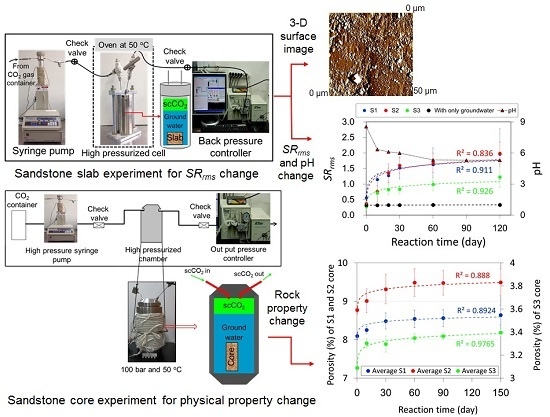
The Use of the Surface Roughness Value to Quantify the Extent of Supercritical CO2 Involved Geochemical Reaction at a CO2 Sequestration Site
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sandstone Cores and Groundwater Samples
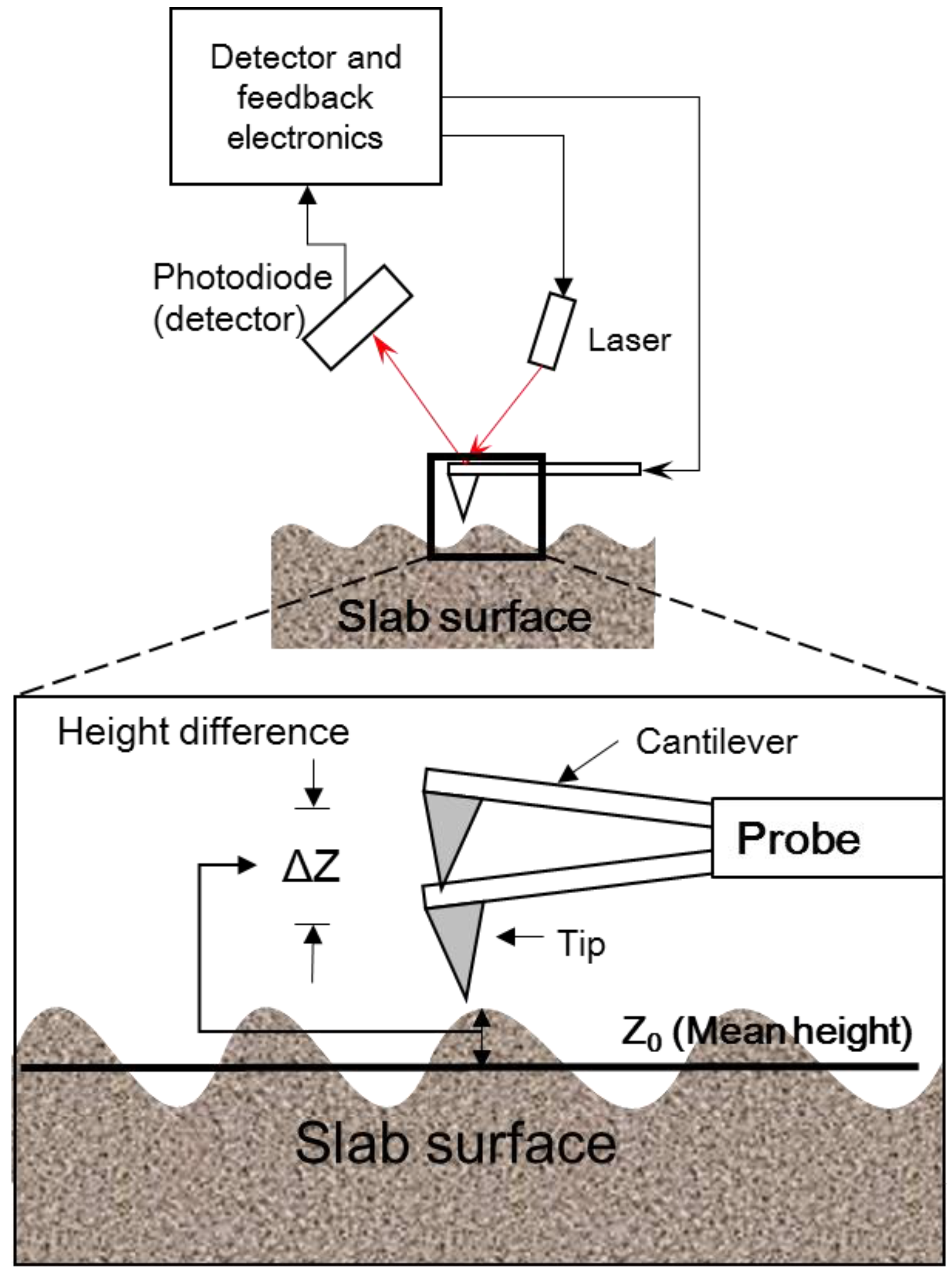
2.2. Measurement of the Surface Roughness Value for Sandstone Slabs
