Experimental Study on Heat Transfer Performance of Vacuum Tube Heat Collector with Thermal Storage
Abstract
:1. Introduction
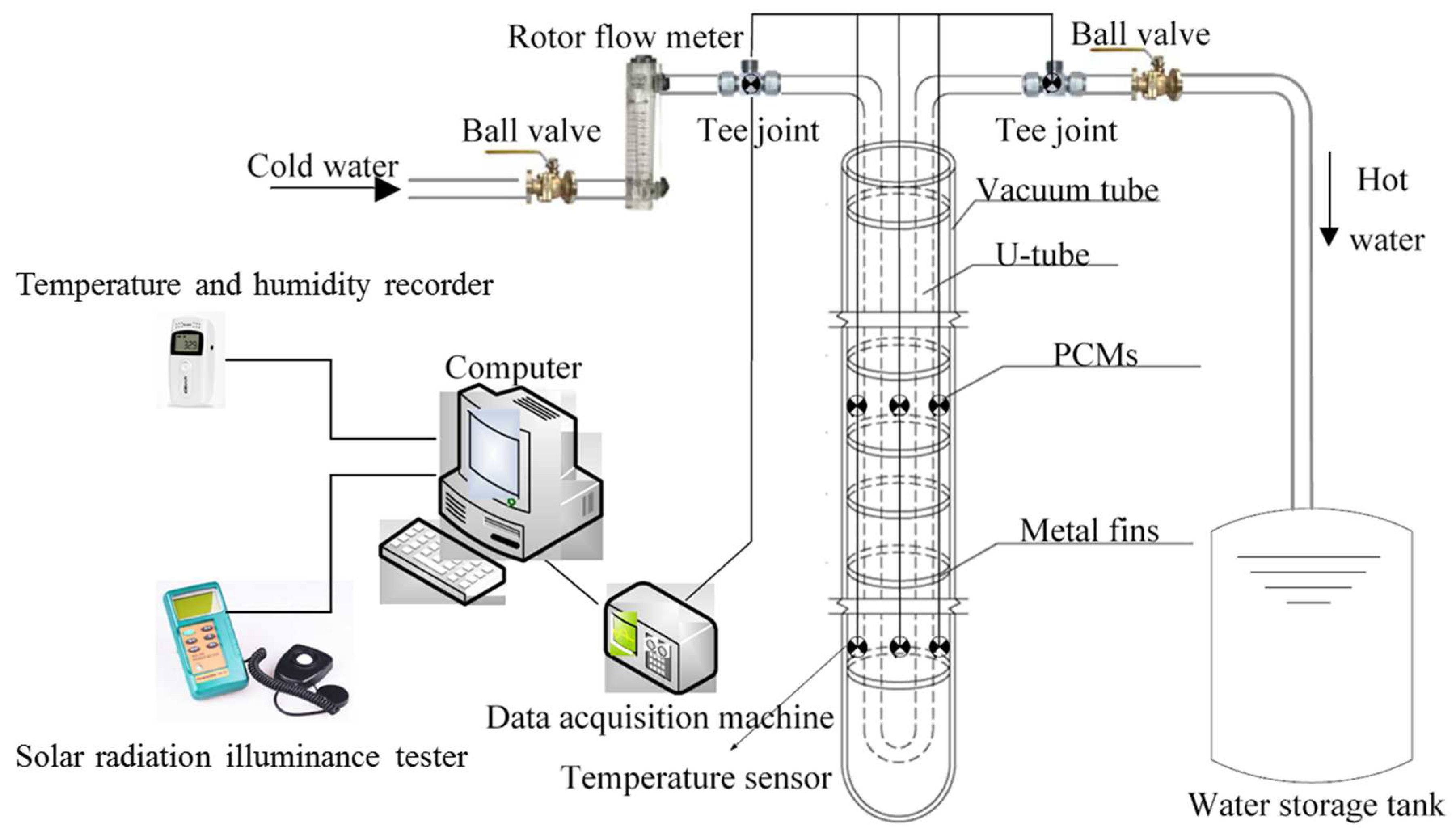
2. The Construction of the Experimental Platform
