Flow Measurement by Lateral Resonant Doppler Optical Coherence Tomography in the Spectral Domain
Abstract
:1. Introduction
2. Phase-Resolved Doppler Model in SD-OCT
2.1. The Relation Between the Doppler Phase Shift and Axial Sample Velocity
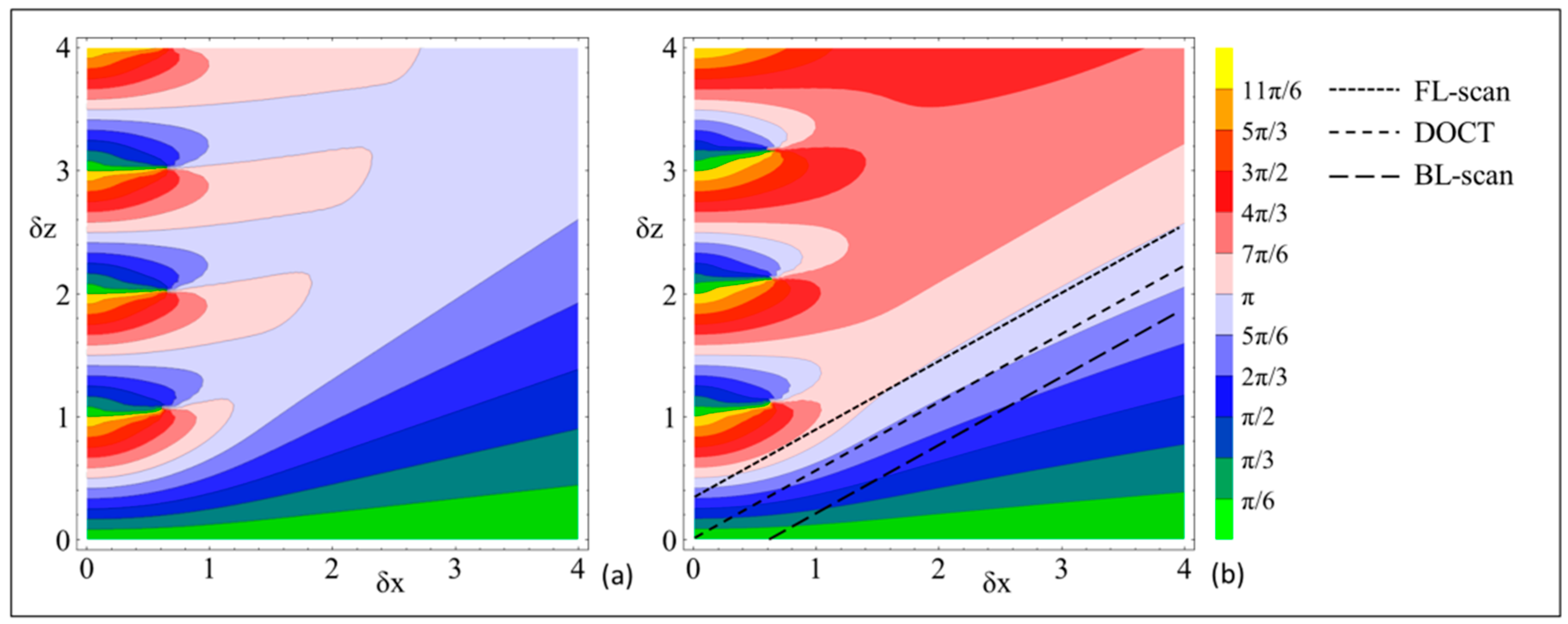
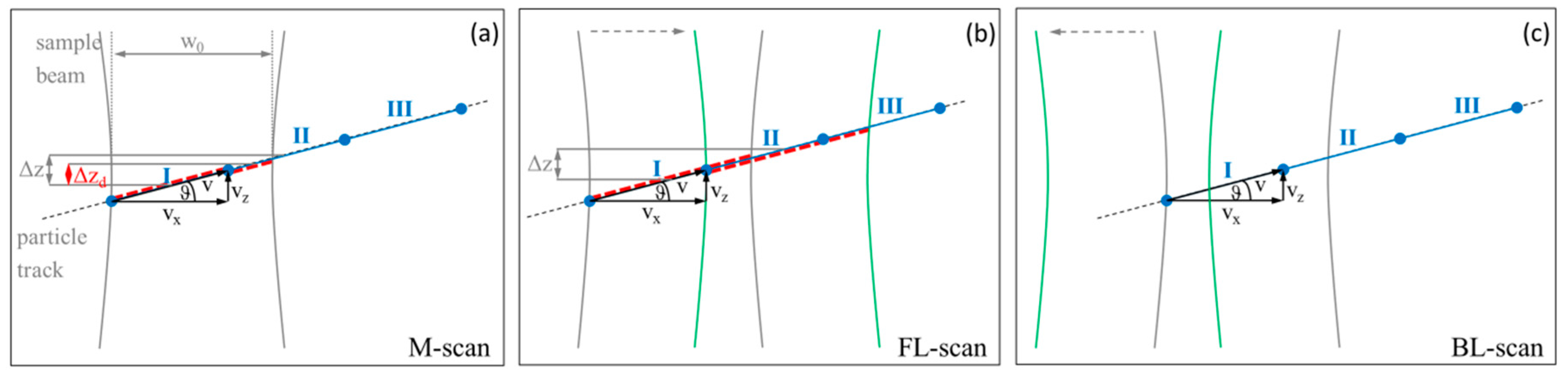
2.2. The Impact of Lateral Resonant Scanning on PR-DOCT
3. Material and Methods
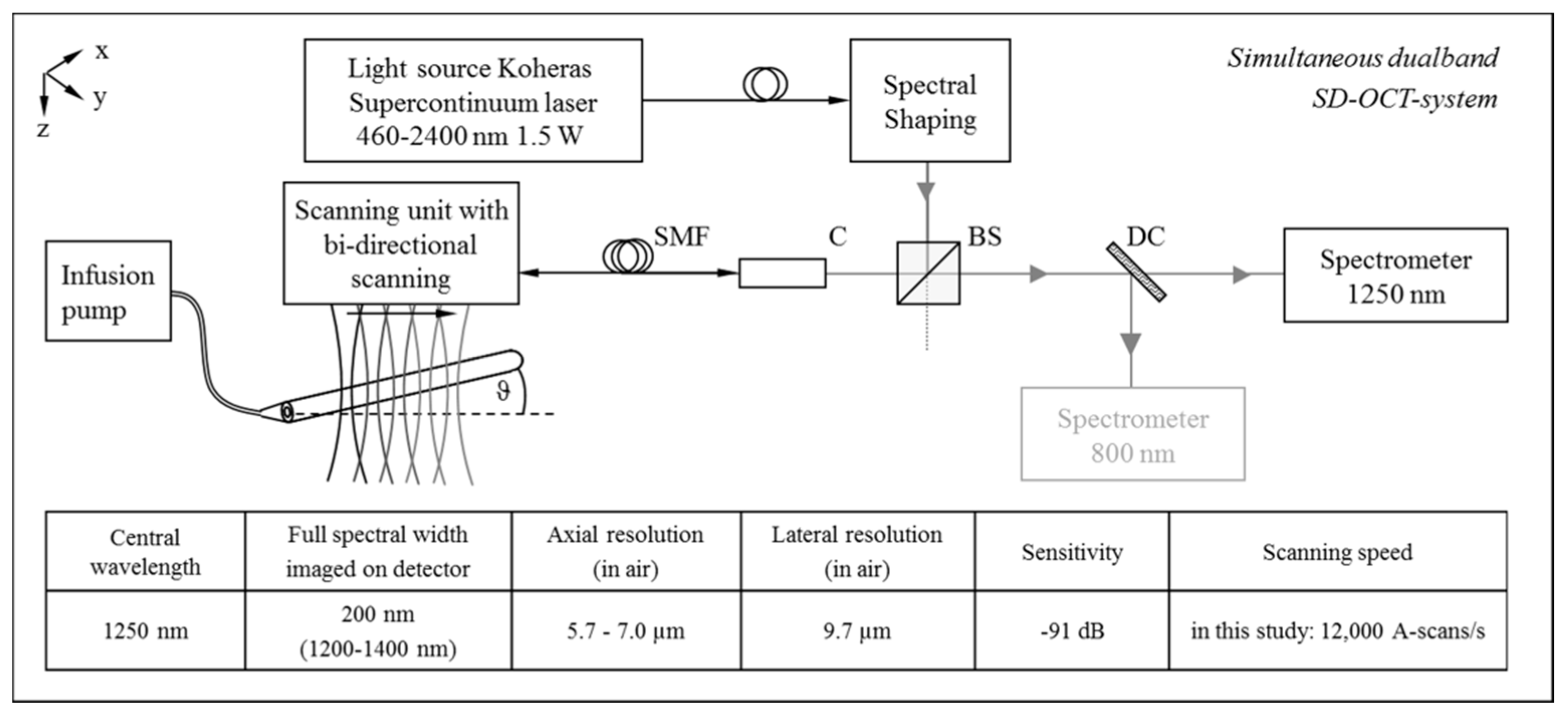
3.1. OCT System Setup and In Vitro Flow Phantom
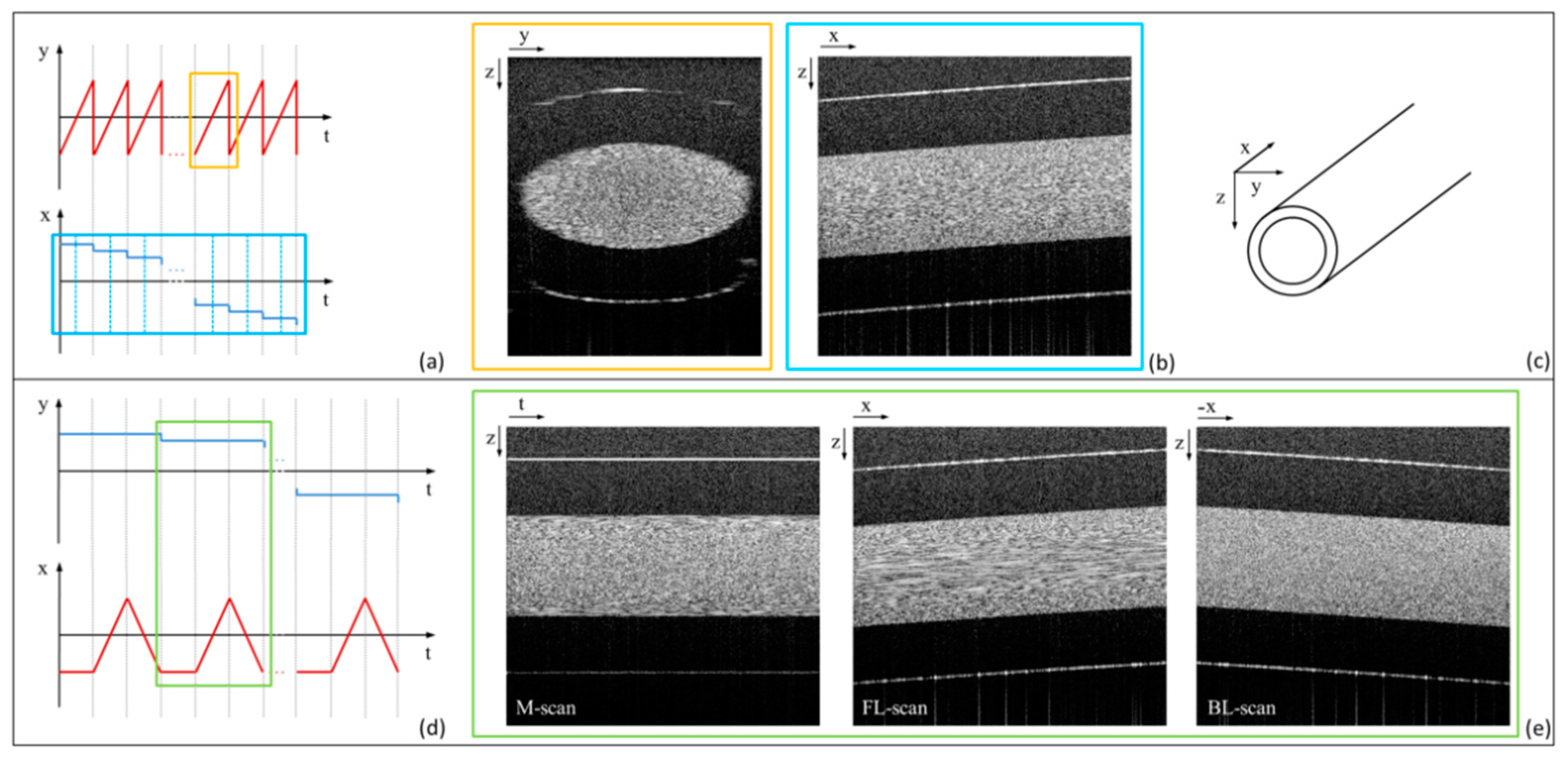
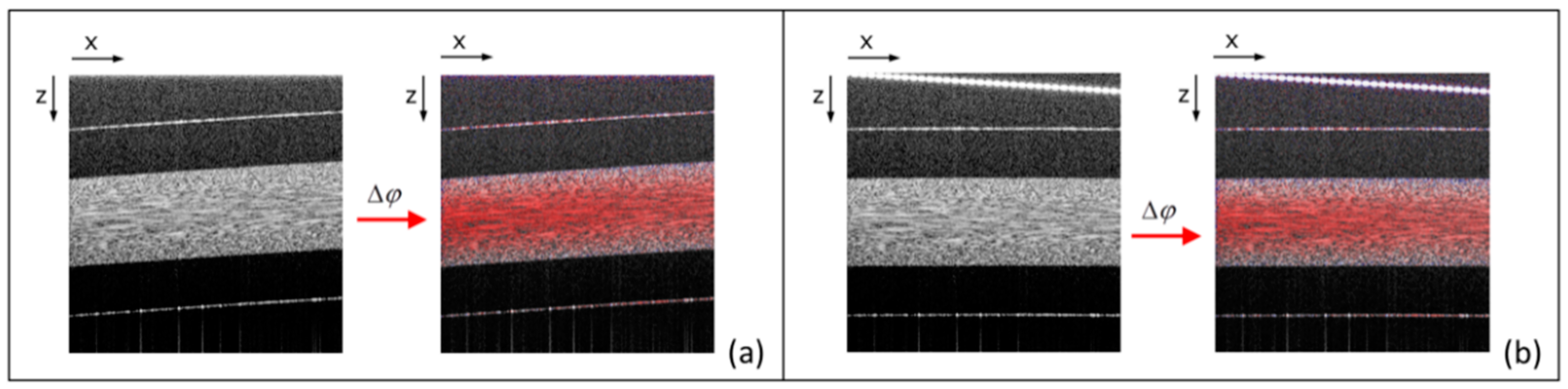
3.2. Bi-Directional Lateral Scanning Protocol and Image Correction for Doppler Analysis
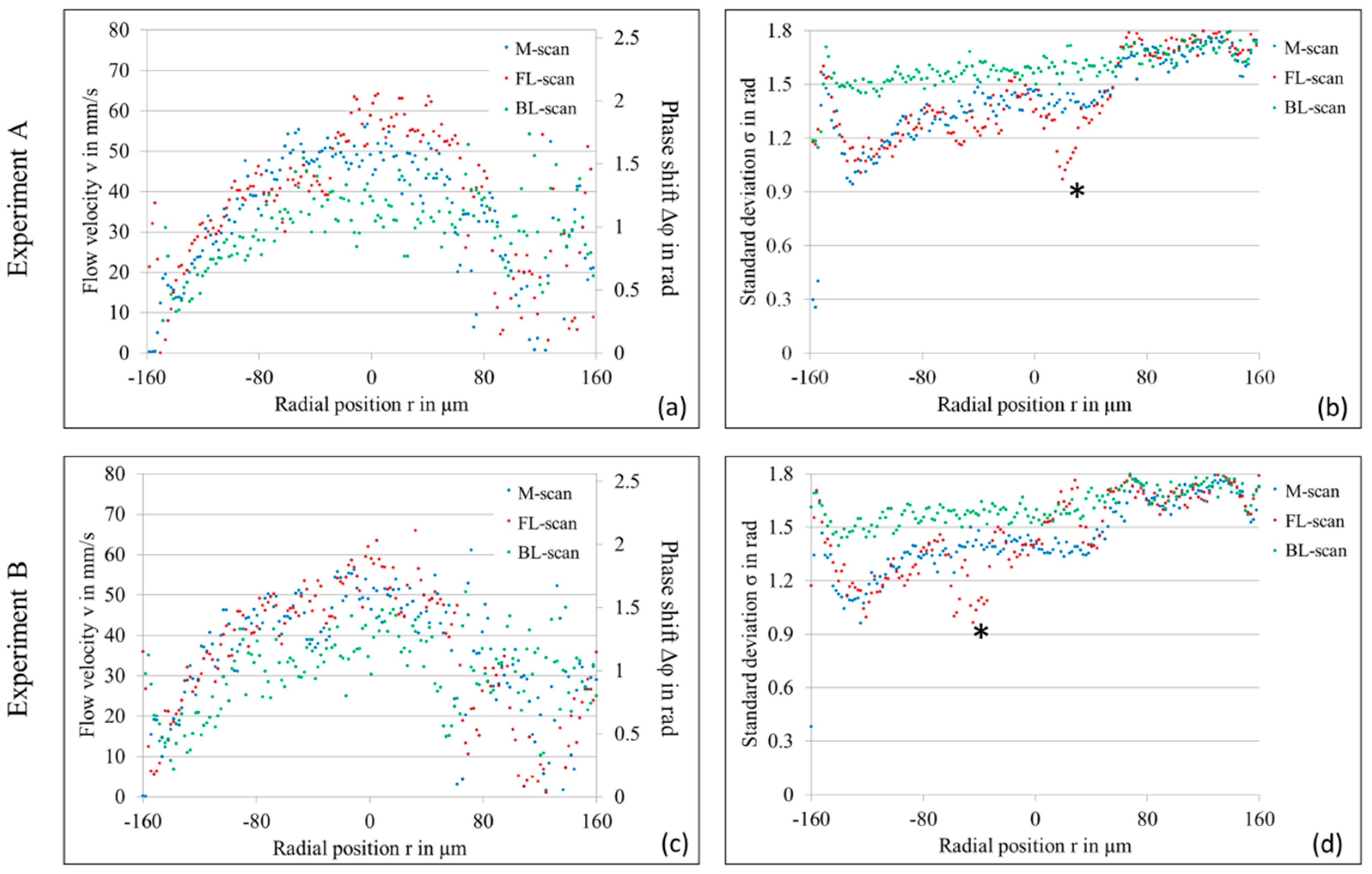
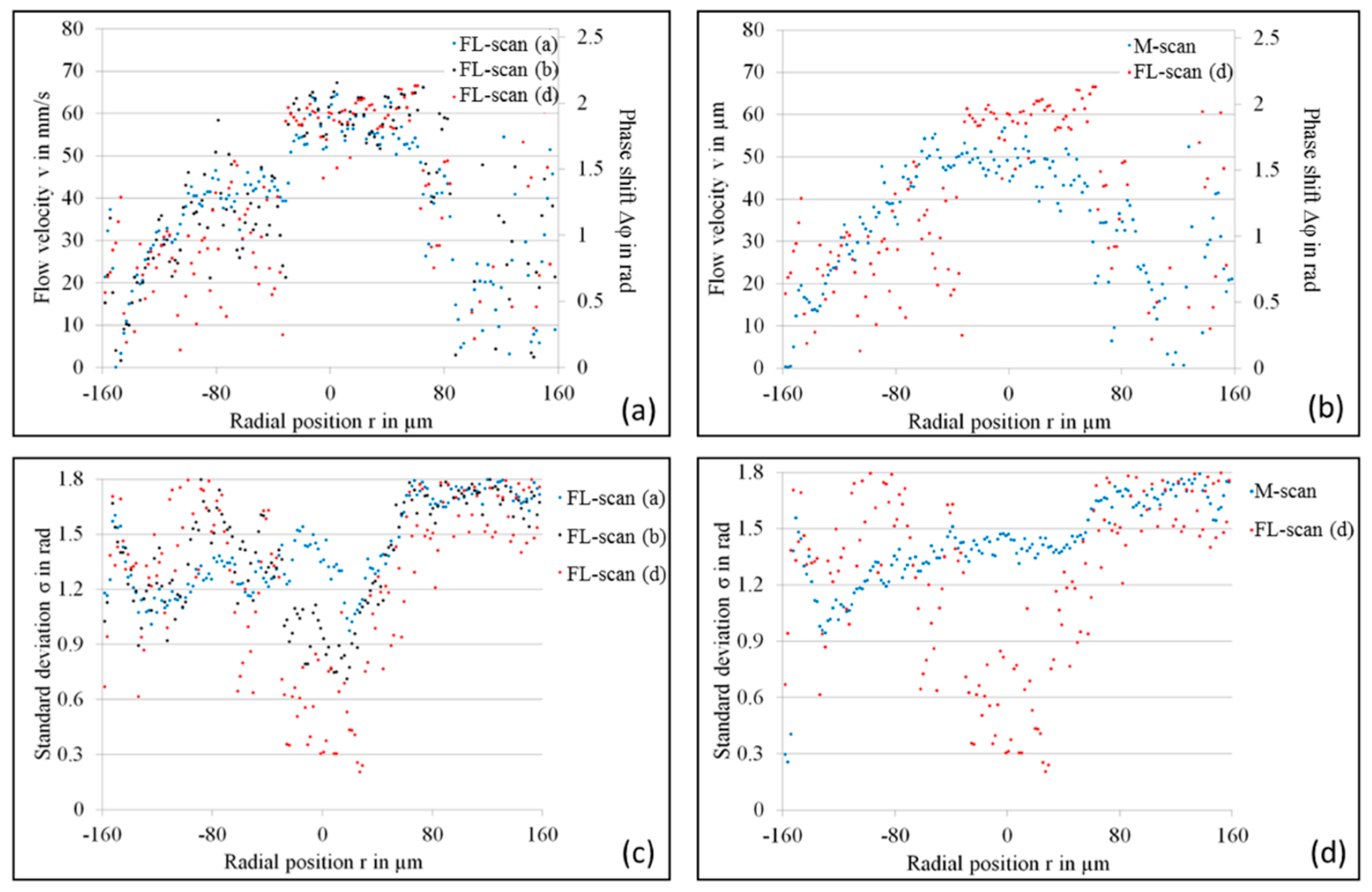
4. Experimental Results
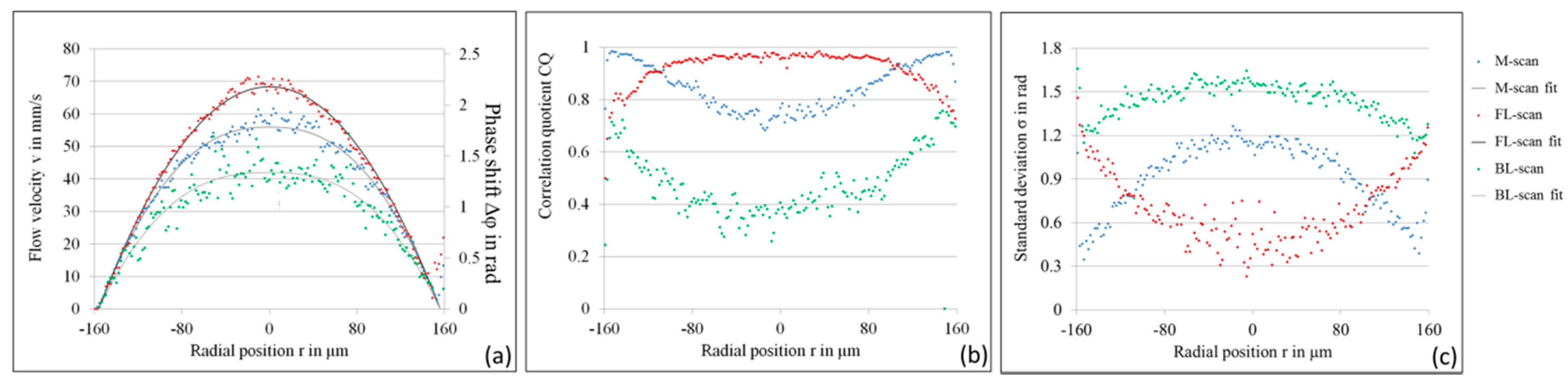
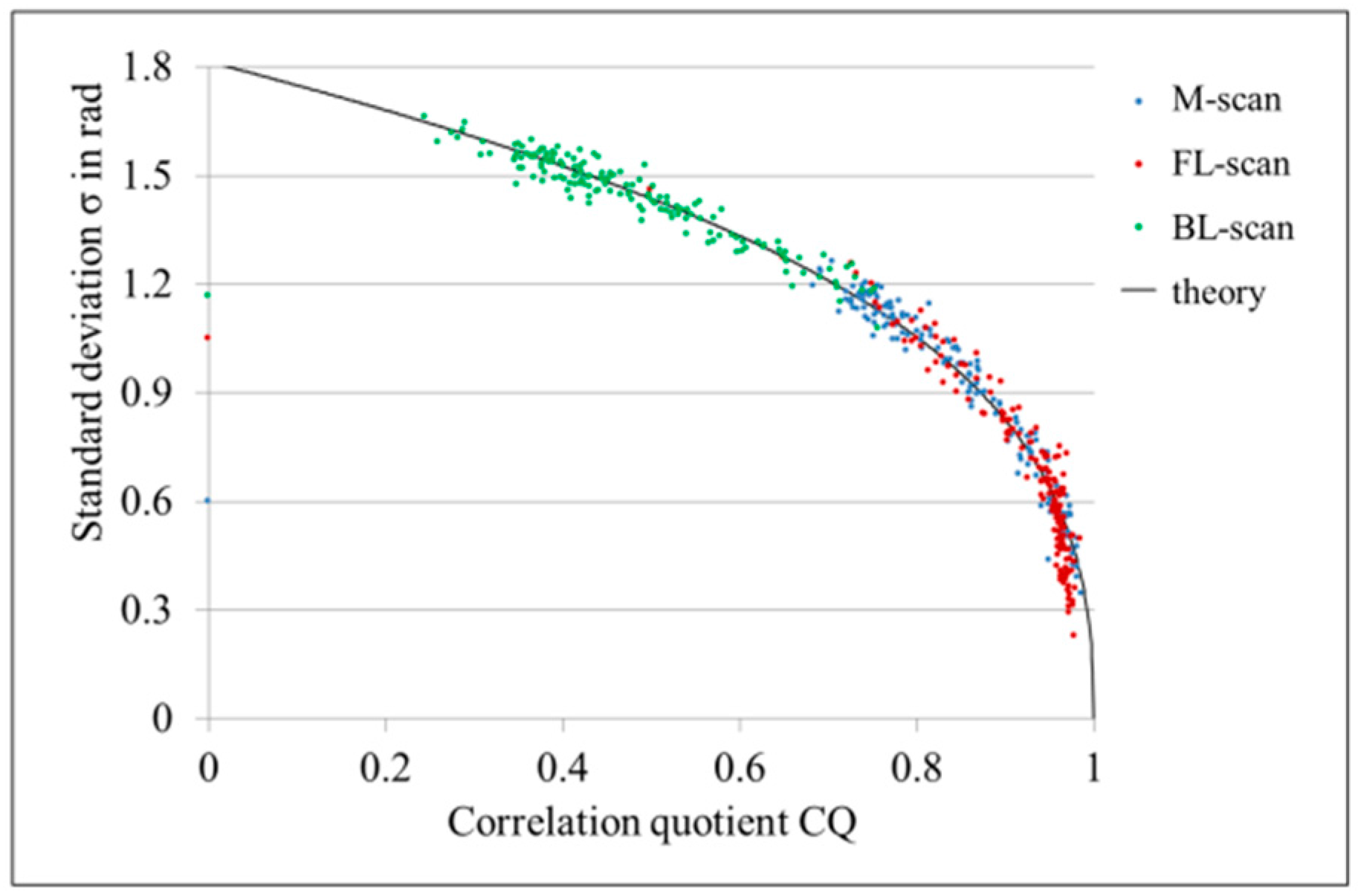
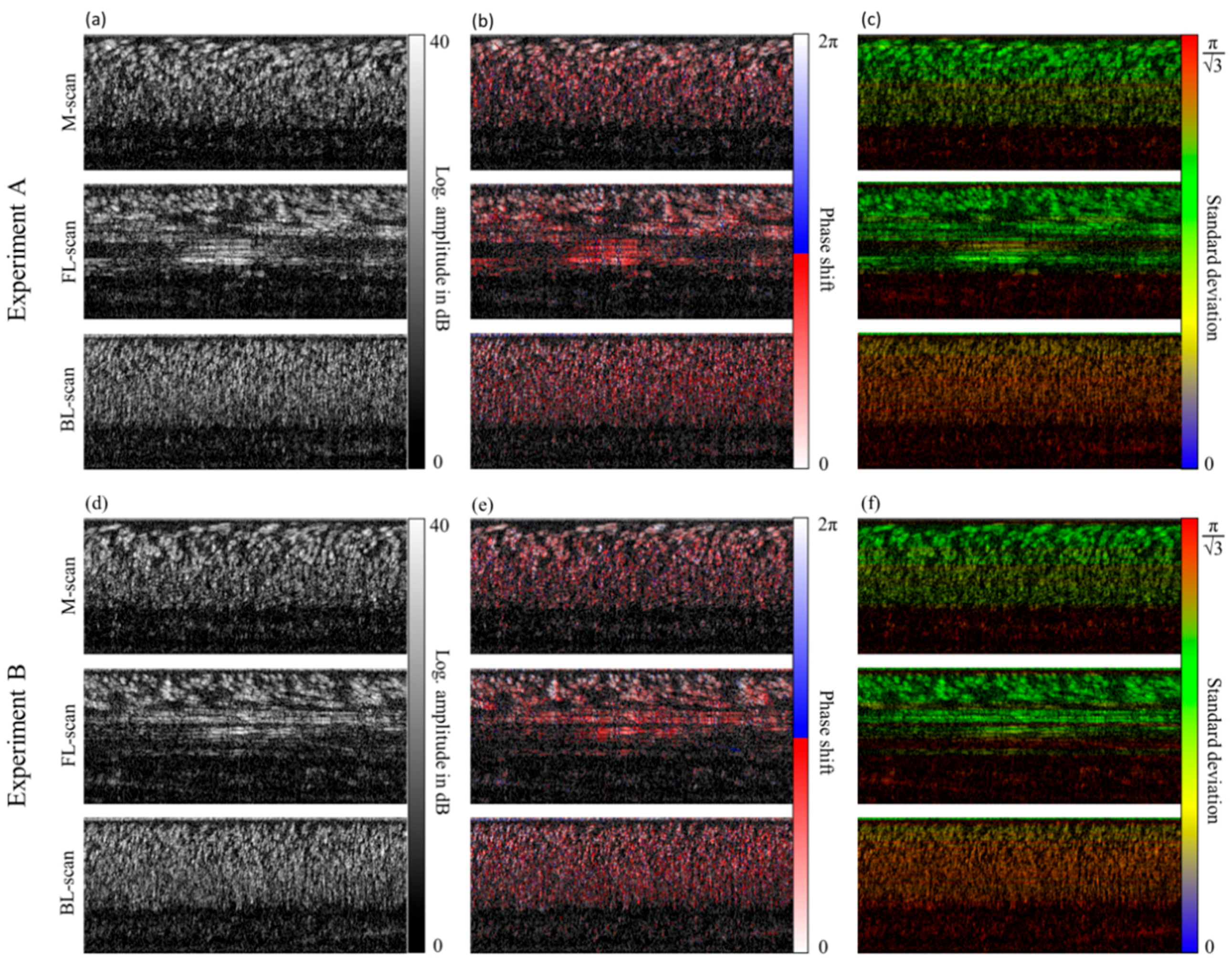
4.1. LR-DOCT with 1% Intralipid Flow
4.2. LR-DOCT with In Vitro Blood Flow
5. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, R.; Hitzenberger, C.; Fercher, A. Performance of Fourier domain vs. time domain optical coherence tomography. Opt. Express 2003, 11, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Choma, M.; Sarunic, M.; Yang, C.; Izatt, J. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt. Express 2003, 11, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.F.; Cense, B.; Park, B.H.; Pierce, M.C.; Tearney, G.J.; Bouma, B.E. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt. Lett. 2003, 28, 2067–2069. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Wojtkowski, M.; Fujimoto, J.G. Fourier domain mode locking (FDML): A new laser operating regime and applications for optical coherence tomography. Opt. Express 2006, 14, 3225–3237. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, L.; Domaschke, T.; Schneider, C.; Walther, J.; Meissner, S.; Hampel, R.; Koch, E. Visualization of dynamic boiling processes using high-speed optical coherence tomography. Exp. Fluids 2015, 56. [Google Scholar] [CrossRef]
- Hitzenberger, C.K.; Gützinger, E.; Sticker, M.; Pircher, M.; Fercher, A.F. Measurement and imaging of birefringence and optic axis orientation by phase resolved polarization sensitive optical coherence tomography. Opt. Express 2001, 9, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Pircher, M.; Hitzenberger, C.K.; Schmidt-Erfurth, U. Polarization sensitive optical coherence tomography in the human eye. Prog. Retin. Eye Res. 2011, 30, 431–451. [Google Scholar] [CrossRef] [PubMed]
- Faber, D.J.; Mik, E.G.; Aalders, M.C.G.; van Leeuwen, T.G. Toward assessments of blood oxygen saturation by spectroscopic optical coherence tomography. Opt. Lett. 2005, 30, 1015–1017. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.J.; Sakadzić, S.; Gorczynska, I.; Ruvinskaya, S.; Wu, W.C.; Fujimoto, J.G.; Boas, D.A. Quantitative cerebral blood flow with optical coherence tomography. Opt. Express 2010, 18, 2477–2494. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, R.A.; Werkmeister, R.M.; Blatter, C.; Schmetterer, L. Doppler optical coherence tomography. Prog. Retin. Eye Res. 2014, 41, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Chen, Z.P.; Saxer, C.; Xiang, S.H.; de Boer, J.F.; Nelson, J.S. Phase-resolved optical coherence tomography and optical Doppler tomography for imaging blood flow in human skin with fast scanning speed and high velocity sensitivity. Opt. Lett. 2000, 25, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, R.; Schmetterer, L.; Drexler, W.; Fercher, A.; Zawadzki, R.; Bajraszewski, T. Real-time assessment of retinal blood flow with ultrafast acquisition by color Doppler Fourier domain optical coherence tomography. Opt. Express 2003, 11, 3116–3121. [Google Scholar] [CrossRef] [PubMed]
- Vakoc, B.; Yun, S.; de Boer, J.; Tearney, G.; Bouma, B. Phase-resolved optical frequency domain imaging. Opt. Express 2005, 13, 5483–5493. [Google Scholar] [CrossRef] [PubMed]
- Grulkowski, I.; Gorczynska, I.; Szkulmowski, M.; Szlag, D.; Szkulmowska, A.; Leitgeb, R.; Kowalczyk, A.; Wojtkowski, M. Scanning protocols dedicated to smart velocity ranging in spectral OCT. Opt. Express 2009, 17, 23736–23754. [Google Scholar] [CrossRef] [PubMed]
- Walther, J.; Koch, E. Relation of joint spectral and time domain optical coherence tomography (jSTdOCT) and phase-resolved Doppler OCT. Opt. Express 2014, 22, 23129–23146. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, A.H.; Villiger, M.L.; Blatter, C.; Lasser, T.; Leitgeb, R.A. Resonant Doppler flow imaging and optical vivisection of retinal blood vessels. Opt. Express 2007, 15, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.; Hammer, D.; Wang, S.; Cuevas, M.; Walther, J. Resonant Doppler imaging with common path OCT. Proc. SPIE 2009, 7372, 737220. [Google Scholar]
- Koch, E.; Walther, J.; Cuevas, M. Limits of Fourier domain Doppler-OCT at high velocities. Sens. Actuators A 2009, 156, 8–13. [Google Scholar] [CrossRef]
- Walther, J.; Koch, E. Transverse motion as a source of noise and reduced correlation of the Doppler phase shift in spectral domain OCT. Opt. Express 2009, 17, 19698–19713. [Google Scholar] [CrossRef] [PubMed]
