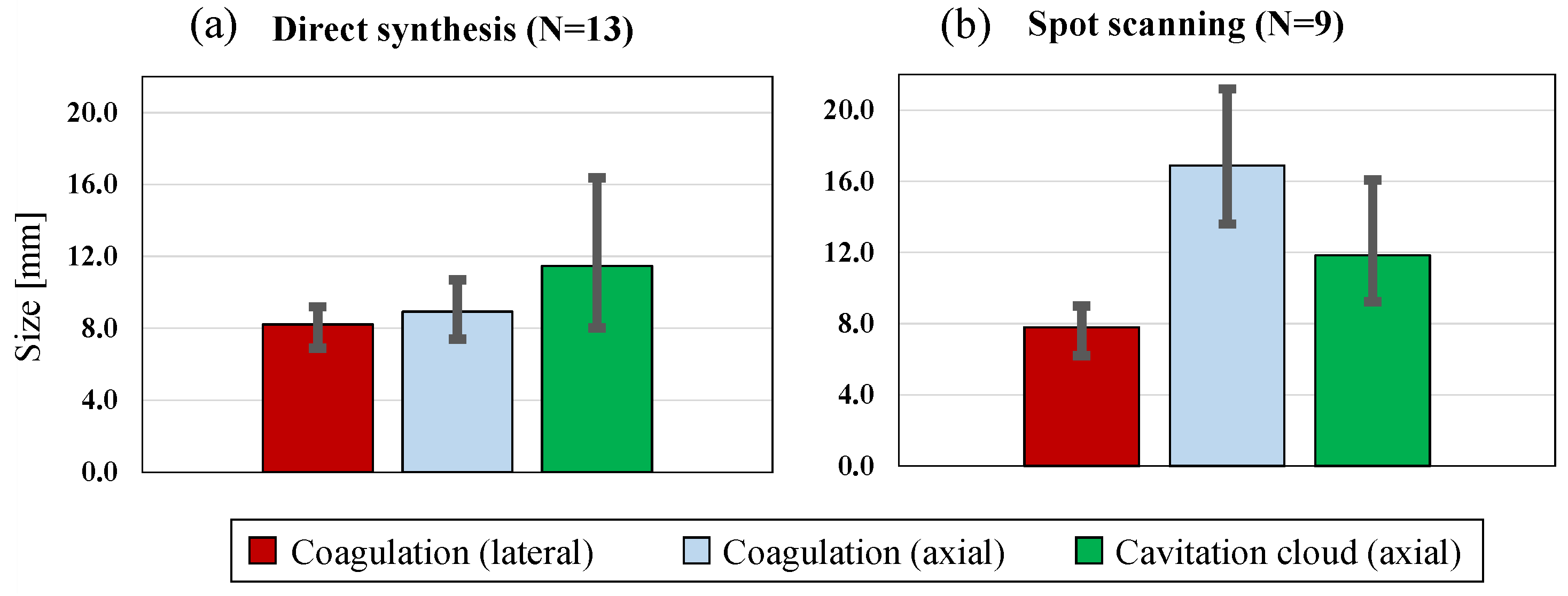
4.2. Coagulation Region Size and Predictability
5. Conclusions
Conflicts of Interest
References
- Kennedy, J.E.; ter Haar, G.; Cranston, D. High intensity focused ultrasound: Surgery of the future? Br. J. Radiol. 2003, 76, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Sanghvi, N.T.; Gardner, T.A.; Koch, M.O.; Ishii, D.; Minei, S.; Satoh, T.; Hyodo, T.; Irie, A.; Baba, S. Transrectal high-intensity focused ultrasound for treatment of patients with stage T1b-2N0M0 localized prostate cancer: A preliminary report. Urology 2002, 59, 394–398. [Google Scholar] [CrossRef]
- Murat, F.J.; Gelet, A. Current status of high-intensity focused ultrasound for prostate cancer: Technology, clinical outcomes, and future. Curr. Urol. Rep. 2008, 9, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Shoji, S.; Nakano, M.; Hongo, S.; Nitta, M.; Murota, A.; Nagata, Y. Transrectal high-intensity focused ultrasound for the treatment of localized prostate cancer: Eight-year experience. Int. J. Urol. 2009, 16, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Nakano, M.; Hongo, S.; Shoji, S.; Nagata, Y.; Satoh, T.; Baba, S.; Usui, Y.; Terachi, T. High-intensity focused ultrasound therapy for prostate cancer. Int. J. Urol. 2012, 19, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, H.; Namba, K.; Thomsen, S.; Akiyama, F.; Bendet, A.; Tanaka, C.; Yasuda, Y.; Nakahara, H. Magnetic resonance–guided focused ultrasound surgery of breast cancer: Reliability and effectiveness. J. Am. Coll. Surg. 2006, 203, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.E.; Wu, F.; ter Haar, G.R.; Gleeson, F.V.; Phillips, R.R.; Middleton, M.R.; Cranston, D. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics 2004, 42, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Fukuda, H.; Ohto, M.; Itou, R.; Nozaki, A.; Kondou, M.; Morimoto, M.; Karasawa, E.; Tanaka, K. Evaluation of the therapeutic efficacy of high-intensity focused ultrasound ablation of hepatocellular carcinoma by three-dimensional sonography with a perflubutane-based contrast agent. Eur. J. Radiol. 2010, 75, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Sofuni, A.; Moriyasu, F.; Sano, T.; Itokawa, F.; Tsuchiya, T.; Kurihara, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Tanaka, R.; et al. Safety trial of high-intensity focused ultrasound therapy for pancreatic cancer. World J. Gastroenterol. 2014, 20, 9570–9577. [Google Scholar] [CrossRef]
