Development of Green Geopolymer Using Agricultural and Industrial Waste Materials with High Water Absorbency
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Geopolymer Preparation and Characterization
3. Result and Discussion
3.1. NaOH Concentration Effect
3.2. Curing Time Effect
3.3. Water Absorption
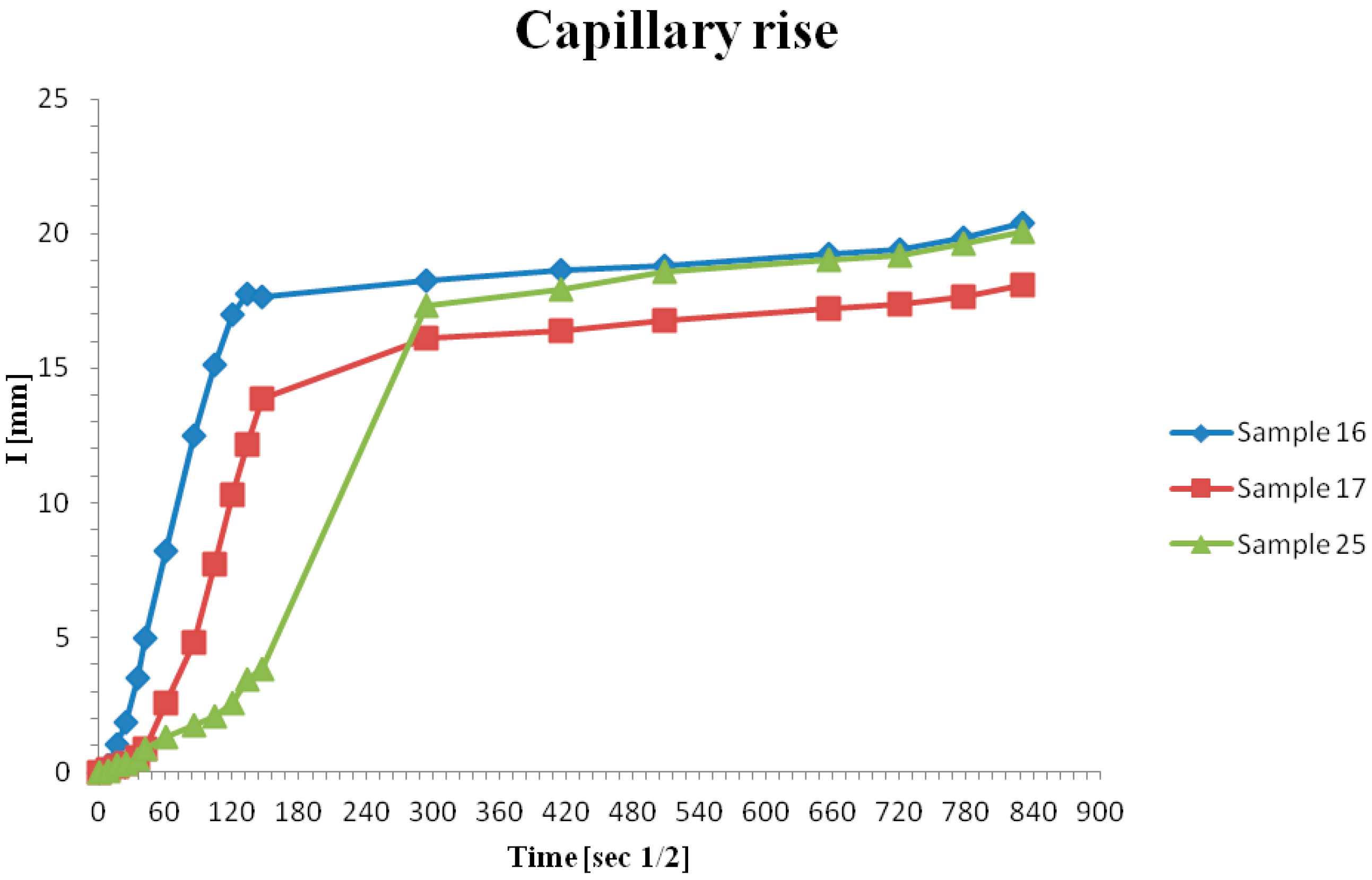
3.4. Capillary Rise Properties
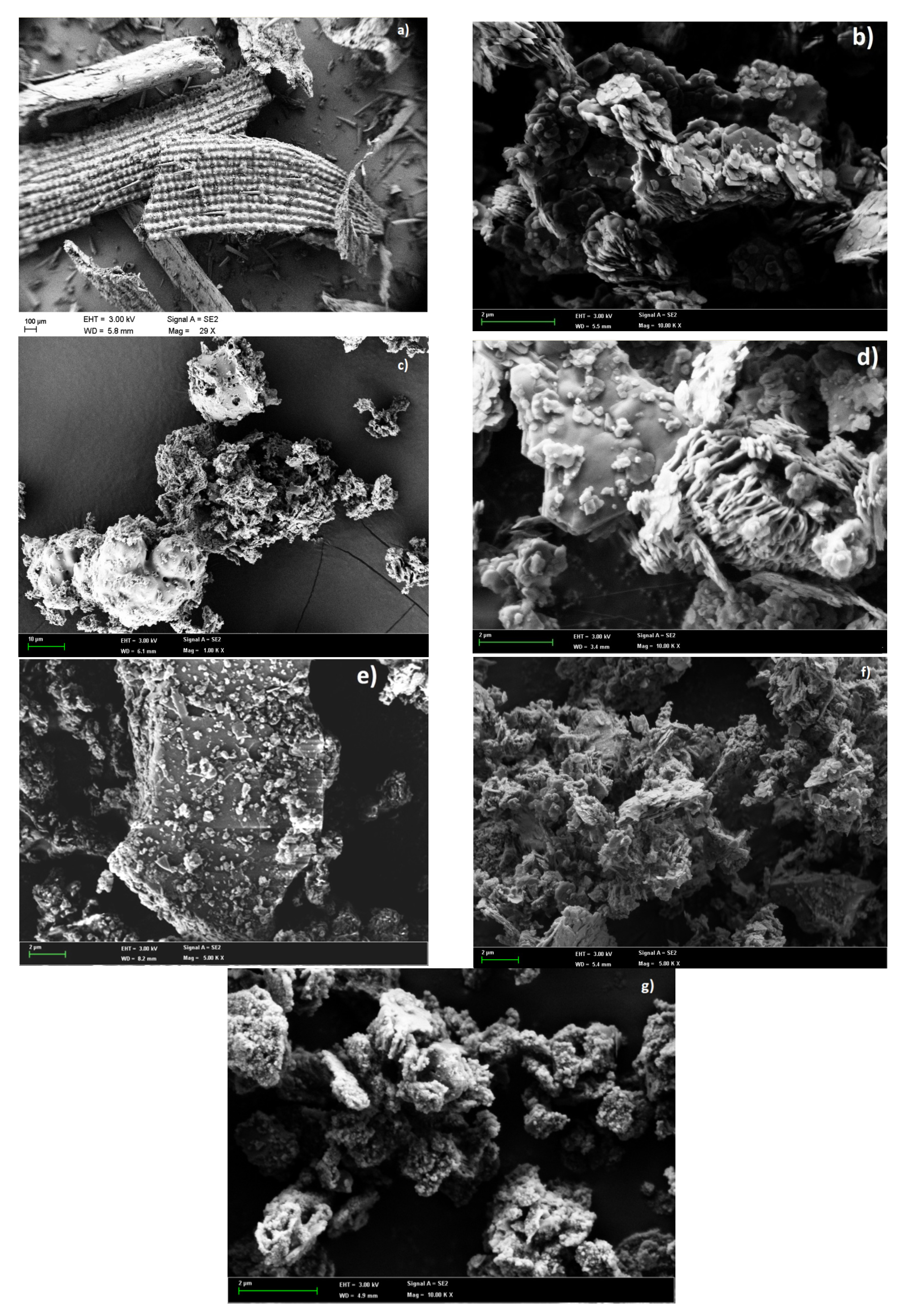
3.5. Microstructure and Morphology of Materials
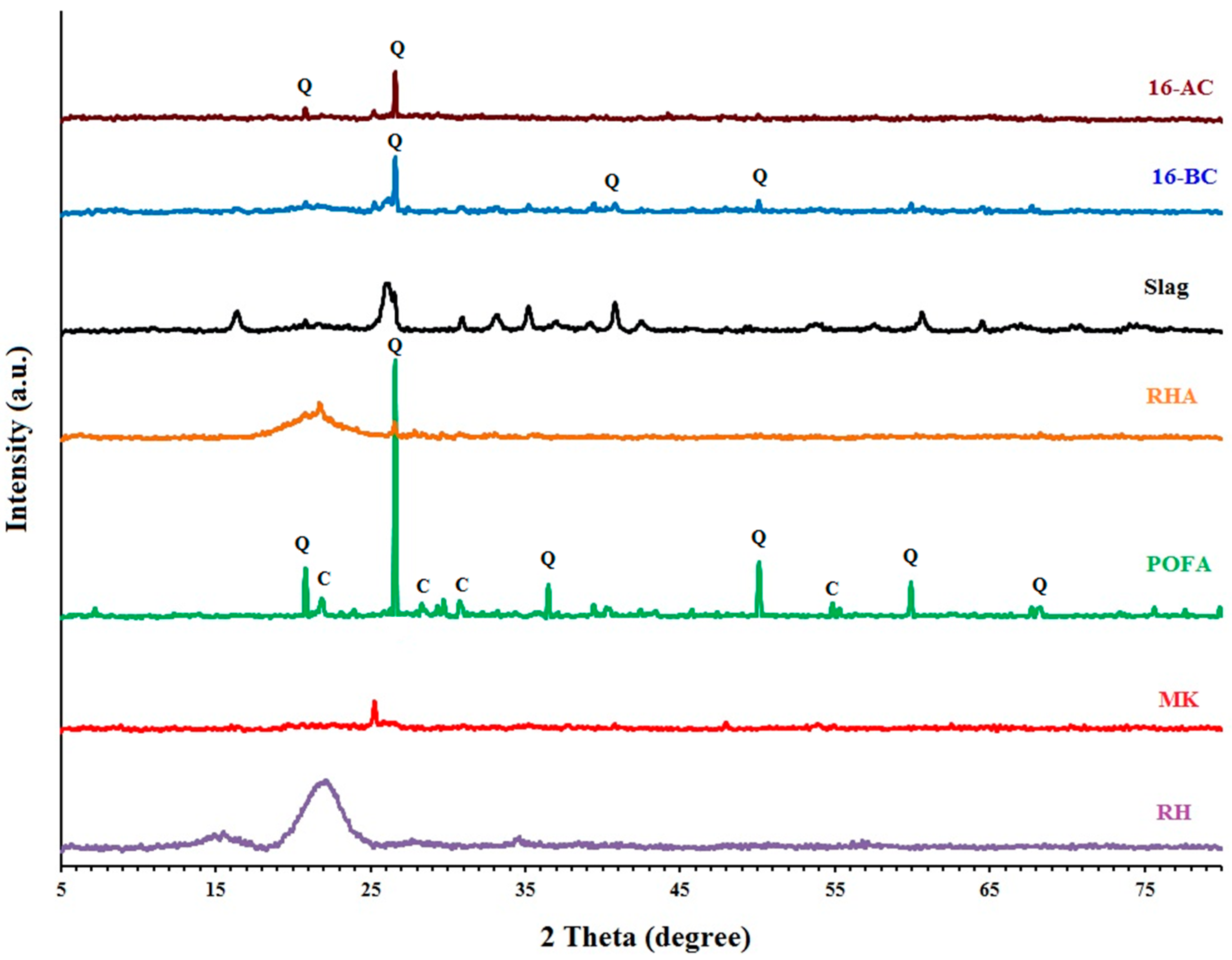
3.6. X-ray Diffraction (XRD)
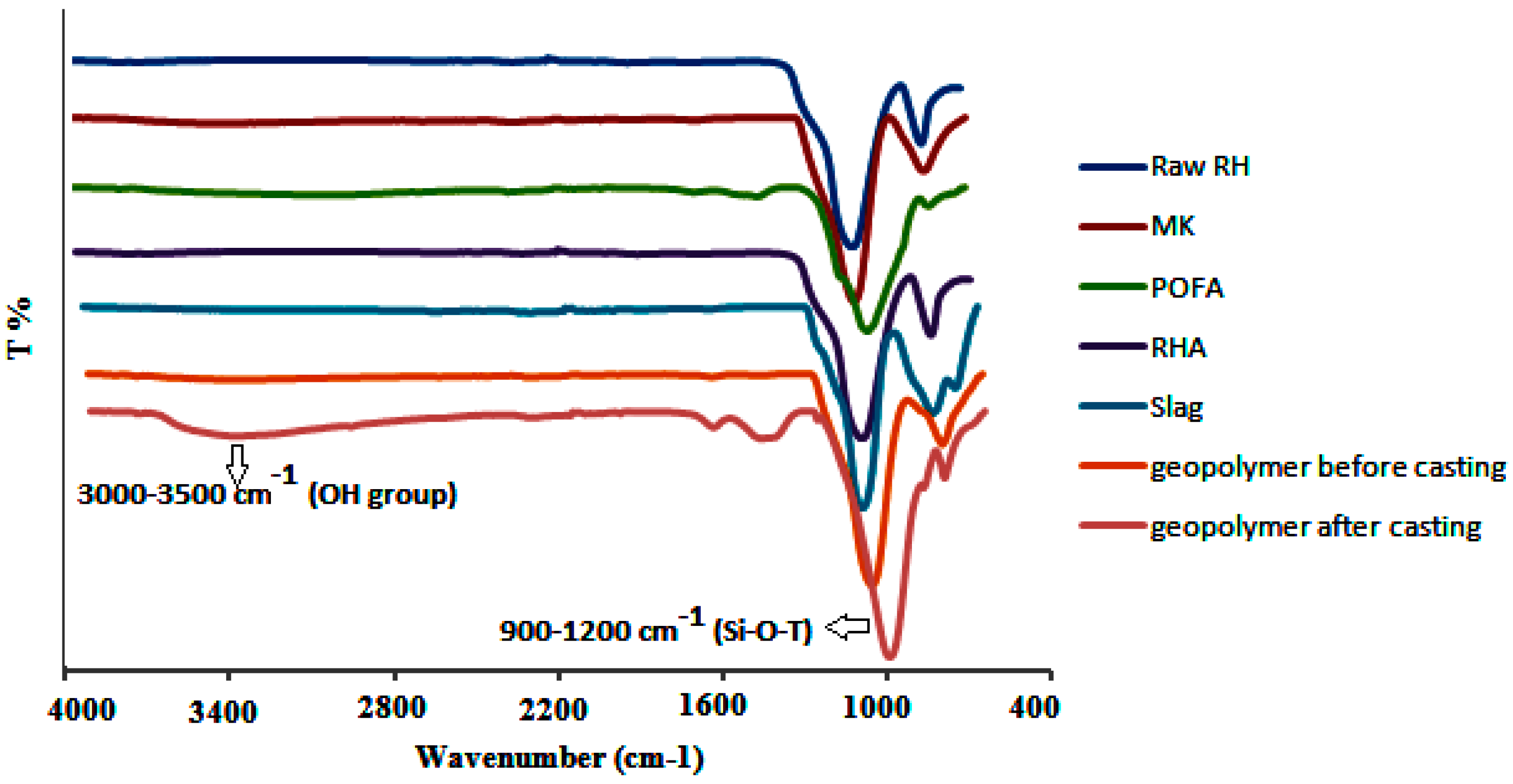
3.7. Fourier Transform Infrared Spectroscopy (FTIR)
3.8. Texture Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zeynab, E.; Asim, N.; Ambar Yarmo, M.; Shamsudin, R.; Mohammad, M.; Sopian, K. Green material prospects for passive evaporative cooling systems: Geopolymers. Energies 2016, 9, 586. [Google Scholar]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. Do geopolymers actually contain nanocrystalline zeolites? A reexamination of existing results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Verdolotti, L.; Iannace, S.; Lavorgna, M.; Lamanna, R. Geopolymerization reaction to consolidate incoherent pozzolanic soil. J. Mater. Sci. 2008, 43, 865–873. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Liberty, J.T.; Ugwuishiwu, B.O.; Pukuma, S.A.; Odo, C.E. Principles and application of evaporative cooling systems for fruits and vegetables preservation. Int. J. Curr. Eng. Technol. 2013, 3, 1000–1006. [Google Scholar]
- Memon, F.A.; Nuruddin, M.F.; Demie, S.; Shafiq, N. Effect of curing conditions on strength of fly ash-based self-compacting geopolymer concrete. World Acad. Sci. Eng. Technol. 2011, 80, 860–863. [Google Scholar]
- Wallah, S.; Rangan, B.V. Low-Calcium Fly Ash-Based Geopolymer Concrete: Long-Term Properties; Research Report-GC2; Curtin University: Bentley, Australia, 2006; pp. 76–80. [Google Scholar]
- Nazari, A.; Bagheri, A.; Riahi, S. Properties of geopolymer with seeded fly ash and rice husk bark ash. Mater. Sci. Eng. A 2011, 528, 7395–7401. [Google Scholar] [CrossRef]