- Walther, J.; Koch, E. Impact of a detector dead time in phase resolved Doppler analysis using spectral domain optical coherence tomography. J. Opt. Soc. Am. A 2017, 34, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, H.; Ruggeri, M.; Jiao, S.; Gregori, G.; Puliafito, C.A.; Zhao, W. Automatic retinal blood flow calculation using spectral domain optical coherence tomography. Opt. Express 2007, 15, 15193–15206. [Google Scholar] [CrossRef] [PubMed]
- Cimalla, P.; Walther, J.; Mehner, M.; Cuevas, M.; Koch, E. Simultaneous dual-band optical coherence tomography in the spectral domain for high resolution in vivo imaging. Opt. Express 2009, 17, 19486–19500. [Google Scholar] [CrossRef] [PubMed]
- Walther, J.; Mueller, G.; Meissner, S.; Cimalla, P.; Homann, H.; Morawietz, H.; Koch, E. Time-resolved blood flow measurement in the in vivo mouse model by optical frequency domain imaging. Proc. SPIE 2009, 7372, 73720J. [Google Scholar]
- Walther, J.; Mueller, G.; Morawietz, H.; Koch, E. Analysis of in vitro and in vivo bidirectional flow velocities by phase-resolved Doppler Fourier-domain OCT. Sens. Actuators A 2009, 156, 14–21. [Google Scholar] [CrossRef]
- Langbein, H.; Brunssen, C.; Hoffmann, A.; Cimalla, P.; Brux, M.; Bornstein, S.R.; Deussen, A.; Koch, E.; Morawietz, H. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. Eur. Heart J. 2016, 37, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Vakoc, B.J.; Tearney, G.J.; Bouma, B.E. Statistical properties of phase-decorrelation in phase-resolved Doppler optical coherence tomography. IEEE Trans. Med. Imaging 2009, 28, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Cimalla, P.; Walther, J.; Mittasch, M.; Koch, E. Shear flow-induced optical inhomogeneity of blood assessed in vivo and in vitro by spectral domain optical coherence tomography in the 1.3 µm wavelength range. J. Biomed. Opt. 2011, 16, 116020. [Google Scholar] [CrossRef] [PubMed]
- Douglas-Hamilton, D.H.; Smith, N.G.; Kuster, C.E.; Vermeiden, J.P.W.; Althouse, G.C. Particle distribution in low-volume capillary-loaded chambers. J. Androl. 2005, 26, 107–114. [Google Scholar] [PubMed]
- Friebel, M.; Helfmann, J.; Müller, G.; Meinke, M. Influence of shear rate on the optical properties of human blood in the spectral range 250 to 1100 nm. J. Biomed. Opt. 2007, 12, 054005. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walther, J.; Koch, E. Flow Measurement by Lateral Resonant Doppler Optical Coherence Tomography in the Spectral Domain. Appl. Sci. 2017, 7, 382. https://doi.org/10.3390/app7040382
Walther J, Koch E. Flow Measurement by Lateral Resonant Doppler Optical Coherence Tomography in the Spectral Domain. Applied Sciences. 2017; 7(4):382. https://doi.org/10.3390/app7040382
Chicago/Turabian StyleWalther, Julia, and Edmund Koch. 2017. "Flow Measurement by Lateral Resonant Doppler Optical Coherence Tomography in the Spectral Domain" Applied Sciences 7, no. 4: 382. https://doi.org/10.3390/app7040382