- Hurwitz, M.D.; Ghanouni, P.; Kanaev, S.V.; Iozeffi, D.; Gianfelice, D.; Fennessy, F.M.; Kuten, A.; Meyer, J.E.; LeBlang, S.D.; Roberts, A.; et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: Phase III trial results. J. Natl. Cancer. Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.G.; Roy, R.A. Measurements of bubble-enhanced heating from focused, MHz-frequency ultrasound in a tissue-mimicking material. Ultrasound Med. Biol. 2001, 27, 1399–1412. [Google Scholar] [CrossRef]
- Bailey, M.R.; Couret, L.N.; Sapozhnikov, O.A.; Khokhlova, V.A.; ter Haar, G.; Vaezy, S.; Shi, X.; Martin, R.; Crum, L.A. Use of overpressure to assess the role of bubbles in focused ultrasound lesion shape in vitro. Ultrasound Med. Biol. 2001, 27, 695–708. [Google Scholar] [CrossRef]
- Sokka, S.D.; King, R.; Hynynen, K. MRI-guided gas bubble enhanced ultrasound heating in in vivo rabbit thigh. Phys. Med. Biol. 2003, 48, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Curiel, L.; Chavrier, F.; Gignoux, B.; Pichardo, S.; Chesnais, S.; Chapelon, J.Y. Experimental evaluation of lesion prediction modelling in the presence of cavitation bubbles: Intended for high-intensity focused ultrasound prostate treatment. Med. Biol. Eng. Comput. 2004, 42, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Maruyama, T.; Takegami, K.; Watanabe, T.; Mitsui, H.; Hanajiri, K.; Nagawa, H.; Matsumoto, Y. Use of a microbubble agent to increase the effects of high intensity focused ultrasound on liver tissue. Eur. Radiol. 2005, 15, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Umemura, S.; Kawabata, K.; Sasaki, K. In vivo acceleration of ultrasonic tissue heating by microbubble agent. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2005, 52, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Razansky, D.; Einziger, P.D.; Adam, D.R. Enhanced heat deposition using ultrasound contrast agent–modeling and experimental observations. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2006, 53, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Chen, W.S.; Chen, J.S.; Shih, T.C.; Chen, Y.Y.; Lin, W.L. Cavitation-enhanced ultrasound thermal therapy by combined low- and high-frequency ultrasound exposure. Ultrasound Med. Biol. 2006, 32, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.S.; Liu, H.L.; Wu, C.C.; Ju, K.C.; Chen, W.S.; Lin, W.L. Contrast-agent-enhanced ultrasound thermal ablation. Ultrasound Med. Biol. 2006, 32, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Farny, C.H.; Holt, R.G.; Roy, R.A. The correlation between bubble-enhanced HIFU heating and cavitation power. IEEE Trans. Biomed. Eng. 2010, 57, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, X.W. Variations of bubble cavitation and temperature elevation during lesion formation by high-intensity focused ultrasound. J. Acoust. Soc. Am. 2013, 134, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Elbes, D.; Denost, Q.; Robert, B.; Köhler, M.O.; Tanter, M.; Bruno, Q. Magnetic resonance imaging for the exploitation of bubble-enhanced heating by high-intensity focused ultrasound: A feasibility study in ex vivo liver. Ultrasound Med. Biol. 2014, 40, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Kopechek, J.A.; Park, E.J.; Zhang, Y.Z.; Vykhodtseva, N.I.; McDannold, N.J.; Porter, T.M. Cavitation-enhanced MR-guided focused ultrasound ablation of rabbit tumors in vivo using phase shift nanoemulsions. Phys. Med. Biol. 2014, 59, 3465–3481. [Google Scholar] [CrossRef] [PubMed]
- Haworth, K.J.; Salgaonkar, V.A.; Corregan, N.M.; Holland, C.K.; Mast, T.D. Using passive cavitation images to classify high-intensity focused ultrasound lesions. Ultrasound Med. Biol. 2015, 41, 2420–2434. [Google Scholar] [CrossRef] [PubMed]
- Chavrier, F.; Chapelon, J.Y.; Gelet, A.; Cathignol, D. Modeling of high- intensity focused ultrasound-induced lesions in the presence of cavitation bubbles. J. Acoust. Soc. Am. 2000, 108, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Sugiyama, K.; Takagi, S.; Matsumto, Y. Microbubble behavior in an ultrasound field for high intensity focused ultrasound therapy enhancement. J. Acoust. Soc. Am. 2013, 134, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Takagi, R.; Yoshizawa, S.; Umemura, S. Enhancement of localized heating by ultrasonically induced cavitation in high intensity focused ultrasound treatment. Jpn. J. Appl. Phys. 2010, 49, 07HF21. [Google Scholar] [CrossRef]
- Inaba, Y.; Yoshizawa, S.; Umemura, S. Coagulation of large regions by creating multiple cavitation clouds for high intensity focused ultrasound treatment. Jpn. J. Appl. Phys. 2010, 49, 07HF22. [Google Scholar] [CrossRef]
- Inaba, Y.; Moriyama, T.; Yoshizawa, S.; Umemura, S. Ultrasonic coagulation of large tissue region by generating multiple cavitation clouds in direction perpendicular to ultrasound propagation. Jpn. J. Appl. Phys. 2011, 50, 07HF13. [Google Scholar] [CrossRef]
