Relationship between Amount of Daily Movement Measured by a Triaxial Accelerometer and Motor Symptoms in Patients with Parkinson’s Disease
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Equipment
2.3. Procedure Applied for Minimization of Interday Variability
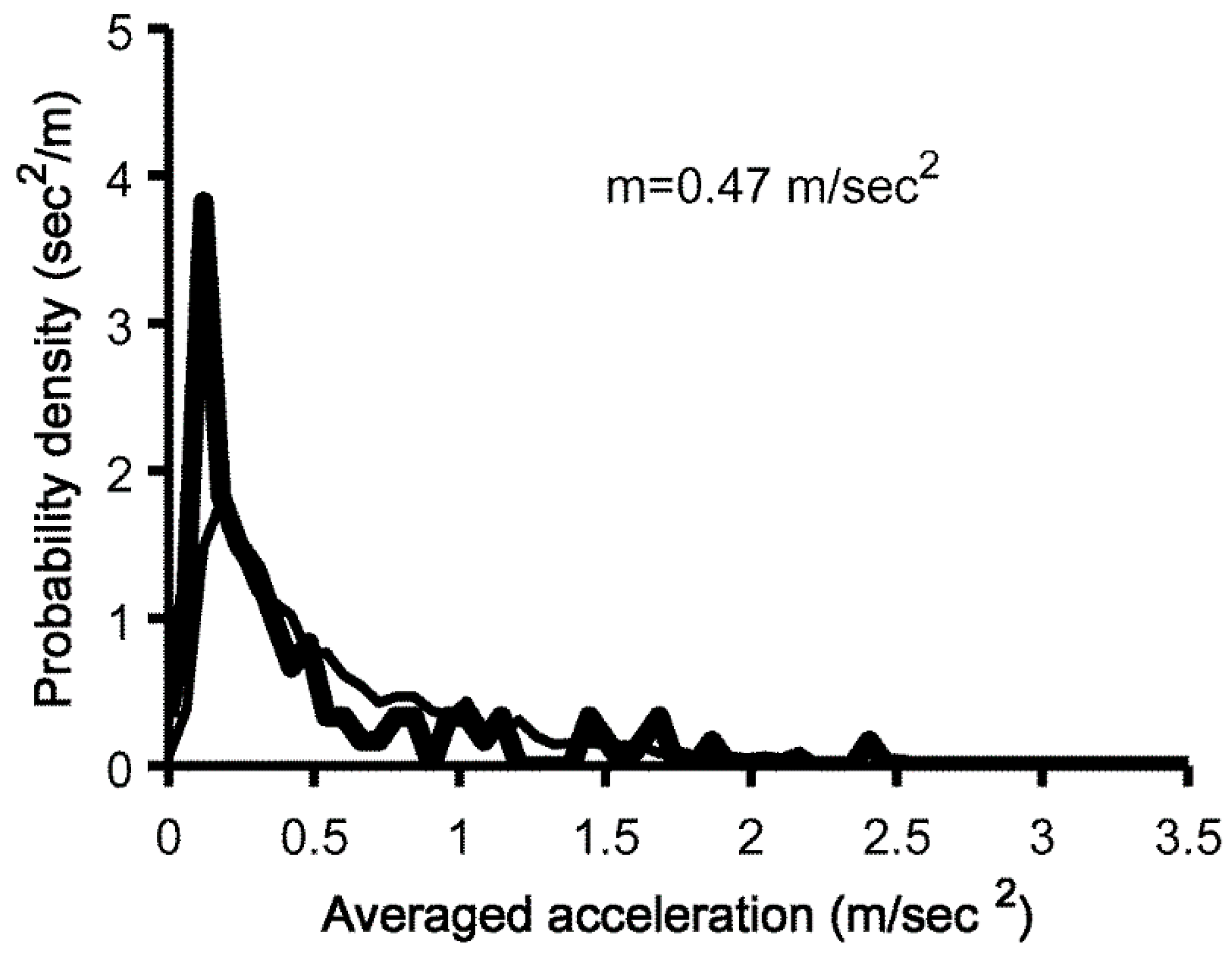
2.4. Calculation of Amount of Overall Movements
2.5. The mH&Y Stage and UPDRS
2.6. Statistical Analysis
3. Results
3.1. Clinical Features of Patients with PD
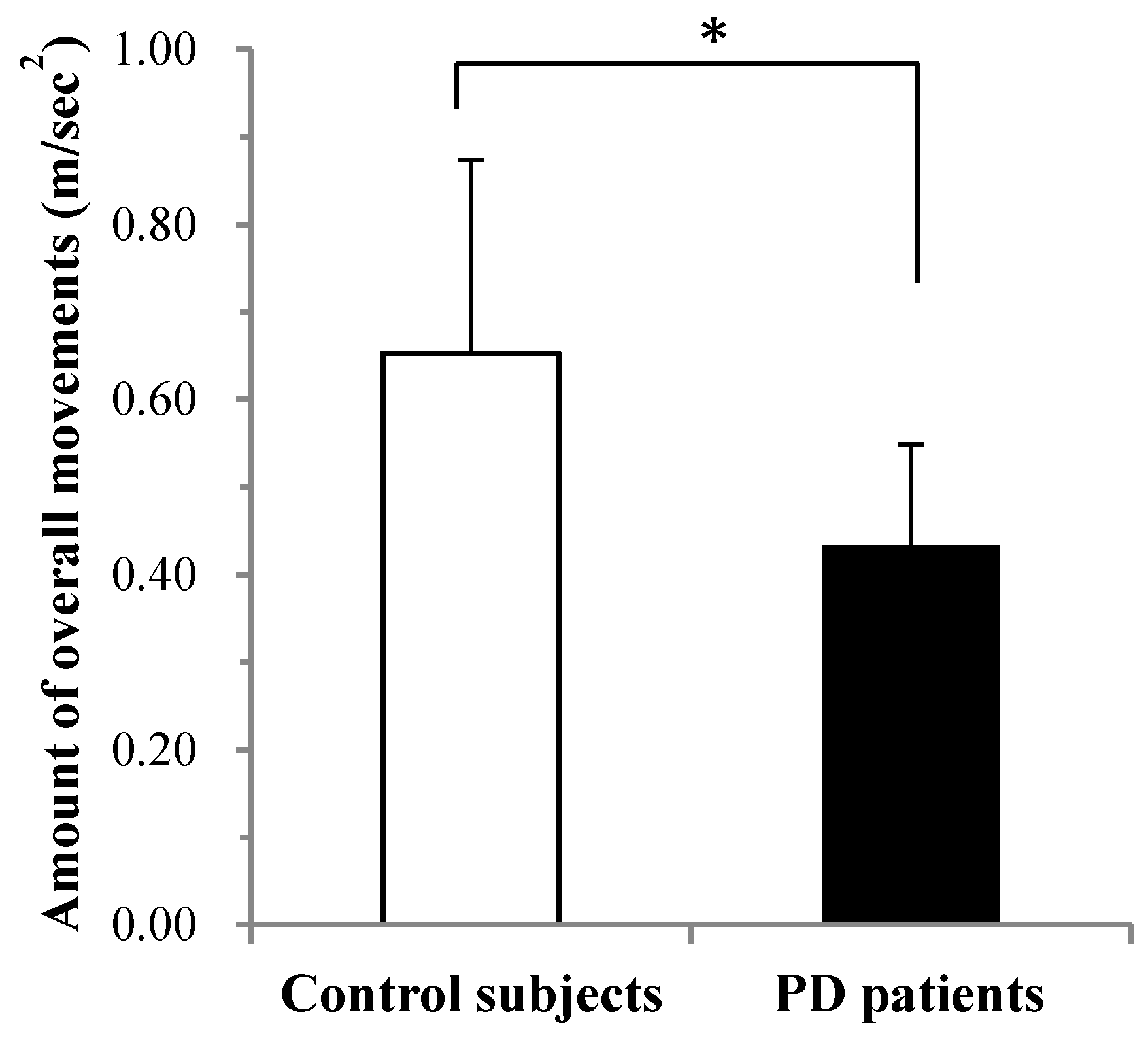
3.2. Comparison of Overall Movement per 24 h between PD and Controls Measured with MIMAMORI-Gait
3.3. Association between Overall Movement per 24 h Measured with MIMAMORI-Gait and UPDRS Scores
4. Discussion
Author Contributions
Conflicts of Interest
References
- Yamawaki, M.; Kusumi, M.; Kowa, H.; Nakashima, K. Changes in prevalence and incidence of Parkinson’s disease in Japan during a quarter of a century. Neuroepidemiology 2009, 32, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S.; Elton, R.L. UPDRS Program Members. Unified Parkinson’s disease rating scale. In Recent Developments in Parkinson’s Disease; Fahn, S., Marsden, C.D., Goldstein, M., Calne, D.B., Eds.; Macmillan Healthcare Information: Florham Park, NJ, USA, 1987; Volume 2, pp. 153–163, 293–304. [Google Scholar]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society UPDRS Revision Task Force. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ferro, Á.; Elshehabi, M.; Godinho, C.; Salkovic, D.; Hobert, M.A.; Domingos, J.; van Uem, J.M.; Ferreira, J.J.; Maetzler, W. New methods for the assessment of Parkinson’s disease (2005 to 2015): A systematic review. Mov. Disord. 