- Mishra, A.; Choudhary, D.; Jain, N.; Kumar, M.; Sharda, N.; Dutt, D. Effect of concentration of alkaline liquid and curing time on strength and water absorption of geopolymer concrete. ARPN J. Eng. Appl. Sci. 2008, 3, 14–18. [Google Scholar]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Shrinkage and strength of alkaline activated ground steel slag/ultrafine palm oil fuel ash pastes and mortars. Mater. Des. 2014, 63, 710–718. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Dick, W.A. Compressive strength and microstructural characteristics of class C fly ash geopolymer. Cem. Concr. Compos. 2010, 32, 142–147. [Google Scholar] [CrossRef]
- Zain, M.; Islam, M.N.; Mahmud, F.; Jamil, M. Production of rice husk ash for use in concrete as a supplementary cementitious material. Constr. Build. Mater. 2011, 25, 798–805. [Google Scholar] [CrossRef]
- ASTM, C. Standard Test Methods for Chemical Analysis of Hydraulic Cement; American Society for Testing of Materials: Philadelphia, PA, USA, 2004. [Google Scholar]
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S. Microstructural characterisation of geopolymers synthesised from kaolinite/stilbite mixtures using XRD, MAS-NMR, SEM/EDX, TEM/EDX, and HREM. Cem. Concr. Res. 2002, 32, 1705–1716. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Evolution of alkaline activated ground blast furnace slag–ultrafine palm oil fuel ash based concrete. Mater. Des. 2014, 55, 387–393. [Google Scholar] [CrossRef]
- Emdadi, Z.; Asim, N.; Yarmo, M.A.; Shamsudin, R. The effect of mix composition on the water absorption, density and compressive strength of rice husk based geopolymers. In Proceedings of the 2015 5th International Conference on Environment Science and Engineering, Istanbul, Turkey, 24–25 April 2015; Volume 83. [Google Scholar]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Investigations about the effect of aggregates on strength and microstructure of geopolymeric mine waste mud binders. Cem. Concr. Res. 2007, 37, 933–941. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Studies about mix composition of alkali-activated mortars using waste mud from Panasqueira. In Proceedings of the Engineering Conference, University of Beira Interior, Covilha, Portugal, 11–13 September 2005. [Google Scholar]