2.1. Development of Energy Storage Vacuum Tube Collector
2.2. Experimental Apparatus and Experimental Platform
3. Error Analysis of Measurement System
4. Result and Discussion
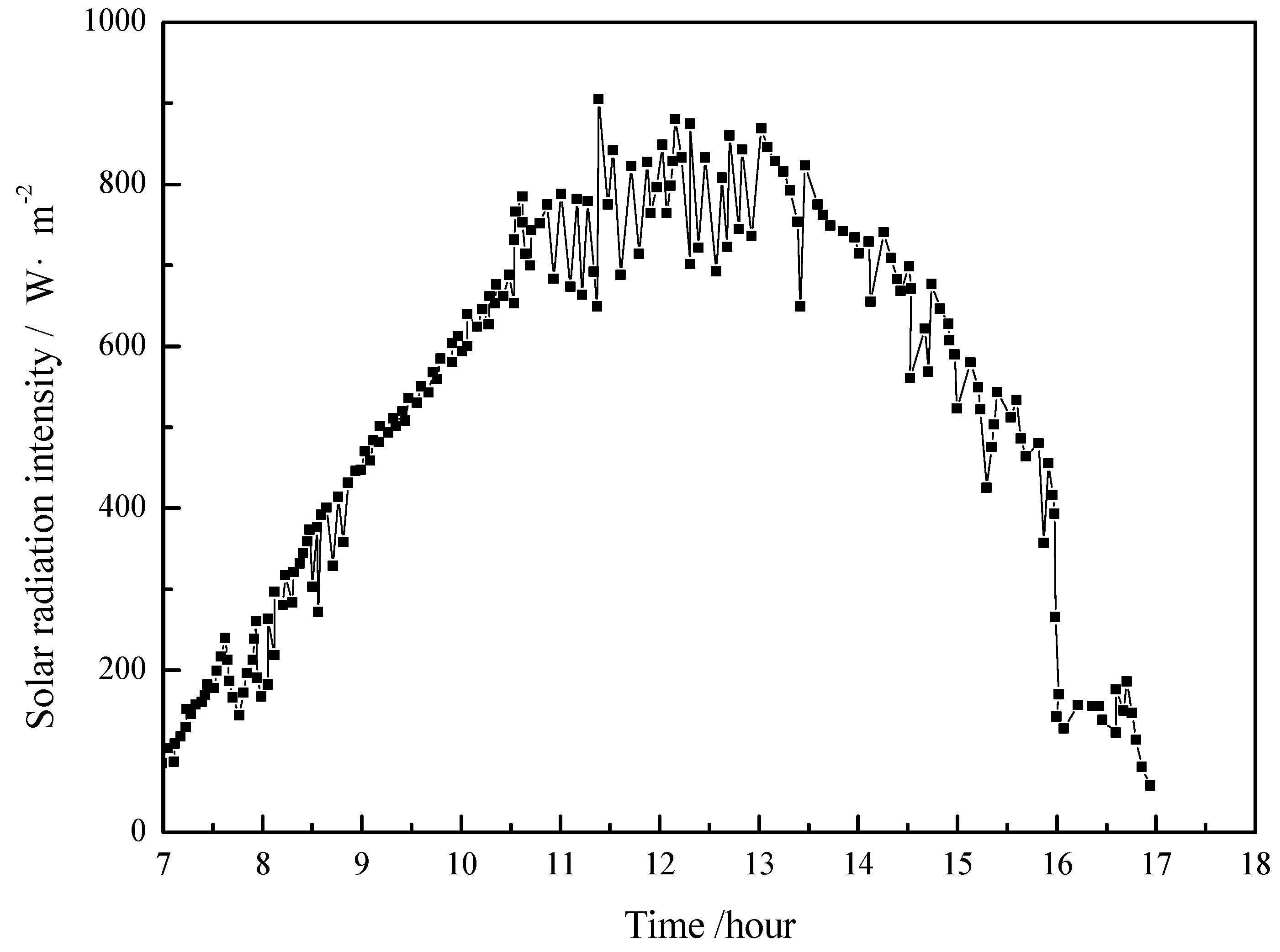