2016, 31, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.; Goetz, C.G. Assessing bradykinesia in parkinsonian disorders. Front. Neurol. 2013, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.S.; Ellis, T.D.; Dibble, L.E.; Earhart, G.M.; Ford, M.P.; Foreman, K.B.; Cavanaugh, J.T. Obtaining reliable estimates of ambulatory physical activity in people with Parkinson’s disease. J. Parkinson’s Dis. 2016, 6, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Ginis, P.; Nieuwboer, A.; Dorfman, M.; Ferrari, A.; Gazit, E.; Canning, C.G.; Rocchi, L.; Chiari, L.; Hausdorff, J.M.; Mirelman, A. Feasibility and effects of home-based smartphone-delivered automated feedback training for gait in people with Parkinson’s disease: A pilot randomized controlled trial. Parkinsonism Relat. Disord. 2016, 22, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Del Din, S.; Godfrey, A.; Mazzà, C.; Lord, S.; Rochester, L. Free-living monitoring of Parkinson’s disease: Lessons from the field. Mov. Disord. 2016, 31, 1293–1313. [Google Scholar] [CrossRef] [PubMed]
- Bonora, G.; Mancini, M.; Carpinella, I.; Chiari, L.; Horak, F.B.; Ferrarin, M. Gait initiation is impaired in subjects with Parkinson’s disease in the OFF state: Evidence from the analysis of the anticipatory postural adjustments through wearable inertial sensors. Gait Posture 2017, 51, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Helbostad, J.L.; Chiari, L.; Chastin, S.; Aminian, K. Advances in long term physical behaviour monitoring. Biomed. Res. Int. 2016, 2016, 6745760. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.; Cavanaugh, J.T.; Earhart, G.M.; Ford, M.P.; Foreman, K.B.; Dibble, L.E. Which measures of physical function and motor impairment best predict quality of life in Parkinson’s disease? Parkinsonism Relat. Disord. 2011, 17, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, H.; Yoneyama, M.; Orimo, S. 24-hour recording of parkinsonian gait using a portable gait rhythmogram. Intern. Med. 2010, 49, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, H.; Terashi, H.; Ishimura, Y.; Takazawa, T.; Hayashi, A.; Mochizuki, H.; Okuma, Y.; Orimo, S.; Takahashi, K.; Yoneyama, M.; et al. Quantitative assessment of gait bradykinesia in Parkinson’s disease using a portable gait rhythmogram. Acta Med. Okayama 2012, 66, 31–40. [Google Scholar] [PubMed]
- Terashi, H.; Utsumi, H.; Ishimura, Y.; Mitoma, H. Independent regulation of the cycle and acceleration in parkinsonian gait analyzed by a long-term daily monitoring system. Eur. Neurol. 2013, 69, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Terashi, H.; Utsumi, H.; Ishimura, Y.; Aizawa, H.; Yoneyama, M.; Mitoma, H. Kinematic analysis of 24-hour recording of walking pattern in patients with vascular parkinsonism. Int. J. Neurosci. 2015, 125, 733–741. [Google Scholar] [CrossRef] [PubMed]
- The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 745–752.