- Temuujin, J.; Williams, R.; van Riessen, A. Effect of mechanical activation of fly ash on the properties of geopolymer cured at ambient temperature. J. Mater. Process. Technol. 2009, 209, 5276–5280. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Wang, K.; Shah, S.; Mishulovich, A. Effects of curing temperature and NaOH addition on hydration and strength development of clinker-free CKD-fly ash binders. Cem. Concr. Res. 2004, 34, 299–309. [Google Scholar] [CrossRef]
- Poon, C.-S.; Azhar, S.; Anson, M.; Wong, Y.L. Performance of metakaolin concrete at elevated temperatures. Cem. Concr. Compos. 2003, 25, 83–89. [Google Scholar] [CrossRef]
- Lee, W.; Van Deventer, J. The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf. A 2002, 211, 115–126. [Google Scholar] [CrossRef]
- Cheng, T.; Chiu, J. Fire-resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 2003, 16, 205–210. [Google Scholar] [CrossRef]
- Martinez-Ramirez, S.; Palomo, A. Microstructure studies on Portland cement pastes obtained in highly alkaline environments. Cem. Concr. Res. 2001, 31, 1581–1585. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vázquez, E.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-) combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Sata, V.; Jaturapitakkul, C.; Rattanashotinunt, C. Compressive strength and heat evolution of concretes containing palm oil fuel ash. J. Mater. Civ. Eng. 2010, 22, 1033–1038. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lukey, G.C. The characterisation of source materials in fly ash-based geopolymers. Mater. Lett. 2003, 57, 1272–1280. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Rangan, B.V. Fly Ash-Based Geopolymer Concrete; Curtin University of Technology: Bentley, Australia, 2008. [Google Scholar]
- Isobe, T.; Kameshima, Y.; Nakajima, A.; Okada, K.; Hotta, Y. Extrusion method using nylon 66 fibers for the preparation of porous alumina ceramics with oriented pores. J. Eur. Ceram. Soc. 2006, 26, 2213–2217. [Google Scholar] [CrossRef]
- Isobe, T.; Kameshima, Y.; Nakajima, A.; Okada, K. Preparation and properties of porous alumina ceramics with uni-directionally oriented pores by extrusion method using a plastic substance as a pore former. J. Eur. Ceram. Soc. 2007, 27, 61–66. [Google Scholar] [CrossRef]
- Isobe, T.; Kameshima, Y.; Nakajima, A.; Okada, K.; Hotta, Y. Gas permeability and mechanical properties of porous alumina ceramics with unidirectionally aligned pores. J. Eur. Ceram. Soc. 2007, 27, 53–59. [Google Scholar] [CrossRef]