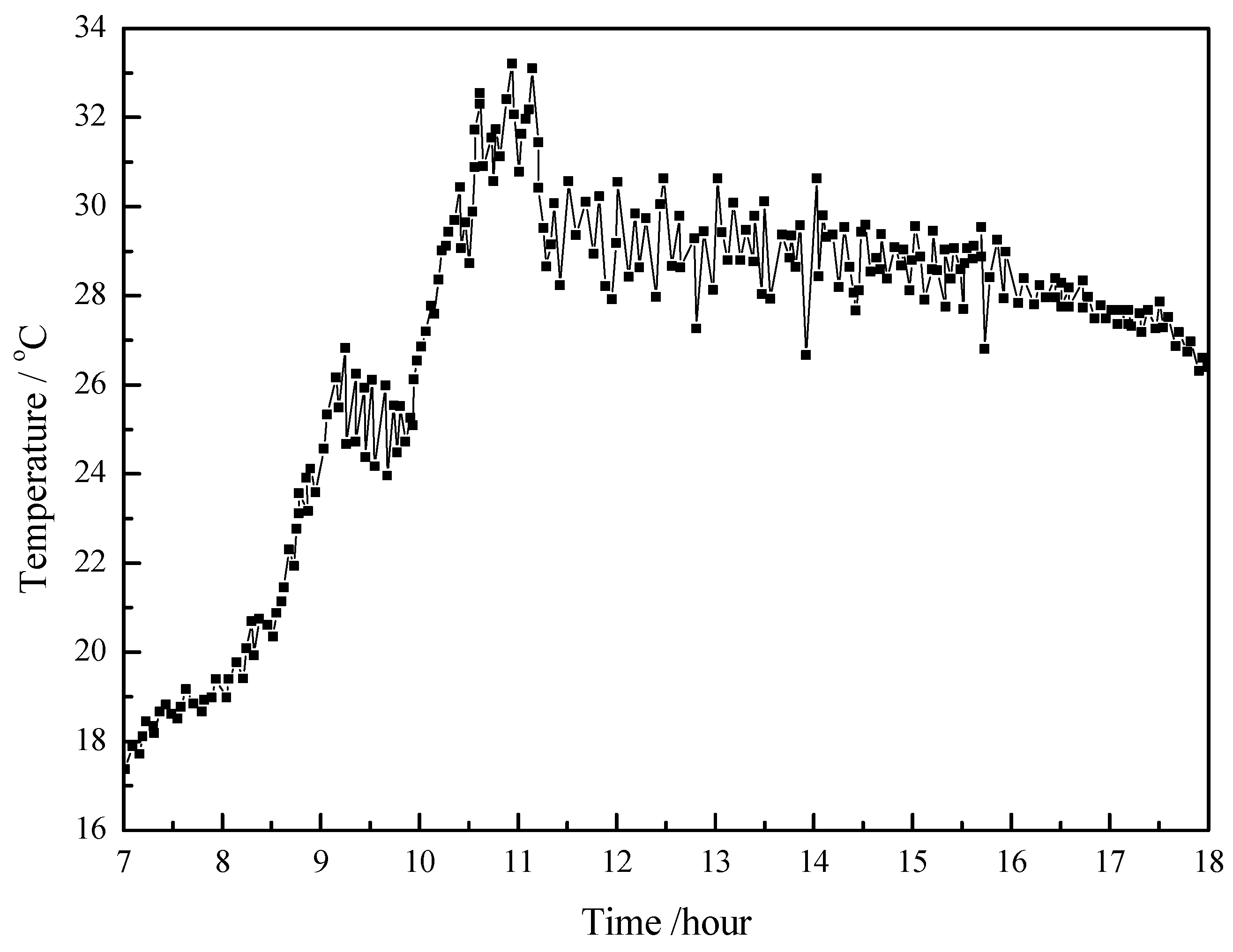
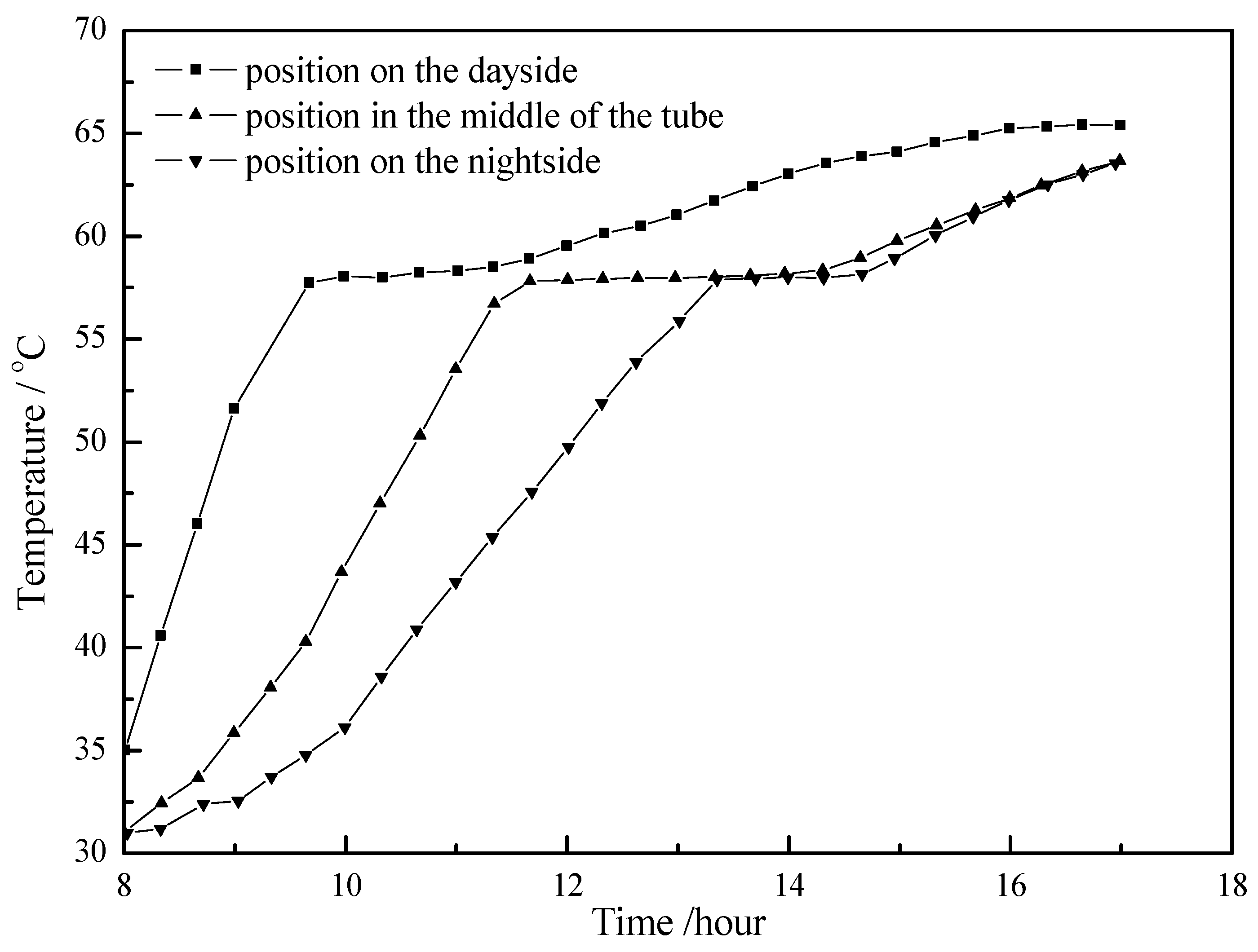
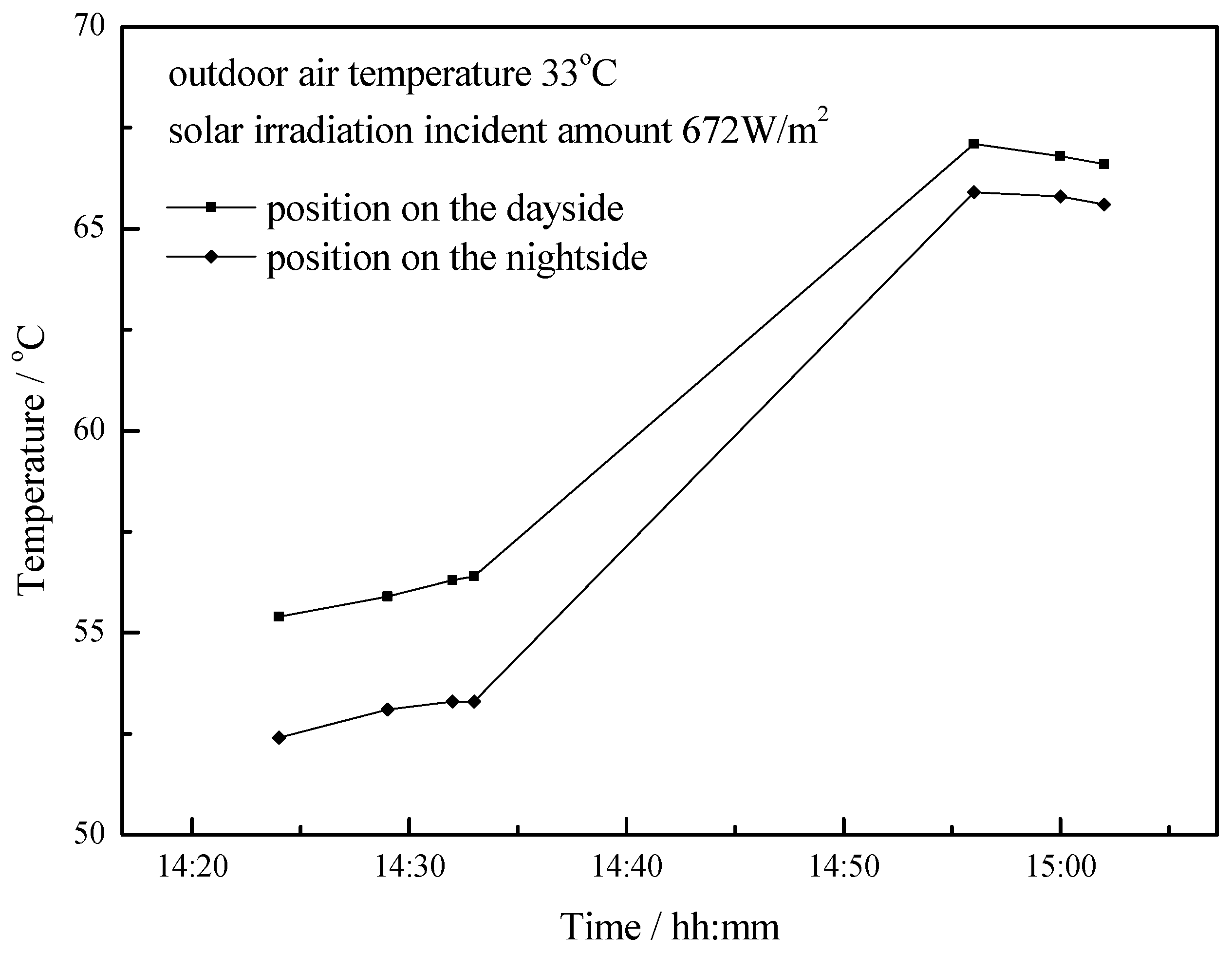
4.1. Working Condition in Daytime of Transitional Season
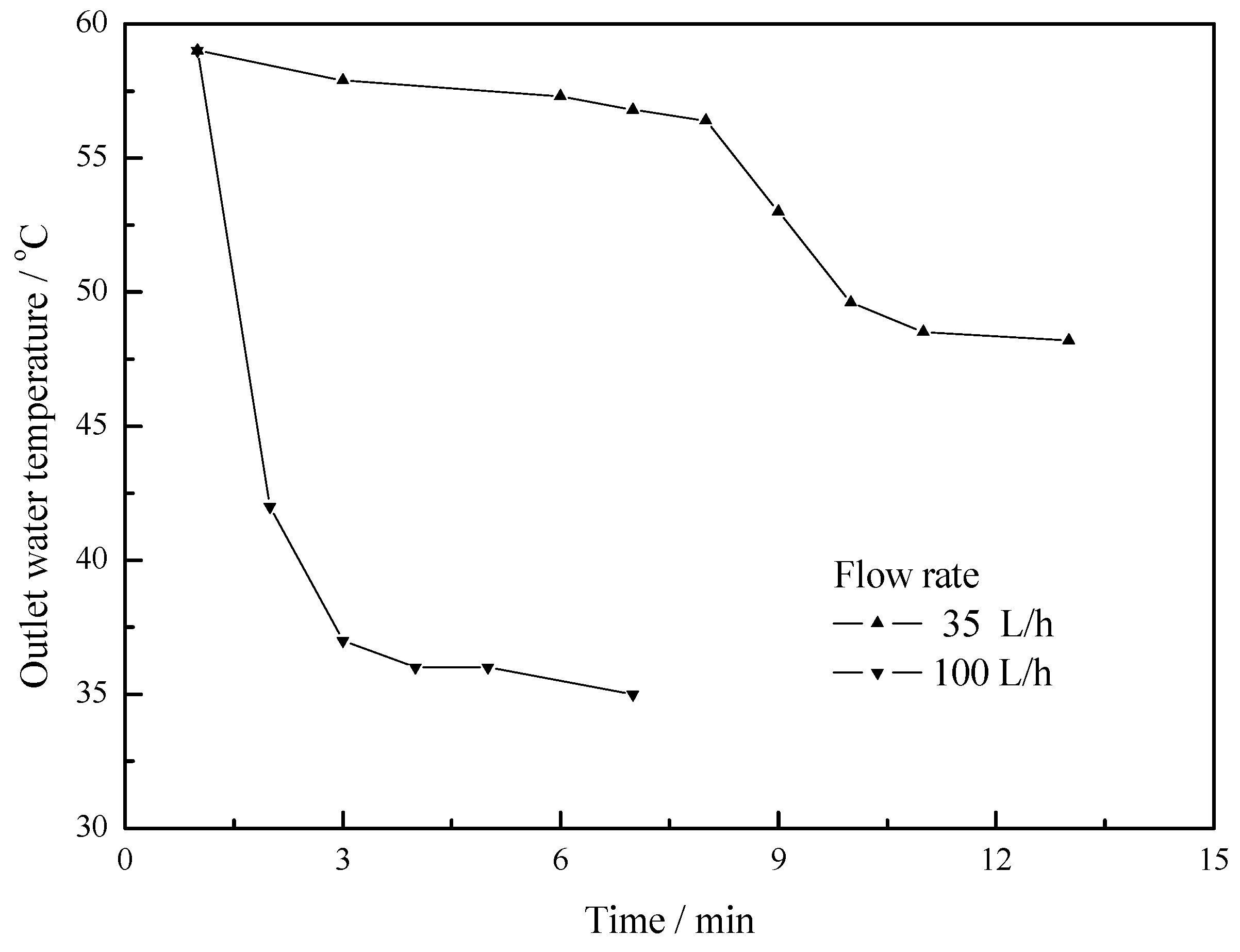
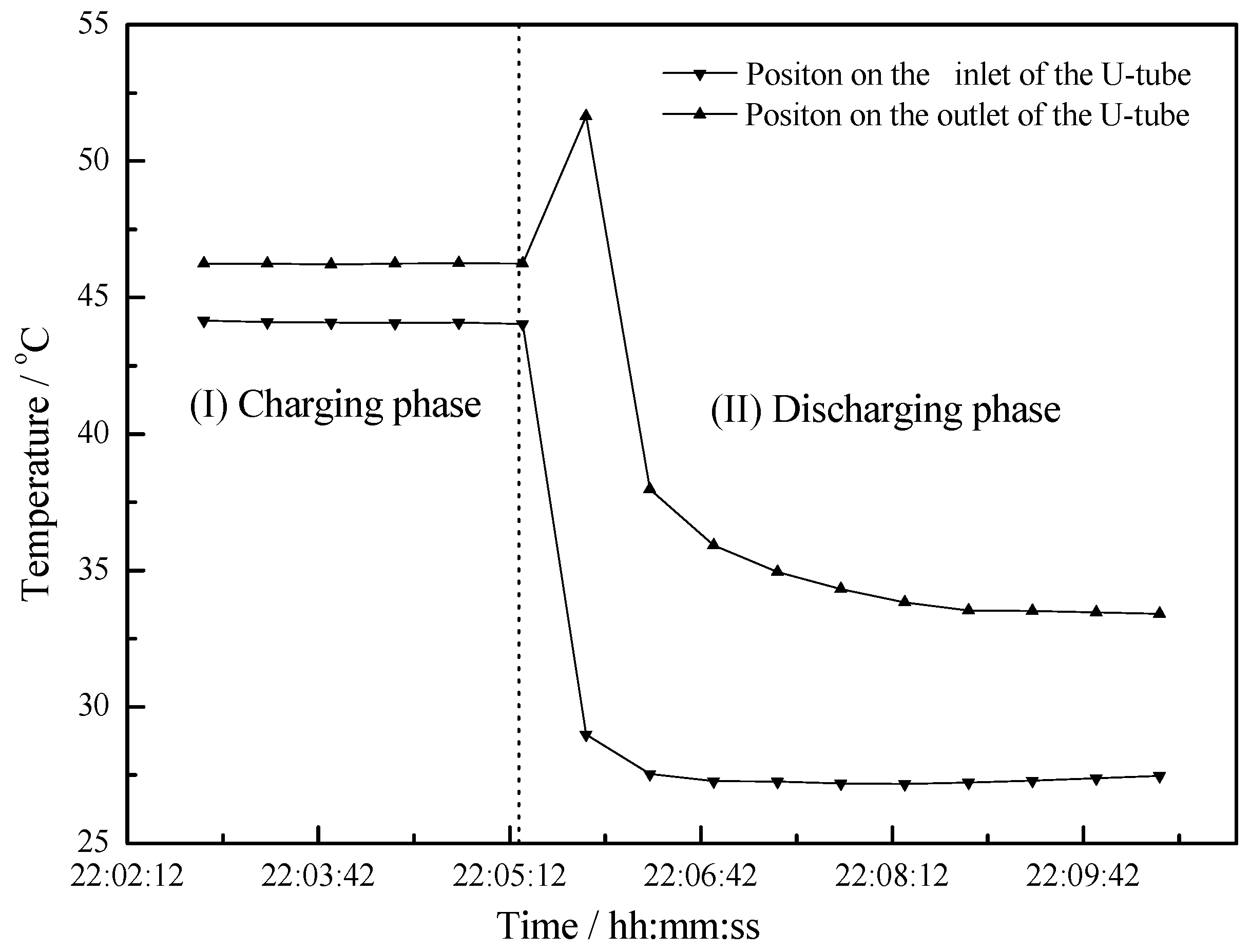