- Moriyama, T.; Yoshizawa, S.; Umemura, S. Thermal simulation of cavitation-enhanced ultrasonic heating verified with tissue-mimicking gel. Jpn. J. Appl. Phys. 2012, 51, 07GF27. [Google Scholar] [CrossRef]
- Asai, A.; Okano, H.; Yoshizawa, S.; Umemura, S. Analysis of temperature rise induced by high-intensity focused ultrasound in tissue-mimicking gel considering cavitation bubbles. Jpn. J. Appl. Phys. 2013, 52, 07HF02. [Google Scholar] [CrossRef]
- Nakamura, K.; Asai, A.; Sasaki, H.; Yoshizawa, S.; Umemura, S. Large volume coagulation utilizing multiple cavitation clouds generated by array transducer driven by 32 channel drive circuits. Jpn. J. Appl. Phys. 2013, 52, 07HF10. [Google Scholar] [CrossRef]
- Sasaki, H.; Yasuda, J.; Takagi, R.; Miyahsita, T.; Goto, K.; Yoshizawa, S.; Umemura, S. Highly efficient cavitation-enhanced heating with dual-frequency ultrasound exposure in high-intensity focused ultrasound treatment. Jpn. J. Appl. Phys. 2014, 53, 07KF11. [Google Scholar] [CrossRef]
- Goto, K.; Takagi, R.; Miyashita, T.; Jimbo, H.; Yoshizawa, S.; Umemura, S. Effect of controlled offset of focal position in cavitation-enhanced high-intensity focused ultrasound treatment. Jpn. J. Appl. Phys. 2015, 54, 07HF12. [Google Scholar] [CrossRef]
- Jimbo, H.; Takagi, R.; Taguchi, K.; Yoshizawa, S.; Umemura, S. Advantage of annular focus generation by sector-vortex array in cavitation-enhanced high-intensity focused ultrasound treatment. Jpn. J. Appl. Phys. 2016, 55, 07KF19. [Google Scholar] [CrossRef]
- Taguchi, K.; Takagi, R.; Yasuda, J.; Yoshizawa, S.; Umemura, S. Study on cavitation behavior during high-intensity focused ultrasound exposure by using optical and ultrasonic imaging. Jpn. J. Appl. Phys. 2016, 55, 07KF22. [Google Scholar] [CrossRef]
- Kawabata, K.; Sugita, N.; Yoshikawa, H.; Azuma, T.; Umemura, S. Nanoparticles with multiple perfluorocarbons for controllable ultrasonically induced phase shifting. Jpn. J. Appl. Phys. 2005, 44, 4548–4552. [Google Scholar] [CrossRef]
- Kawabata, K.; Asami, R.; Yoshikawa, H.; Azuma, T.; Umemura, S. Sustaining microbubbles derived from phase change nanodroplet by low-amplitude ultrasound exposure. Jpn. J. Appl. Phys. 2010, 49, 07HF20. [Google Scholar] [CrossRef]