- Okada, K.; Imase, A.; Isobe, T.; Nakajima, A. Capillary rise properties of porous geopolymers prepared by an extrusion method using polylactic acid (PLA) fibers as the pore formers. J. Eur. Ceram. Soc. 2011, 31, 461–467. [Google Scholar] [CrossRef]
- Okada, K.; Ooyama, A.; Isobe, T.; Kameshima, Y.; Nakajima, A.; MacKenzie, K.J. Water retention properties of porous geopolymers for use in cooling applications. J. Eur. Ceram. Soc. 2009, 29, 1917–1923. [Google Scholar] [CrossRef]
- Onutai, S.; Jiemsirilers, S.; Thavorniti, P.; Kobayashi, T. Fast microwave syntheses of fly ash based porous geopolymers in the presence of high alkali concentration. Ceram. Int. 2016, 42, 9866–9874. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Ascensão, G.; Seabra, M.P.; Labrincha, J.A. Porous biomass fly ash-based geopolymers with tailored thermal conductivity. J. Clean. Prod. 2016, 119, 99–107. [Google Scholar] [CrossRef]
- Ducman, V.; Korat, L. Characterization of geopolymer fly-ash based foams obtained with the addition of Al powder or H2O2 as foaming agents. Mater. Charact. 2016, 113, 207–213. [Google Scholar] [CrossRef]
- He, P.; Jia, D.; Lin, T.; Wang, M.; Zhou, Y. Effects of high-temperature heat treatment on the mechanical properties of unidirectional carbon fiber reinforced geopolymer composites. Ceram. Int. 2010, 36, 1447–1453. [Google Scholar] [CrossRef]
- Okada, K.; Uchiyama, S.; Isobe, T.; Kameshima, Y.; Nakajima, A.; Kurata, T. Capillary rise properties of porous mullite ceramics prepared by an extrusion method using organic fibers as the pore former. J. Eur. Ceram. Soc. 2009, 29, 2491–2497. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Lynd, L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 2004, 88, 797–824. [Google Scholar] [CrossRef] [PubMed]
- Della, V.P.; Kühn, I.; Hotza, D. Rice husk ash as an alternate source for active silica production. Mater. Lett. 2002, 57, 818–821. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Santos, P.L.; Bonacin, J.A.; Passos, R.R.; Pocrifka, L.A. Rice husk reuse in the preparation of SnO2/SiO2 nanocomposite. Mater. Res. 2015, 18, 639–643. [Google Scholar] [CrossRef]