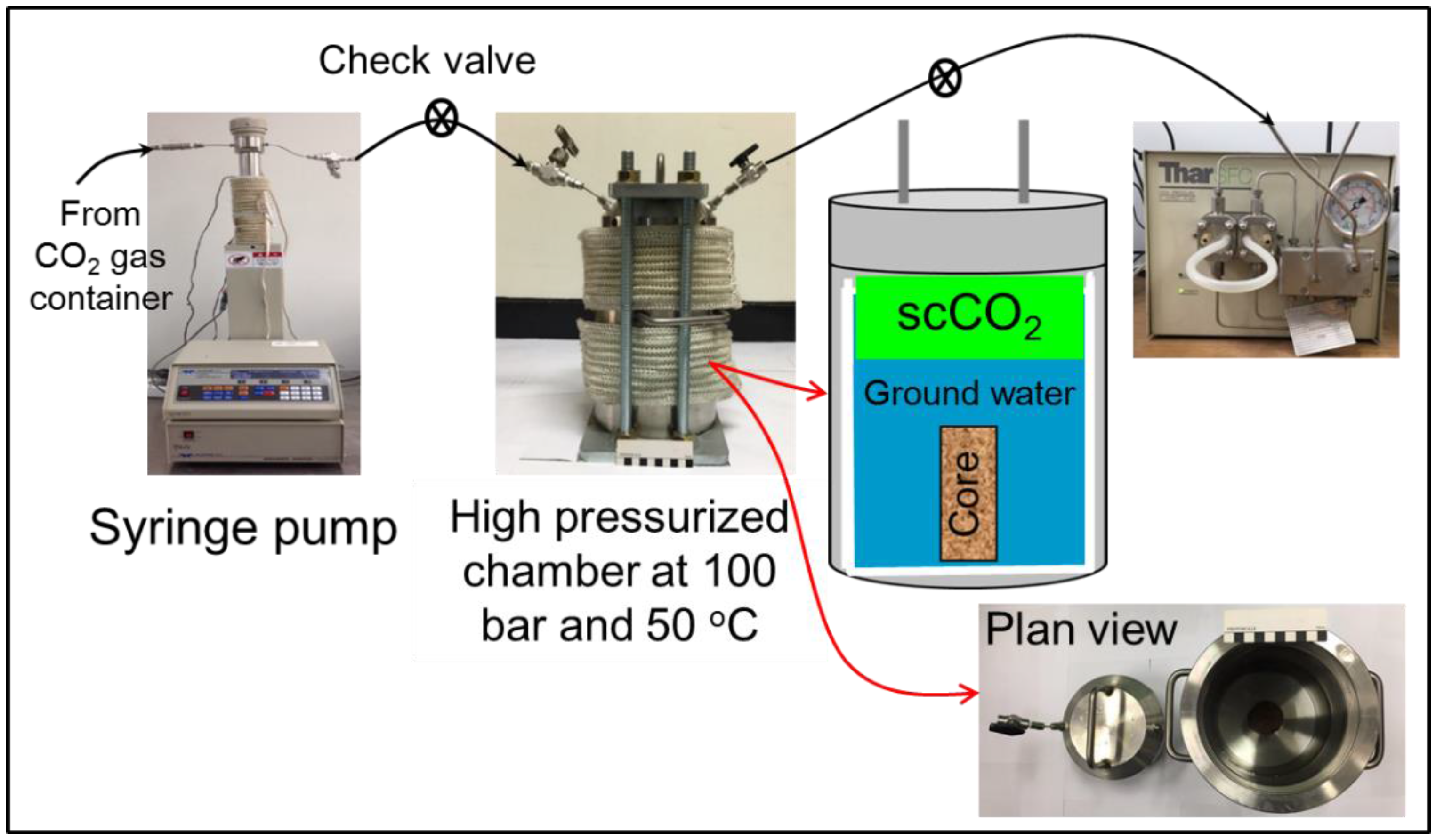
2.3. Measurement of Physical Property Changes for the Sandstone Cores
3. Results and Discussion
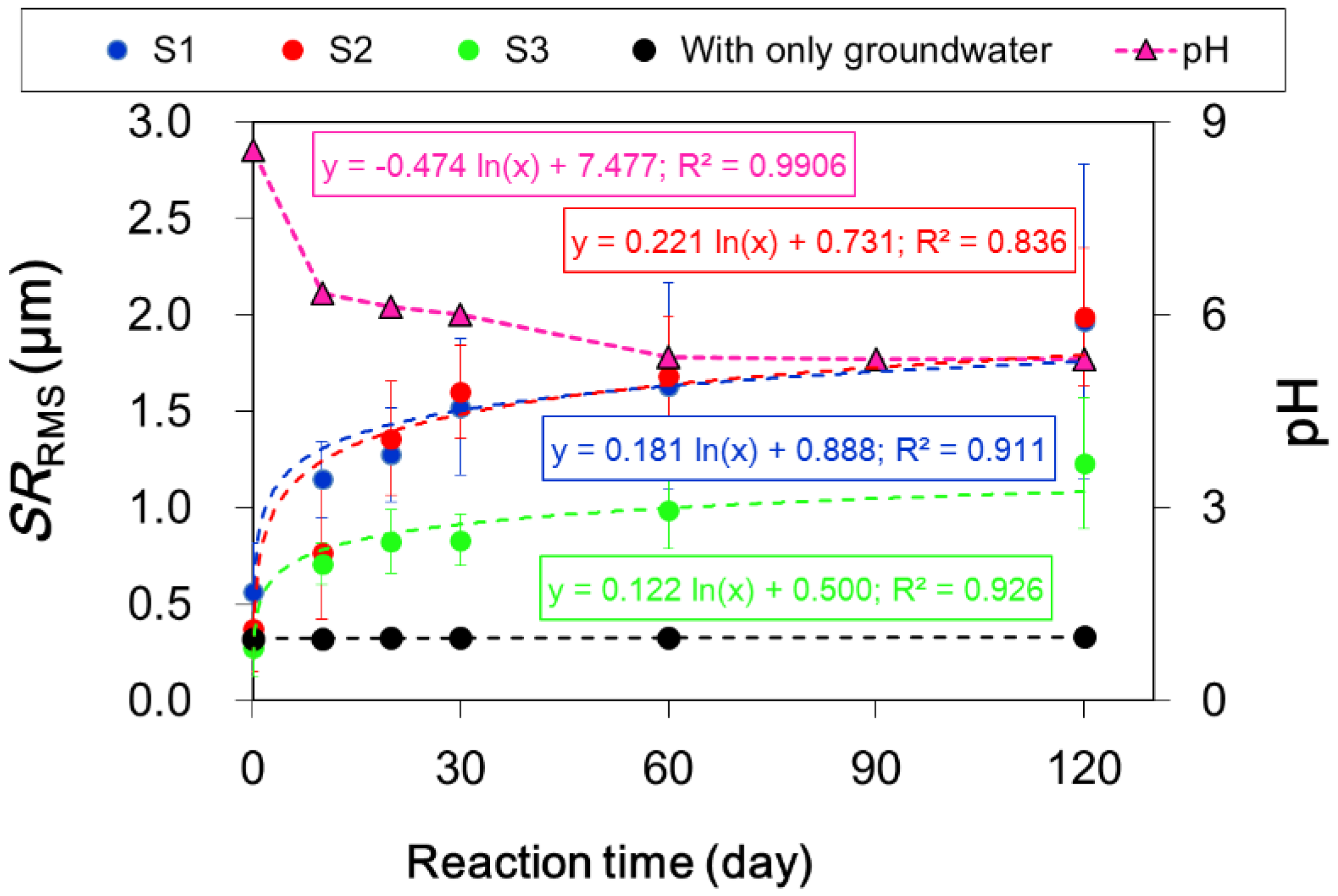
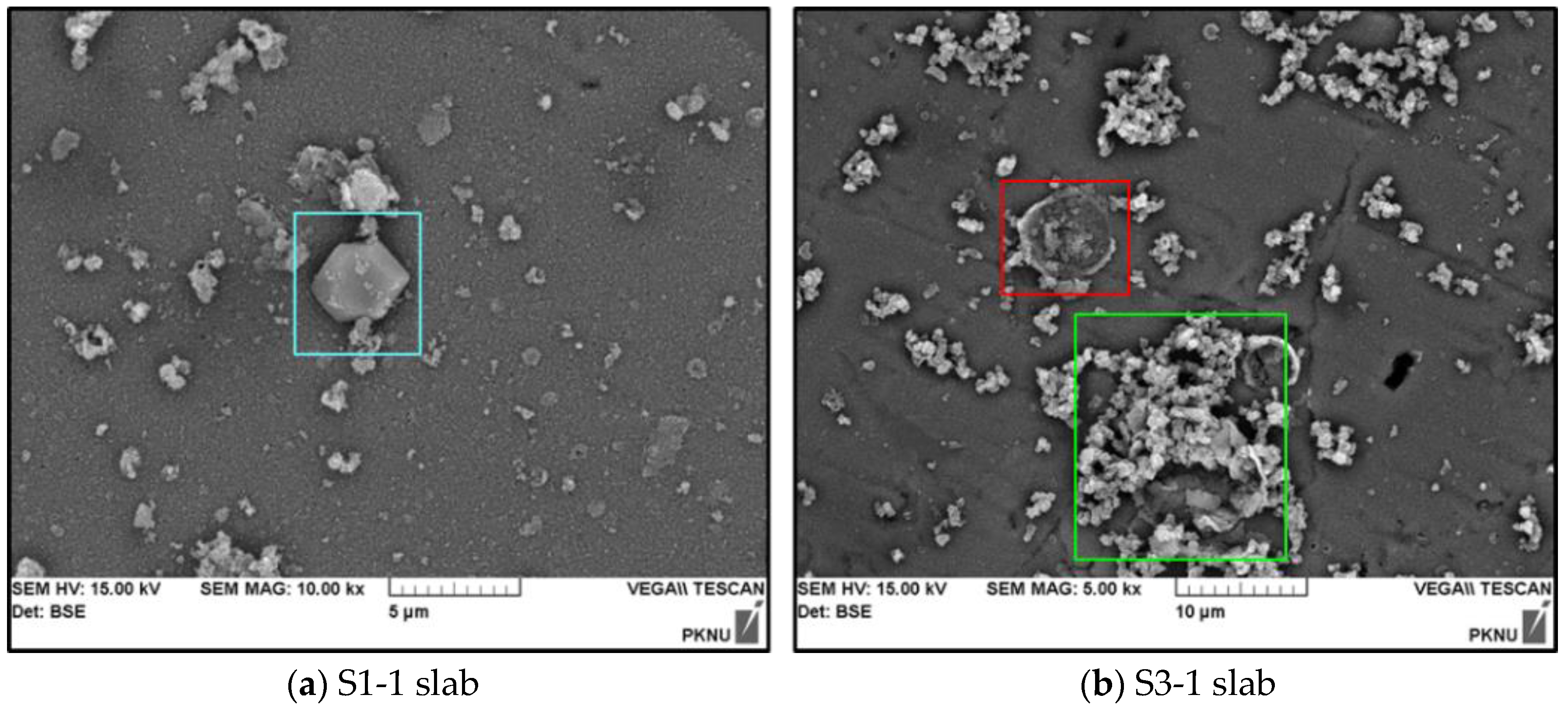
3.1. Measurement of the Surface Roughness Value for the Sandstone Slabs
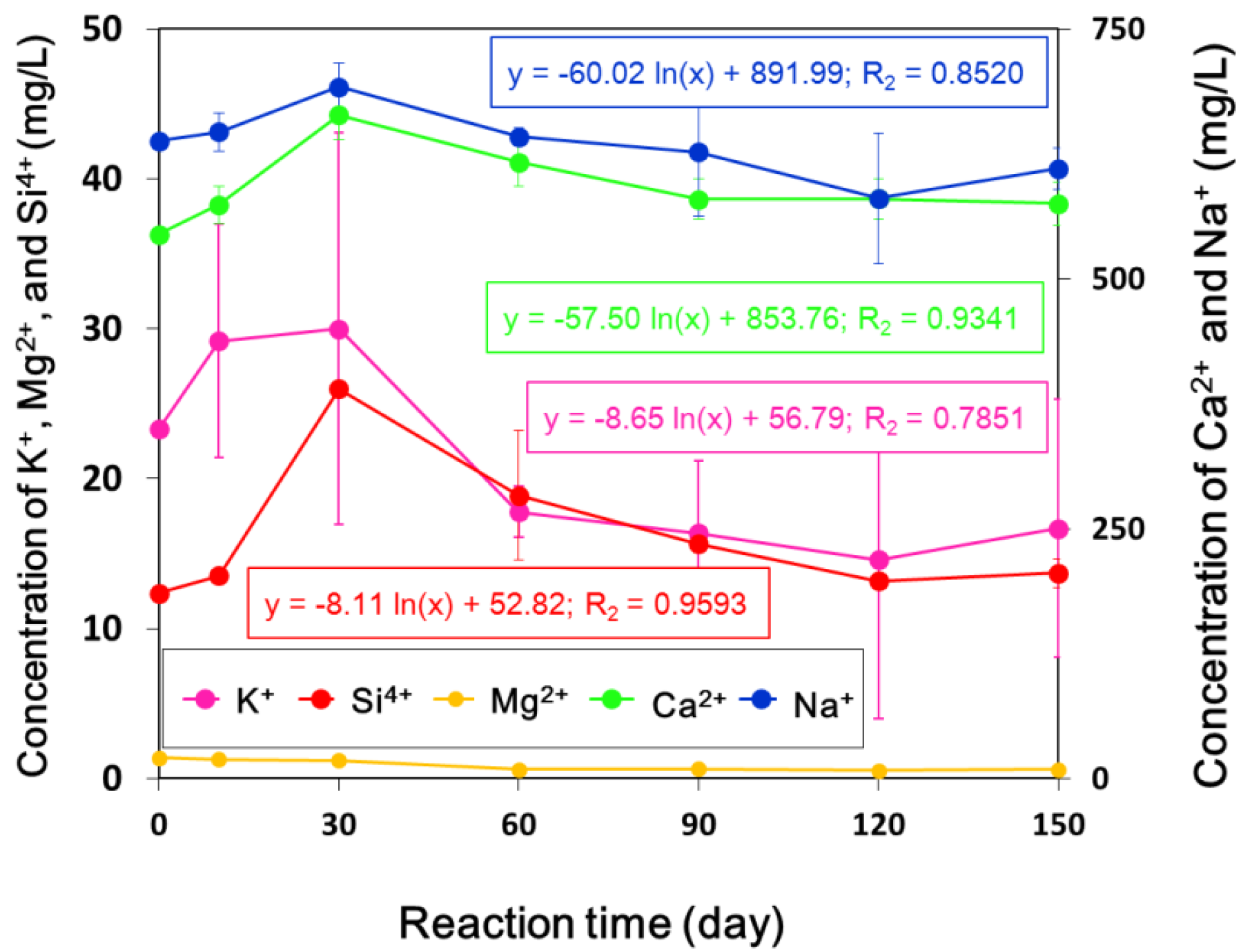
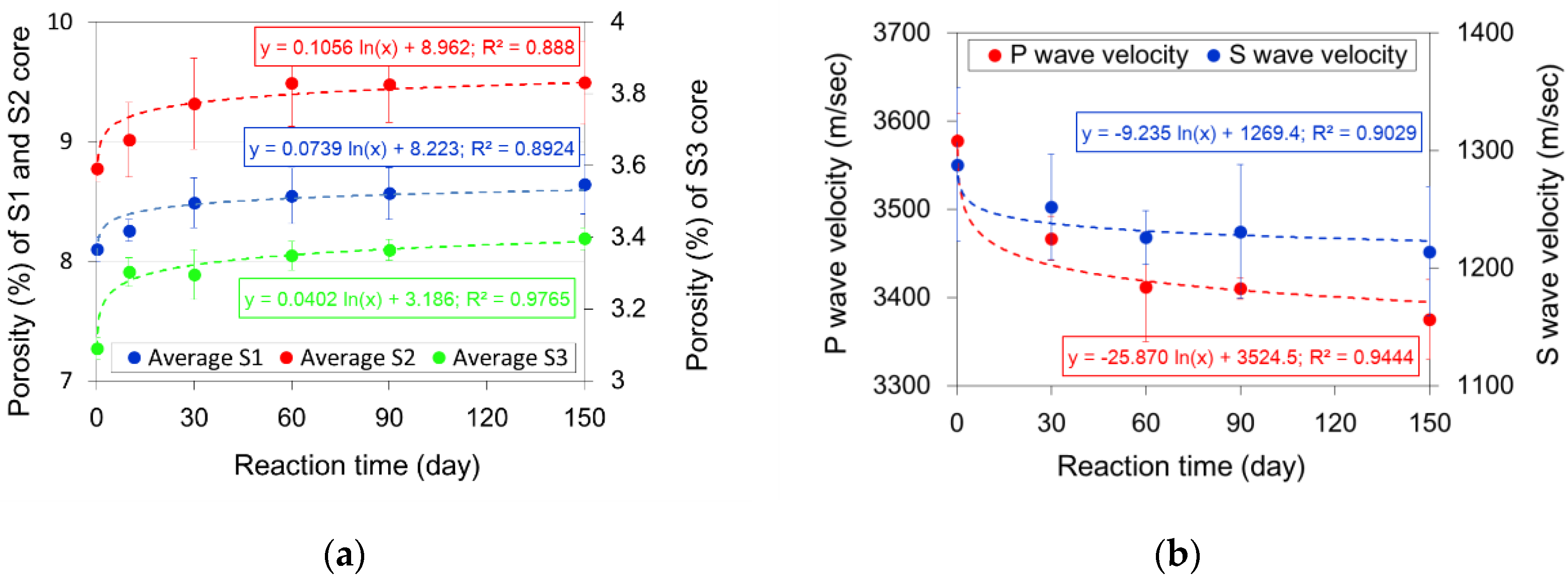