4.2. Working Condition at Night of Transitional Season
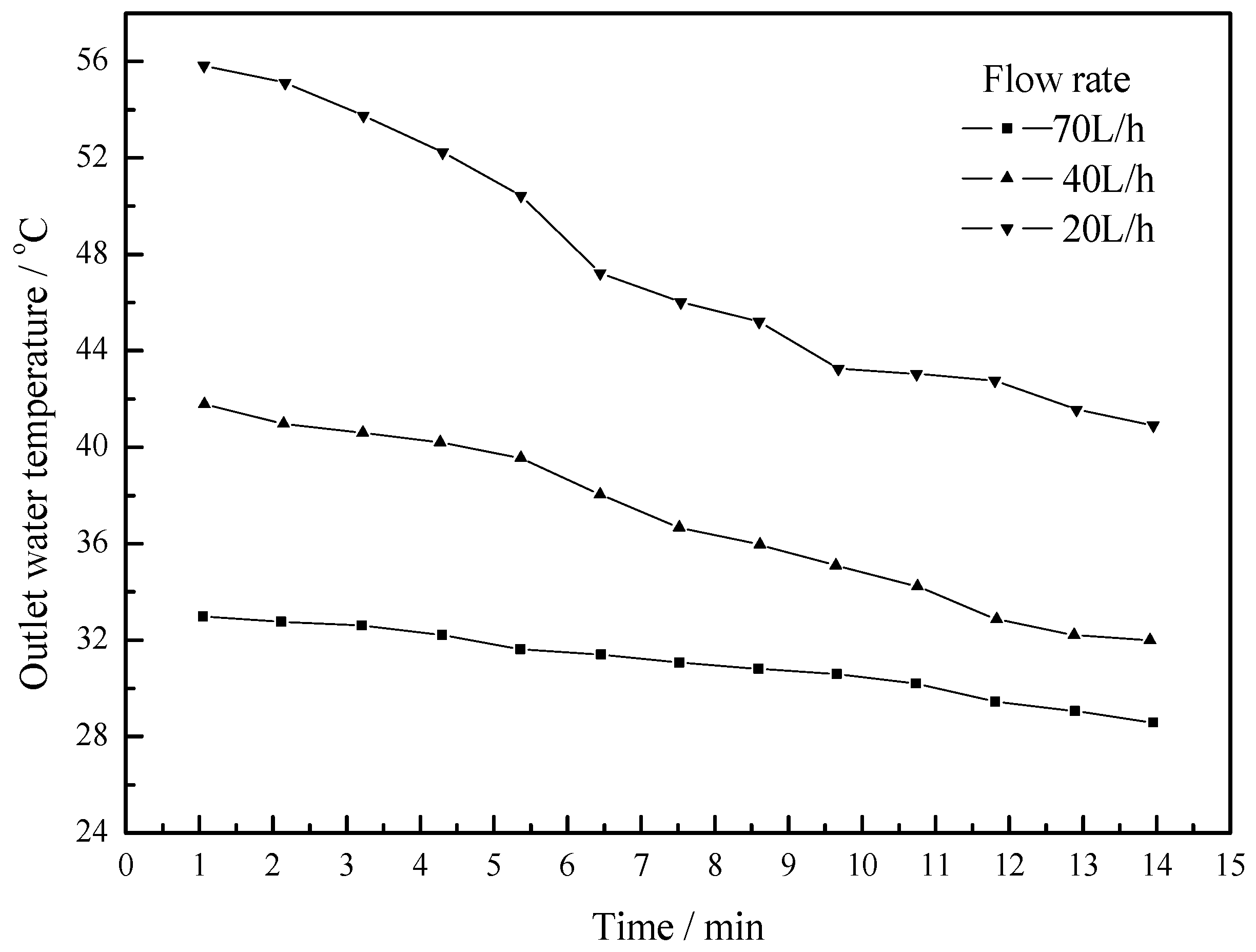
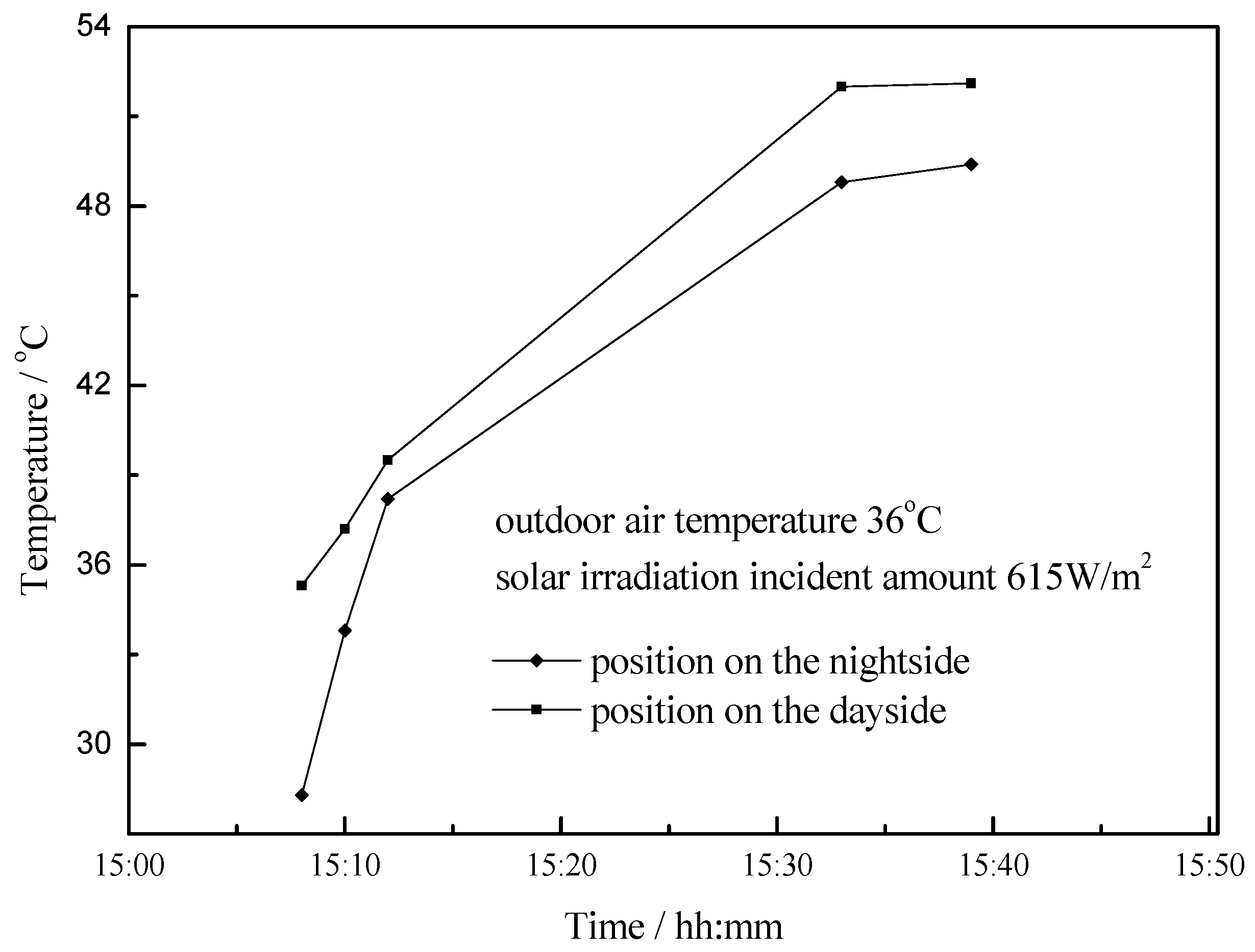
4.3. Working Condition in Daytime of Summer Season
4.4. Working Condition at Night of Summer Season
5. Conclusions
- (1)
- The paraffin phase transformation occurs on sunny days of the transition season and summer. It can be found that the maximum hot water temperature reaches 67 °C and 53 °C with the average solar irradiation amount of 672 W/m2 and 615 W/m2 respectively in summer. Solar radiation has a greater influence on the temperature of the heat collector than ambient temperature during the charging process.