- Mohamed Nacer, G.; Abdesselam, Z.; Samia, H. Investigating the local granulated blast furnace slag. Open J. Civ. Eng. 2012, 2, 17807. [Google Scholar]
- Nurdeen, M.; Johari, M.A.M.; Hashim, S.F.S. Strength activity index and microstructural characteristics of treated palm oil fuel ash. Int. J. Civ. Environ. Eng. 2011, 11, 5. [Google Scholar]
- Inoubli, A.; Kahlaoui, M.; Sobrados, I.; Chefi, S.; Madani, A.; Sanz, J.; Ben Haj Amara, A. Influence of anionic vacancies on the conductivity of La9.33Si6−xAlxO26−x/2 oxide conductors with an oxyapatite structure. J. Power Sources 2014, 271, 203–212. [Google Scholar] [CrossRef]
- Madani, A.; Aznar, A.; Sanz, J.; Serratosa, J.M. 29Si and 27Al NMR study of zeolite formation from alkali-leached kaolinites. Influence of thermal preactivation. J. Phys. Chem. 1990, 94, 760–765. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Granizo, M.L.; Blanco-Varela, M.T.; Martínez-Ramírez, S. Alkali activation of metakaolins: Parameters affecting mechanical, structural and microstructural properties. J. Mater. Sci. 2007, 42, 2934–2943. [Google Scholar] [CrossRef]
- Davdovits, J. Geopolymer Chemistry and Applications; Institute Geopolymere: Saint Quentin, France, 2008. [Google Scholar]
- Alonso, S.; Palomo, A. Calorimetric study of alkaline activation of calcium hydroxide–metakaolin solid mixtures. Cem. Concr. Res. 2001, 31, 25–30. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]


| Chemical Composition (wt %) | RH | RHA | MK | POFA | Slag | WG | Sample 16 before Casting | Sample 16 after Casting |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 23.77 | 91.04 | 50.29 | 48 | 29.24 | 26.5 | 54.46 | 51.97 |
| Al2O3 | 0.06 | 0.33 | 45.75 | 2.30 | 11.71 | - | 30.95 | 19.88 |
| Fe2O3 | 0.13 | 1.89 | 0.38 | 2.70 | 0.34 | - | 0.86 | 1.32 |
| CaO | 0.25 | 0.54 | - | 4.95 | 42.43 | - | 0.86 | 7.58 |
| MgO | 0.08 | 0.58 | - | 4.64 | 5.06 | - | 0.64 | 1.13 |
| K2O | 1.08 | 2.4 | 0.17 | 10.99 | 0.27 | - | 2.00 | 1.65 |
| SO3 | 0.27 | 0.53 | 0.08 | 2.47 | 4.67 | - | 0.42 | 0.89 |
| TiO2 | - | 0.02 | 1.56 | 0.15 | 0.46 | - | 0.82 | 0.67 |
| Na2O | - | 0.05 | 0.27 | 0.12 | 0.2 | 10.6 | 0.17 | 8.46 |
| Others | 0.81 | 2.62 | 0.3 | 5.47 | 0.14 | 62.9 (water) | 1.16 | 0.7 |
| Loss on ignition (LOI) | 78 | 2.86 | 1.27 | 8.73 | 0.6 | - | 5.36 | 5.81 |