- Zhang, P.; Porter, T. An in vitro study of a phase-shift nanoemulsion: A potential nucleation agent for bubble-enhanced HIFU tumor ablation. Ultrasound Med. Biol. 2010, 36, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.D.; Cain, C.A.; Hall, T.L.; Fowlkes, J.B.; Xu, Z. Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound Med. Biol. 2013, 39, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.W.; Kim, Y.; Maxwell, A.D.; Wang, T.Y.; Hall, T.L.; Xu, Z.; Fowlkes, J.B.; Cain, C.A. Histotripsy beyond the intrinsic cavitation threshold using very short ultrasound pulses: Microtripsy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2014, 61, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Sankin, G.; Teslenko, V. Two-threshold cavitation regime. Dokl. Phys. 2003, 48, 665–668. [Google Scholar] [CrossRef]
- Zhang, X.; Owens, G.E.; Gurm, H.S.; Ding, Y.; Cain, C.A.; Xu, Z. Noninvasive thrombolysis using histotripsy beyond the intrinsic threshold (microtripsy). IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2015, 62, 1342–1355. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.W.; Hall, T.L.; Xu, Z.; Cain, C.A. Histotripsy lesion formation using an ultrasound imaging probe enabled by a low-frequency pump transducer. Ultrasound Med. Biol. 2015, 41, 2148–2160. [Google Scholar] [CrossRef] [PubMed]
- Sukovich, J.; Xu, Z.; Kim, Y.; Cao, H.; Nguyen, T.S.; Pandey, A.; Hall, T.; Cain, C. Targeted lesion generation through the skull without aberration correction using histotripsy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2016, 63, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.D.; Wang, T.Y.; Cain, C.A.; Fowlkes, J.B.; Sapozhnikov, O.A.; Bailey, M.R.; Xu, Z. Cavitation clouds created by shock scattering from bubbles during histotripsy. J. Acoust. Soc. Am. 2011, 130, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.L.; Gregory, D.W.; Shammari, M.; Watmough, D.J.; Wheatley, D.N. Differing effects of ultrasound-irradiation on suspension and monolayer cultured HeLa cells, investigated by scanning electron microscopy. Ultrasound Med. Biol. 1989, 15, 397–401. [Google Scholar] [CrossRef]
- Azuma, T.; Kawabata, K.; Umemura, S.; Ogihara, M.; Kubota, J.; Sasaki, A.; Furuhata, H. Bubble generation by standing wave in water surrounded by cranium with transcranial ultrasonic beam. Jpn. J. Appl. Phys. 2005, 44, 4625–4630. [Google Scholar] [CrossRef]
- Cain, C.A.; Umemura, S. Concentric-ring and sector-vortex phased-array applicators for ultrasound hyperthermia. IEEE Trans. Microwave Theory Tech. 1986, 34, 542–551. [Google Scholar] [CrossRef]