- (2)
- The amount of heat absorbed by PCM in the daytime of the summer season reaches 3300 kJ and 2934 KJ with the average solar irradiation amount of 672 W/m2 and 615 W/m2 respectively in summer. It can also be concluded that, as the solar radiation increases, the heat absorbed by the PCM also increases.
- (3)
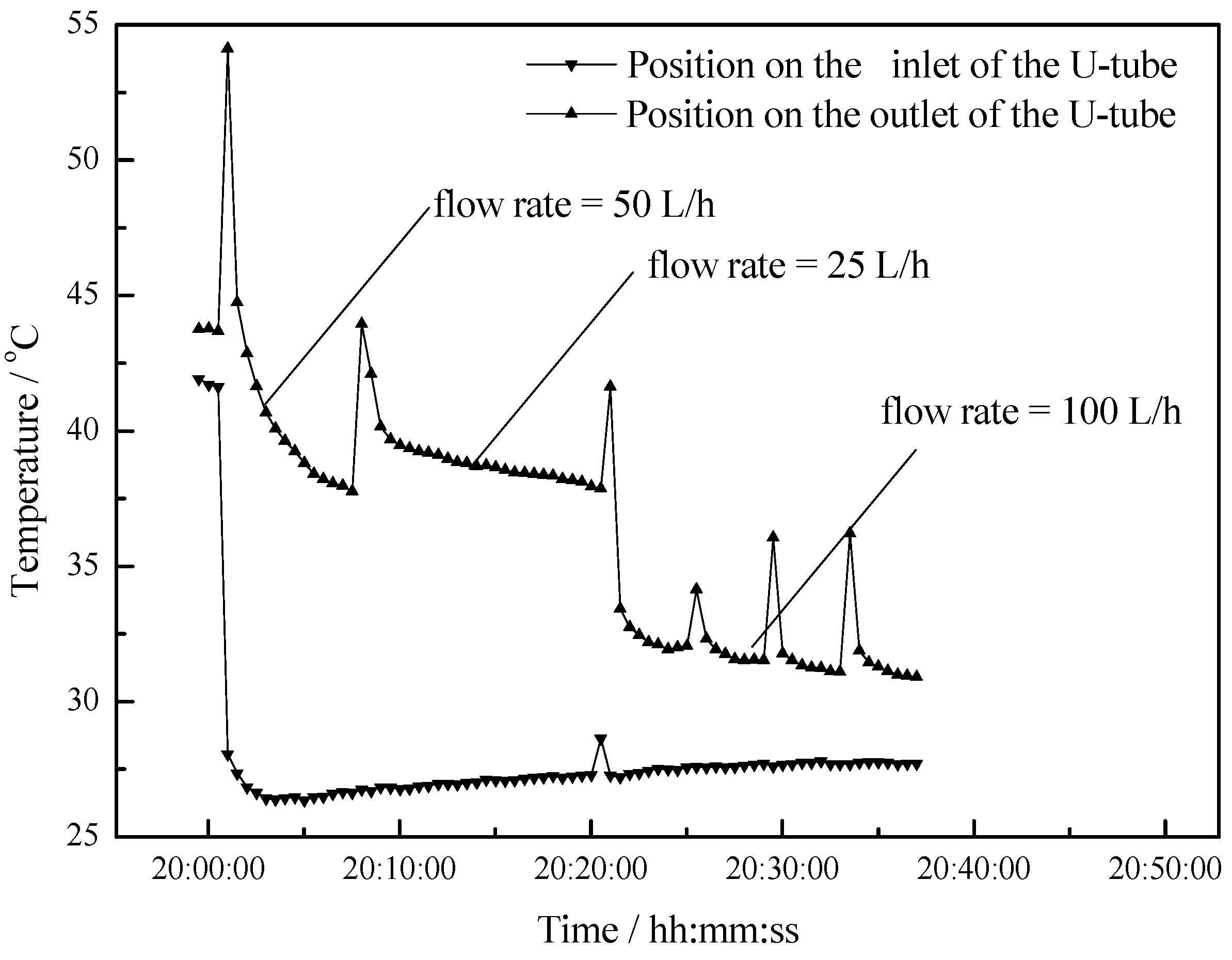
- The maximum temperature difference reaches 11 °C, 10 °C and 5 °C at flow rates of 25 L/h, 50 L/h and 100 L/h respectively. As the flow rate increases, the outlet water temperature of the U-type copper tube decreases. As the flow rate decreases, the temperature difference between the outlet water and inlet water increases, but the changing rate of temperature difference is becoming slower.