| Sample | NaOH Concentration (Molar) | Sodium Silicate/NaOH | Liquid/Solid | % Water Absorption after 24 h | Density g/cm3 | Compressive Strength (MPa) |
|---|---|---|---|---|---|---|
| 28 Days | ||||||
| 1 | 8 | 2.5 | 0.5 | 40.9 | 1.166 | 3.3 |
| 2 | 10 | 1.75 | 0.4 | 46.2 | 1.181 | 1.4 |
| 3 | 14 | 1 | 0.5 | - | 1.277 | 1.9 |
| 4 | 14 | 2.5 | 0.3 | 45.4 | 1.202 | 2.3 |
| 5 | 8 | 1 | 0.3 | 49.9 | 1.162 | 2.2 |
| 6 | 8 | 1 | 0.5 | 45.9 | 1.216 | 2.2 |
| 7 | 14 | 1 | 0.3 | 49.0 | 1.213 | 2.2 |
| 8 | 14 | 2.5 | 0.5 | 40.8 | 1.173 | 2.3 |
| 9 | 8 | 2.5 | 0.3 | 48.2 | 1.111 | 1.4 |
| 10 | 14 | 1.75 | 0.4 | 45.0 | 1.216 | 2.3 |
| 11 | 8 | 1.5 | 0.4 | 43.2 | 1.181 | 2.4 |
| 12 | 10 | 1 | 0.4 | 48.0 | 1.240 | 1.4 |
| 13 | 10 | 1.75 | 0.5 | 42.7 | 1.293 | 2.8 |
| 14 | 10 | 2.5 | 0.5 | 37.9 | 1.1 | 4.9 |
| 15 | 10 | 2.5 | 0.4 | 44.3 | 1.207 | 1.9 |
| 16 | 10 | 1.75 | 0.3 | 46.2 | 1.171 | 1.3 |
| 17 | 14 | 1.5 | 0.5 | 42.5 | 1.310 | 2.0 |
| 18 | 14 | 1 | 0.4 | 47.0 | 1.120 | 2.0 |
| 19 | 14 | 2.5 | 0.4 | 42.3 | 1.121 | 2.4 |
| 20 | 14 | 1.5 | 0.3 | 44.11 | 1.09 | 1.7 |
| 21 | 10 | 1 | 0.5 | 43.9 | 1.128 | 1.7 |
| 22 | 10 | 1.5 | 0.5 | 30.9 | 1.278 | 1.8 |
| 23 | 10 | 1.5 | 0.4 | 26.6 | 1.287 | 2.2 |
| 24 | 10 | 1 | 0.3 | 34.7 | 1.186 | 1.4 |
| 25 | 10 | 1.5 | 0.3 | 32.2 | 1.286 | 1.6 |
| 26 | 10 | 2.5 | 0.3 | 29.3 | 1.316 | 0.8 |
| 27 | 8 | 1.5 | 0.5 | 25.8 | 1.380 | 2.0 |
| 28 | 8 | 1 | 0.4 | 31.0 | 1.368 | 1.8 |
| 29 | 8 | 1.5 | 0.4 | 29.2 | 1.343 | 1.9 |
| 30 | 8 | 2.5 | 0.4 | - | 1.387 | 2.0 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | Yield |
|---|---|---|---|---|---|---|
| Model | 8.90 | 6 | 1.48 | 5.38 | 0.0013 | significant |
| NaOH Concentration (Molar) | 0.029 | 1 | 0.029 | 0.11 | 0.7469 | - |
| Sodium Silicate/NaOH | 1.25 | 1 | 1.25 | 4.54 | 0.0439 | - |
| Liquid/Solid | 3.69 | 1 | 3.69 | 13.39 | 0.0013 | - |
| Lack of Fit | 6.21 | 22 | 0.28 | 2.26 | 0.4873 | not significant |
| Number | % Contents | % Water Absorption | % Porosity Calculated by ASTM) | Density g/cm3 | Compressive Strength (MPa) | ||||
|---|---|---|---|---|---|---|---|---|---|
| RH | RHA | MK | POFA | Slag | 24 h | 28 Days | |||
| Sample 1 | 10 | 20 | 40 | 10 | 20 | 37.31 | 35.3 | 1.06 | 2.3 |
| Sample 2 | 10 | 25 | 35 | 10 | 20 | 41.99 | 31.7 | 1.08 | 2.4 |
| Sample 3 | 10 | 30 | 30 | 10 | 20 | 38.21 | 29.1 | 1.088 | 3.4 |