- Umemura, S.; Cain, C.A. The sector-vortex phased array: Acoustic field synthesis for hyperthermia. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1989, 36, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Umemura, S.; Kawabata, K.; Sasaki, K. In vitro and in vivo enhancement of sonodynamically active cavitation by second-harmonic superimposition. J. Acoust. Soc. Am. 1997, 101, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Takagi, R.; Yoshizawa, S.; Umemura, S. Cavitation inception by dual-frequency excitation in high-intensity focused ultrasound treatment. Jpn. J. Appl. Phys. 2011, 50, 07HF14. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Yasuda, J.; Umemura, S. High-speed observation of bubble cloud generation near a rigid wall by second-harmonic superimposed ultrasound. J. Acoust. Soc. Am. 2013, 134, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, J.; Asai, A.; Yoshizawa, S.; Umemura, S. Efficient generation of cavitation bubbles in gel phantom by ultrasound exposure with negative-followed by positive-peak-pressure-emphasized waves. Jpn. J. Appl. Phys. 2013, 52, 07HF11. [Google Scholar] [CrossRef]
- Chen, W.S.; Matula, T.J.; Crum, L.A. The disappearance of ultrasound contrast bubbles: Observations of bubble dissolution and cavitation nucleation. Ultrasound Med. Biol. 2002, 28, 793–803. [Google Scholar] [CrossRef]
- Tanter, M.; Fink, M. Ultrafast imaging in biomedical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2014, 61, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Gateau, J.; Aubry, J.F.; Pernot, M.; Fink, M.; Tanter, M. Combined passive detection and ultrafast active imaging of cavitation events induced by short pulses of high-intensity ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2011, 58, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Prieur, F.; Zorgani, A.; Catheline, S.; Souchon, R.; Mestas, J.L.; Lafond, M.; Lafon, C. Observation of a cavitation cloud in tissue using correlation between ultrafast ultrasound images. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2015, 62, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Eller, A.; Flynn, H.G. Rectified diffusion during nonlinear pulsations of cavitation bubble. J. Acoust. Soc. Am. 1965, 37, 493–503. [Google Scholar] [CrossRef]
- Crum, L.A. Measurements of the growth of air bubbles by rectified diffusion. J. Acoust. Soc. Am. 1980, 68, 203–211. [Google Scholar] [CrossRef]