- Yoneyama, M.; Kurihara, Y.; Watanabe, K.; Mitoma, H. Accelerometry-based gait analysis and its application to Parkinson’s disease assessment—Part 1: Detection of stride event. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kurihara, Y.; Watanabe, K.; Mitoma, H. Accelerometry-based gait analysis and its application to Parkinson’s disease assessment—Part 2: A new measure for quantifying walking behavior. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Mitoma, H.; Okuma, Y. Accelerometry-based long-term monitoring of movement disorders: from diurnal gait behavior to nocturnal bed mobility. J. Mech. Med. Biol. 2013, 13, 1350041. [Google Scholar] [CrossRef]
- Rascol, O.; Brooks, D.J.; Korczyn, A.D.; De Deyn, P.P.; Clarke, C.E.; Lang, A.E. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N. Engl. J. Med. 2000, 18, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S.; Oakes, D.; Shoulson, I.; Kieburtz, K.; Rudolph, A.; Lang, A.; Olanow, C.W.; Tanner, C.; Marek, K.; Parkinson Study Group. Levodopa and the progression of Parkinson’s disease. N. Engl. J. Med. 2004, 351, 2498–2508. [Google Scholar] [PubMed]
- Stocchi, F.; Rascol, O.; Kieburtz, K.; Poewe, W.; Jankovic, J.; Tolosa, E.; Barone, P.; Lang, A.E.; Olanow, C.W. Initiating levodopa/carbidopa therapy with and without entacapone in early Parkinson disease: The STRIDE-PD study. Ann. Neurol. 2010, 68, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Khoo, T.K.; Yarnall, A.J.; Duncan, G.W.; Coleman, S.; O’Brien, J.T.; Brooks, D.J.; Barker, R.A.; Burn, D.J. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 2013, 80, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, X.Y.; Wei, Q.Q.; Ou, R.W.; Song, W.; Cao, B.; Zhao, B.; Shang, H.F. Non-motor symptoms and quality of life in tremor dominant vs postural instability gait disorder Parkinson’s disease patients. Acta Neurol. Scand. 2016, 133, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.P.; Evans, J.R.; Farrell, K.; Brayne, C.; Barker, R.A. Determinants of delayed diagnosis in Parkinson’s disease. J. Neurol. 2013, 260, 1978–1981. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, N.; Sato, K.; Hishikawa, N.; Takemoto, M.; Ohta, Y.; Yamashita, T.; Abe, K. Comparative gait analysis in progressive supranuclear palsy and Parkinson’s disease. Eur. Neurol. 2016, 75, 282–299. [Google Scholar] [CrossRef] [PubMed]
| Age (years) | 65.7 ± 6.5 |
| Male/female (n) | 29/21 |
| Height (m) | 1.61 ± 0.09 |
| Disease duration (years) | 1.4 ± 0.9 |
| Mini-Mental State Examination | 28.0 ± 2.0 |
| Modified Hoehn and Yahr stage | 2.2 ± 0.7 |
| Unified Parkinson’s Disease Rating Scale part II score | 7.9 ± 4.5 |
| Unified Parkinson’s Disease Rating Scale part III score | 15.6 ± 8.7 |
| Assessment of Motor Symptoms | B (SE) | β | p Value |
|---|---|---|---|
| Modified Hoehn and Yahr stage | −0.044(0.023) | −0.260 | 0.059 |
| Unified Parkinson’s Disease Rating Scale part II score | −0.013 (0.003) | −0.506 | <0.001 |
| Unified Parkinson’s Disease Rating Scale part III score | −0.005 (0.002) | −0.347 | 0.010 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terashi, H.; Mitoma, H.; Yoneyama, M.; Aizawa, H. Relationship between Amount of Daily Movement Measured by a Triaxial Accelerometer and Motor Symptoms in Patients with Parkinson’s Disease. Appl. Sci. 2017, 7, 486. https://doi.org/10.3390/app7050486
Terashi H, Mitoma H, Yoneyama M, Aizawa H. Relationship between Amount of Daily Movement Measured by a Triaxial Accelerometer and Motor Symptoms in Patients with Parkinson’s Disease. Applied Sciences. 2017; 7(5):486. https://doi.org/10.3390/app7050486
Chicago/Turabian StyleTerashi, Hiroo, Hiroshi Mitoma, Mitsuru Yoneyama, and Hitoshi Aizawa. 2017. "Relationship between Amount of Daily Movement Measured by a Triaxial Accelerometer and Motor Symptoms in Patients with Parkinson’s Disease" Applied Sciences 7, no. 5: 486. https://doi.org/10.3390/app7050486