| Sample 4 | 10 | 35 | 25 | 10 | 20 | 36.83 | 29.5 | 1.106 | 3.2 |
| Sample 5 | 10 | 40 | 20 | 10 | 20 | 39.6 | 33.8 | 0.95 | 2.7 |
| Sample 6 | 10 | 15 | 40 | 15 | 20 | 40.20 | 32.3 | 0.99 | 2.2 |
| Sample 7 | 10 | 20 | 35 | 15 | 20 | 42.22 | 35.2 | 0.98 | 2.3 |
| Sample 8 | 10 | 25 | 30 | 15 | 20 | 36.40 | 28.8 | 1.11 | 3.22 |
| Sample 9 | 10 | 30 | 25 | 15 | 20 | 41.23 | 28.15 | 0.95 | 2.5 |
| Sample 10 | 10 | 35 | 20 | 15 | 20 | 39.85 | 28.63 | 1.03 | 2.9 |
| Sample 11 | 10 | 40 | 15 | 15 | 20 | 33.78 | 28.4 | 1.11 | 4.86 |
| Sample 12 | 10 | 15 | 30 | 15 | 30 | 38.6 | 33.8 | 1.05 | 2.5 |
| Sample 13 | 10 | 20 | 25 | 15 | 30 | 31.8 | 27.1 | 1.16 | 4.7 |
| Sample 14 | 10 | 25 | 20 | 15 | 30 | 48.40 | 37.3 | 1.22 | 2.43 |
| Sample 15 | 10 | 30 | 15 | 15 | 30 | 37.9 | 31.1 | 1.06 | 4.5 |
| Sample 16 | 10 | 15 | 35 | 10 | 30 | 37.9 | 32.8 | 1.1 | 4.9 |
| Sample 17 | 10 | 20 | 30 | 10 | 30 | 41.9 | 33.3 | 1.07 | 5.7 |
| Sample 18 | 10 | 25 | 25 | 10 | 30 | 38.2 | 29.8 | 1.07 | 4.5 |
| Sample 19 | 10 | 30 | 20 | 10 | 30 | 38.9 | 30.9 | 1.06 | 2.8 |
| Sample 20 | 10 | 35 | 15 | 10 | 30 | 37.9 | 28.5 | 1.1 | 4.1 |
| Sample 21 | 5 | 30 | 20 | 15 | 30 | 44.21 | 33.7 | 1.26 | 2.7 |
| Sample 22 | 10 | 25 | 25 | 15 | 25 | 34.25 | 28.3 | 1.147 | 4.87 |
| Sample 23 | 5 | 30 | 35 | 10 | 20 | 34.21 | 28.9 | 1.12 | 3.32 |
| Sample 24 | 5 | 35 | 30 | 10 | 20 | 35.51 | 27.5 | 1.1 | 3.6 |
| Sample 25 | 5 | 25 | 30 | 10 | 30 | 42.71 | 28.9 | 1.26 | 5.7 |
| Sample 26 | 5 | 40 | 15 | 10 | 30 | 21.83 | 21.1 | 1.1 | 2.57 |
| Sample 27 | 10 | 30 | 15 | 15 | 30 | 30.11 | 26.5 | 1.16 | 4.99 |
| Sample 28 | 5 | 40 | 25 | 10 | 20 | 28.5 | 26.8 | 1.02 | 2.4 |
| Sample 29 | 5 | 40 | 20 | 10 | 25 | 33.0 | 27.1 | 1.04 | 3.2 |
| Sample 30 | 5 | 40 | 15 | 10 | 30 | 24.63 | 17.4 | 1.12 | 3.65 |
| Materials | Properties & Performance | Reference | |||
|---|---|---|---|---|---|
| Mechanical Strength | Water Absorption | Porosity | Application | ||
| Porous geopolymer: Metakaolin + sodium silicate solution + polyacetic acid (PLA) | Bending strength: 36 MPa | Capillary rise: 1125 mm | - | Passive cooling | [36] |
| Porous geopolymer: Metakaolin + sodium silicate solution + NaOH | Compressive strength: 14.2 MPa (H2O/AL2O3↓) to <5 MPa (H2O/AL2O3↑) | 27% (H2O/AL2O3↓)–51.1% (H2O/AL2O3↑) | Improving | Passive cooling | [37] |