- Church, C.C. Prediction of rectified diffusion during nonlinear bubble pulsations at biomedical frequencies. J. Acoust. Soc. Am. 1988, 83, 2210–2217. [Google Scholar] [CrossRef] [PubMed]
- Bjerknes, V. Fields of Force; Colombia University Press: New York, NY, USA, 1906. [Google Scholar]
- Doinikov, A.A. Acoustic radiation interparticle forces in a compressible fluid. J. Fluid Mech. 2001, 444, 1–21. [Google Scholar] [CrossRef]
- Brennen, C.E. Fission of collapsing cavitation bubbles. J. Fluid Mech. 2002, 472, 153–166. [Google Scholar] [CrossRef]
- Moro, K.; Yoshizawa, S.; Umemura, S. Staircase-voltage metal–oxide–semiconductor field-effect transistor driver circuit for therapeutic ultrasound. Jpn. J. Appl. Phys. 2010, 49, 07HF02. [Google Scholar] [CrossRef]
- Simpson, D.H.; Chin, C.T.; Burns, P.N. Pulse inversion Doppler: A new method for detecting nonlinear echoes from microbubble contrast agents. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1999, 46, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Mougenot, C.; Quesson, B.; de Senneville, B.D.; de Oliveira, P.L.; Sprinkhuizen, S.; Palussière, J.; Grenier, N.; Moonen, C.T. Three-dimensional spatial and temporal temperature control with MR thermometry-guided focused ultrasound (MRgHIFU). Magn. Reson. Med. 2009, 61, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Köhler, M.O.; Mougenot, C.; Quesson, B.; Enholm, J.; Le Bail, B.; Laurent, C.; Moonen, C.T.; Ehnholm, G.J. Improved Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med. Phys. 2009, 36, 3521–3535. [Google Scholar] [CrossRef] [PubMed]
- Enholm, J.; Kohler, M.; Quesson, B.; Mougenot, C.; Moonen, C.; Sokka, S. Improved volumetric MR-HIFU ablation by robust binary feedback control. IEEE Trans. Biomed. Eng. 2010, 57, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Keserci, B.; Partanen, A.; Rhim, H.; Lim, H.K.; Park, M.J.; Köhler, M.O. Volumetric MR-HIFU ablation of uterine fibroids: Role of treatment cell size in the improvement of energy efficiency. Eur. J. Radiol. 2012, 81, 3652–3659. [Google Scholar] [CrossRef] [PubMed]
- Casper, A.; Liu, D.; Ebbini, E.S. Realtime control of multiple-focus phased array heating patterns based on noninvasive ultrasound thermography. IEEE Trans. Biomed. Eng. 2012, 59, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, S.; Matsuura, K.; Takagi, R.; Yamamoto, M.; Umemura, S. Detection of tissue coagulation by decorrelation of ultrasonic echo signals in cavitation-enhanced high-intensity focused ultrasound treatment. J. Ther. Ultrasound. 