- (4)
- Due to the heat stored in the PCM, a uniform discharging process takes a long time which provides more comfortable hot water application.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khodadadi, J.M.; Hosseinizadeh, S.F. Nanoparticle-enhanced phase change materials (NEPCM) with great potential for improved thermal energy storage. Int. Commun. Heat Mass Transf. 2007, 34, 534–543. [Google Scholar] [CrossRef]
- Elgafy, A.; Lafdi, K. Effect of carbon nanofiber additives on thermal behavior of phase change materials. Carbon 2005, 43, 3067–3074. [Google Scholar] [CrossRef]
- Tiari, S.; Qiu, S.; Mahdavi, M. Numerical study of finned heat pipe-assisted thermal energy storage system with high temperature phase change material. Energy Convers. Manag. 2015, 89, 833–842. [Google Scholar] [CrossRef]
- Sharifi, N.; Bergman, T.L.; Allen, M.J.; Faghri, A. Melting and solidification enhancement using a combined heat pipe, foil approach. Int. J. Heat Mass Transf. 2014, 78, 930–941. [Google Scholar] [CrossRef]
- Eftekhar, J.; Hajisheikh, A.; Lou, D. Heat-transfer enhancement in a paraffin wax thermal storage-system. J. Sol. Energy Eng. 1984, 106, 299–306. [Google Scholar] [CrossRef]
- Zalba, B.; Marin, J.M.; Cabeza, L.F. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Yazici, M.Y.; Avci, M.; Aydin, O. Effect of eccentricity on melting behavior of paraffin in a horizontal tube-in-shell storage unit: An experimental study. Sol. Energy 2014, 101, 291–298. [Google Scholar] [CrossRef]
- Reyes, A.; Henriquez-Vargas, L.; Aravena, R. Experimental analysis, modeling and simulation of a solar energy accumulator with paraffin wax as PCM. Energy Convers. Manag. 2015, 105, 189–196. [Google Scholar] [CrossRef]
- Kumar, R.A.; Babu, B.G.; Mohanraj, M. Thermodynamic performance of forced convection solar air heaters using pin-fin absorber plate packed with latent heat storage materials. J. Therm. Anal. Calorim. 2016, 126, 1657–1678. [Google Scholar] [CrossRef]
- Padmanabhan, P.V.; Murthy, M.V.K. Outward phase-change in a cylindrical annulus with axial fins on the inner tube. Int. J. Heat Mass Transf. 1986, 29, 1855–1868. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, Z.N.; Zhu, H. Melting heat transfer characteristics of a composite phase change material fabricated by paraffin and metal foam. Appl. Energy 2017, 185, 1971–1983. [Google Scholar] [CrossRef]
- Yang, J.; Yang, L.; Xu, C. Experimental study on enhancement of thermal energy storage with phase-change material. Appl. Energy 2016, 169, 164–176. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P. Numerical and experimental study of heat transfer characteristics of a shell-tube latent heat storage system: Part I—Charging process. Energy 2015, 79, 337–350. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P. Numerical and experimental study of heat transfer characteristics of a shell-tube latent heat storage system: Part II—Discharging process. Energy 2015, 80, 177–189. [Google Scholar] [CrossRef]
- Baby, R.; Balaji, C. Experimental investigations on thermal performance enhancement and effect of orientation on porous matrix filled PCM based heat sink. Int. Commun. Heat Mass Transf. 2013, 46, 27–30. [Google Scholar] [CrossRef]
- Reddy, K.S. Thermal modeling of PCM-based solar integrated collector storage water heating system. J. Sol. Energy Eng. 2007, 129, 458–464. [Google Scholar] [CrossRef]
- Mahfuz, M.H.; Anisur, M.R.; Kibria, M.A. Performance investigation of thermal energy storage system with Phase Change Material (PCM) for solar water heating application. Int. Commun. Heat Mass Transf. 2014, 57, 132–139. [Google Scholar] [CrossRef]
- Murali, G.; Mayilsamy, K. Effect of Latent Thermal Energy storage and inlet locations on enhancement of stratification in a solar water heater under discharging mode. Appl. Therm. Eng. 2016, 106, 354–360. [Google Scholar] [CrossRef]
- Sakhrieh, A.; Abdullat, Y.; Hamdan, M.A. Enhancement of Thermal Energy Storage Using Phase-Change Material under Jordanian Climate. J. Infrastruct. Syst. 2016, 22, A40140011–A40140015. [Google Scholar] [CrossRef]
- El Khadraoui, A.; Bouadila, S.; Kooli, S. Solar air heater with phase change material: An energy analysis and a comparative study. Appl. Therm. Eng. 2016, 107, 1057–1064. [Google Scholar] [CrossRef]




| No. | Physical Properties | Unit | Value |
|---|---|---|---|
| 1 | Specific heat C | J/(kg·K) | 3350 (solid)/2700 (liquid) |
| 2 | Thermal conductivity k | W/(m·K) | 0.35 (solid)/0.15 (liquid) |
| 3 | Density ρ | kg/m3 | 920 (solid)/795 (liquid) |
| 4 | Latent heat H | kJ/kg | 158.2 |
| 5 | Phase-transition temperature | °C | 55~61 |
| Name | Type and Specification | Measuring Range and Measuring Frequency | Accuracy |
|---|---|---|---|
| Data acquisition instrument | Agilent 34970A | up to 60 channels per second | - |
| Solar radiation illuminance tester | TENMARS TM-207 | 0~2000 W/m2 | 7~12 μV/W·m2 |
| Anemometer | FS-R4-30 | 0~30 m/s | 0.5 m/s |
| Rotor flow meter | JUNHAI LZB-10 | 10~100 L/h | 1.5 level |
| Thermocouple | T-type (copper-constantan) | −40 °C~200 °C | 0.2 °C |
| Testing Stage Name | Period |
|---|---|
| Water filling stage | 7:00~7:30 |
| Heat accumulation and heat preservation stage | 7:30~19:00 |
| Heat release stage | 19:00~20:00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Chen, Z.; Shi, J. Experimental Study on Heat Transfer Performance of Vacuum Tube Heat Collector with Thermal Storage. Appl. Sci. 2017, 7, 448. https://doi.org/10.3390/app7050448
Huang X, Chen Z, Shi J. Experimental Study on Heat Transfer Performance of Vacuum Tube Heat Collector with Thermal Storage. Applied Sciences. 2017; 7(5):448. https://doi.org/10.3390/app7050448
Chicago/Turabian StyleHuang, Xinpeng, Zhenqian Chen, and Juan Shi. 2017. "Experimental Study on Heat Transfer Performance of Vacuum Tube Heat Collector with Thermal Storage" Applied Sciences 7, no. 5: 448. https://doi.org/10.3390/app7050448