| Porous geopolymer: fly ash + sodium silicate solution + NaOH | - | - | Improving | - | [38] |
| Porous geopolymer: fly ash + metakaolin sodium silicate solution + NaOH + hydrogen peroxide (H2O2) | - | 50.44% | 72.5% | Building | [39] |
| Porous geopolymer: fly ash + metakaolin sodium silicate solution + NaOH + hydrogen peroxide (H2O2) + Al powder | Compressive strength: 3.3–4.3 MPa (Al powder additional) Compressive strength: 2.9–9.3 MPa (H2O2 additional) | - | AL additional: 59% H2O2 additional: 48% | Thermal insulation | [40] |
| Geopolymer resin + metakaolin + carbon fiber + potassium silicate solution | Flexural strength: 234.2 ± 22.6 MPa (with heat treated 110 °C) 12.3 ± 1.2 MPa (Without fiber and heat treatment) | - | Improving | - | [41] |
| Geopolymer paste: rice husk + rice husk ash + metakaolin + palm oil fuel ash + slag + NaOH + sodium silicate solution | Compressive strength: ~5 MPa | ~38% | ~33% | - | Current study |
| Physical Properties | Raw RH | MK | POFA | Raw RH Ash | Slag | Geopolymer Sample 16 before Casting | Geopolymer Sample 16 after Casting |
|---|---|---|---|---|---|---|---|
| Surface area (m2/g) | 1.11 | 12.26 | 3.45 | 16.55 | 4.22 | 13.22 | 5.78 |
| Pore size (nm) | 16.3 | 7.0 | 6.4 | 8.8 | 10.3 | 6.3 | 7.6 |
| Total pore volume of pores (cm3/g) | 0.004 | 0.053 | 0.011 | 0.038 | 0.022 | 0.043 | 0.025 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emdadi, Z.; Asim, N.; Amin, M.H.; Ambar Yarmo, M.; Maleki, A.; Azizi, M.; Sopian, K. Development of Green Geopolymer Using Agricultural and Industrial Waste Materials with High Water Absorbency. Appl. Sci. 2017, 7, 514. https://doi.org/10.3390/app7050514
Emdadi Z, Asim N, Amin MH, Ambar Yarmo M, Maleki A, Azizi M, Sopian K. Development of Green Geopolymer Using Agricultural and Industrial Waste Materials with High Water Absorbency. Applied Sciences. 2017; 7(5):514. https://doi.org/10.3390/app7050514
Chicago/Turabian StyleEmdadi, Zeynab, Nilofar Asim, Mohamad Hassan Amin, Mohd Ambar Yarmo, Ali Maleki, Mojtaba Azizi, and Kamaruzzaman Sopian. 2017. "Development of Green Geopolymer Using Agricultural and Industrial Waste Materials with High Water Absorbency" Applied Sciences 7, no. 5: 514. https://doi.org/10.3390/app7050514