2016, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Yumita, N.; Nishigaki, R.; Umemura, K.; Umemura, S. Synergistic effect of ultrasound and hematoporphyrin on sarcoma 180. Jpn. J. Cancer Res. 1990, 81, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Y.N.; Khokhlova, T.D.; D’Andrea, S.; Starr, F.; Chen, H.; McCune, J.S.; Risler, L.J.; Mashadi-Hossein, A.; Hwang, J.H. Pulsed high-intensity focused ultrasound enhances delivery of doxorubicin in a preclinical model of pancreatic cancer. Cancer Res. 2015, 75, 3738–3746. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, J.; Miyashita, T.; Taguchi, K.; Yoshizawa, S.; Umemura, S. Quantitative assessment of reactive oxygen sonochemically generated by cavitation bubbles. Jpn. J. Appl. Phys. 2015, 54, 07HF21. [Google Scholar] [CrossRef]
- Yasuda, J.; Yoshizawa, S.; Umemura, S. Efficient generation of cavitation bubbles and reactive oxygen species using triggered high-intensity focused ultrasound sequence for sonodynamic treatment. Jpn. J. Appl. Phys. 2016, 55, 07KF24. [Google Scholar] [CrossRef]
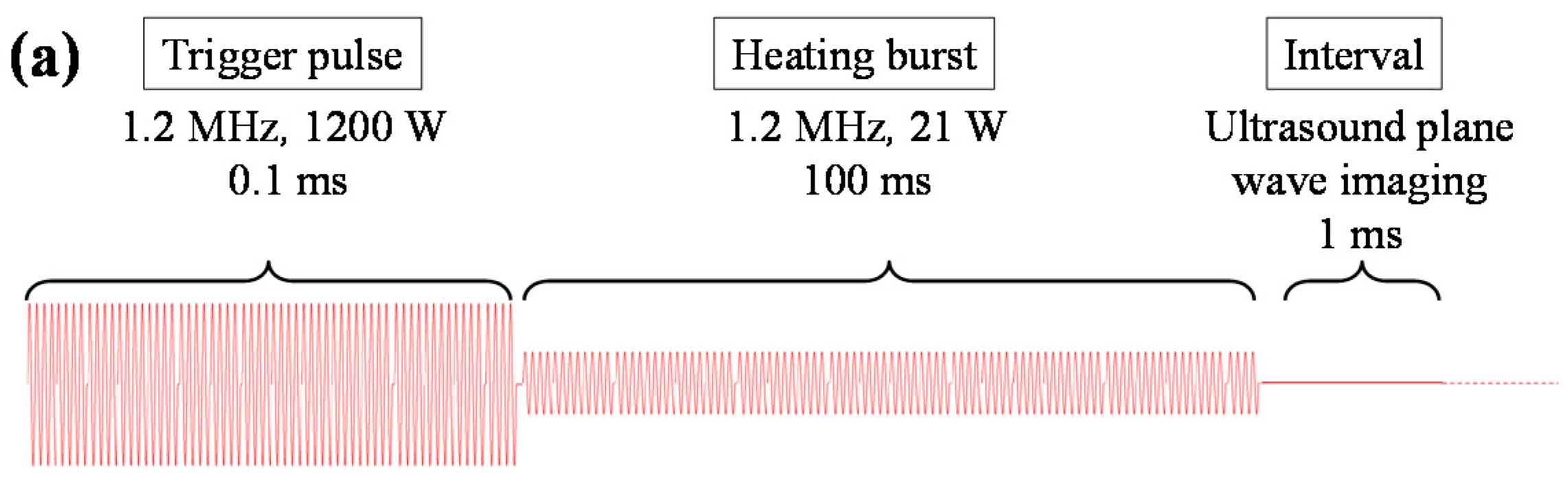
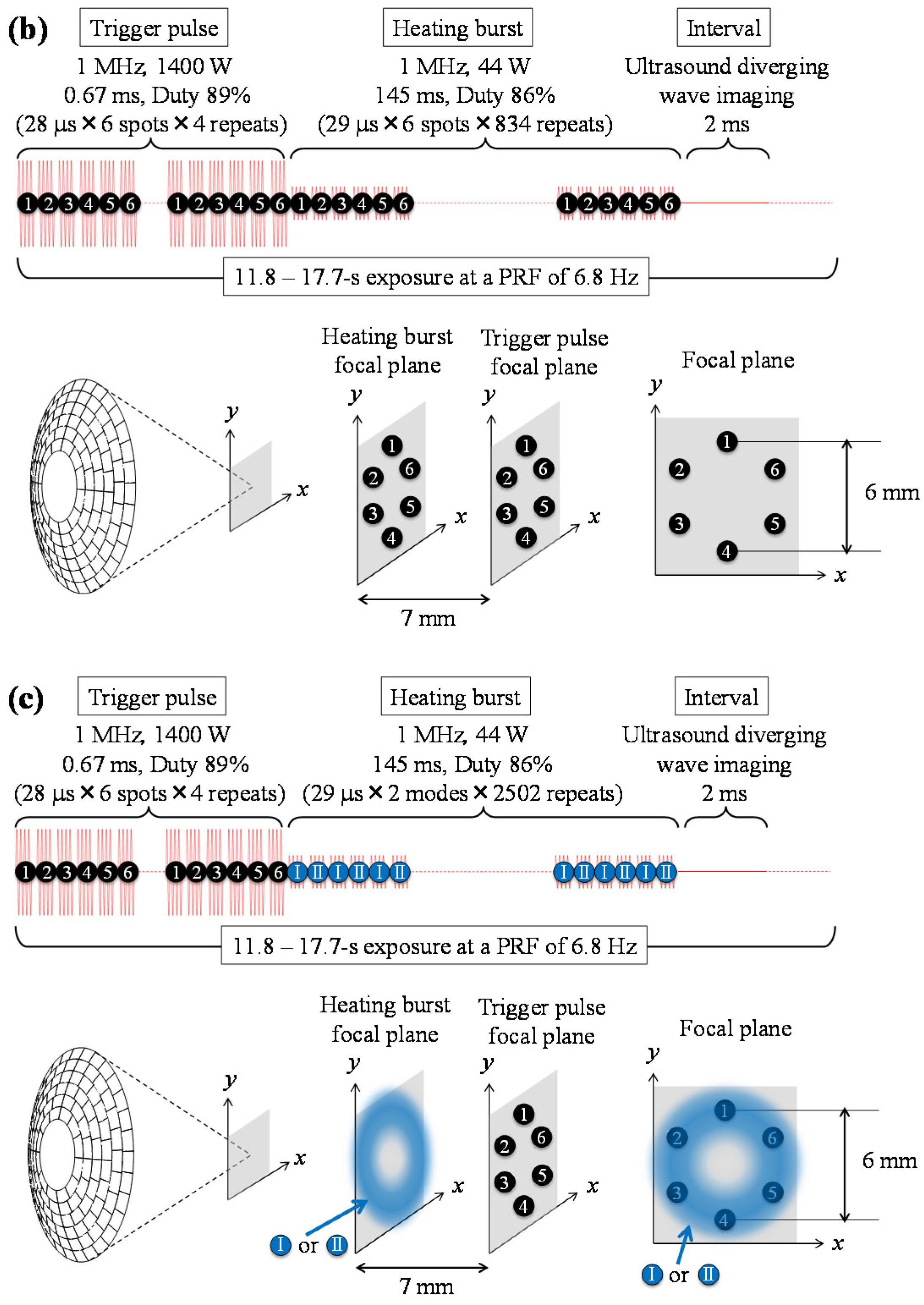


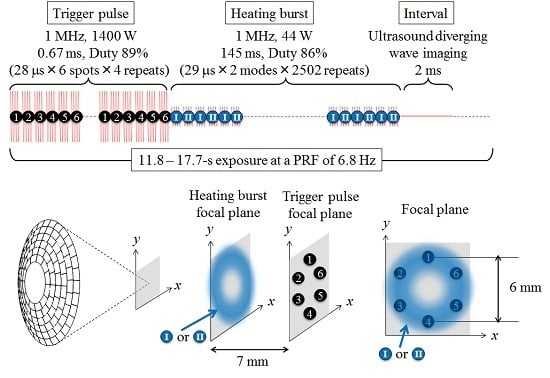
| Corresponding Sequence in Figure 1 | Trigger Pulse | Heating Burst | ||
|---|---|---|---|---|
| Spot Scanning Focus | Focal Plane Shift | Spot Scanning Focus | Wide Focus | |
| Figure 1a | ||||
| Figure 1b | √ | √ | √ | |
| Figure 1c | √ | √ | √ | |
| Trigger Pulse Focal Shift (mm) | Thickness of Intermediate Brightness Region at the Backward End of Coagulation Region (mm) | Thickness of Intermediate Brightness Region at the Forward End of Coagulation Region (mm) |
|---|---|---|
| 0 | 5.65 | 6.30 |
| 10 | 4.25 | 2.95 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshizawa, S.; Takagi, R.; Umemura, S.-i. Enhancement of High-Intensity Focused Ultrasound Heating by Short-Pulse Generated Cavitation. Appl. Sci. 2017, 7, 288. https://doi.org/10.3390/app7030288
Yoshizawa S, Takagi R, Umemura S-i. Enhancement of High-Intensity Focused Ultrasound Heating by Short-Pulse Generated Cavitation. Applied Sciences. 2017; 7(3):288. https://doi.org/10.3390/app7030288
Chicago/Turabian StyleYoshizawa, Shin, Ryo Takagi, and Shin-ichiro Umemura. 2017. "Enhancement of High-Intensity Focused Ultrasound Heating by Short-Pulse Generated Cavitation" Applied Sciences 7, no. 3: 288. https://doi.org/10.3390/app7030288