3.2. Measurement of Physical Property Changes for the Sandstone Cores
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Safdarmejad, M.; Hedengren, D.; Baxter, L. Dynamic optimization of a hybrid system of energy-storing cryogenic carbon capture and a baseline power generation unit. Appl. Energy 2016, 172, 66–79. [Google Scholar] [CrossRef]
- Gopan, A.; Kumfer, M.; Phillips, J.; Thimsen, D.; Smith, R.; Axelbaum, R.L. Process design and performance analysis of a Staged, Pressurized Oxy-Combustion (SPOC) power plant for carbon capture. Appl. Energy 2014, 125, 179–188. [Google Scholar] [CrossRef]
- Cohen, M.; Rochelle, T.; Webber, E. Optimizing postcombustion CO2 capture in response to volatile electricity prices. Int. J. Greenh. Gas Control 2012, 8, 180–195. [Google Scholar] [CrossRef]
- Bachu, S.; Gunter, D.; Perkins, H. Aquifer disposal of CO2: Hydrodynamic and mineral trapping. Energy Convers. Manag. 1994, 35, 269–279. [Google Scholar] [CrossRef]
- Gunter, D.; Perkins, H.; McCann, J. Aquifer disposal of CO2-rich gases, reaction design for added capacity. Energy Convers. Manag. 1993, 3, 941–948. [Google Scholar] [CrossRef]
- International Energy Agency(IEA). World Energy Outlook 2010. Annual Report; OECD/IEA: Paris, France, 2010. [Google Scholar]
- Holloway, S. An overview of the underground disposal of CO2. Energy Convers. Manag. 1997, 3, 193–198. [Google Scholar] [CrossRef]
- Klara, M.; Srivastava, D.; Mcllvried, G. Integrated collaborative technology development program for CO2 sequestration in geologic formations-United States Department of Energy R&D. Energy Convers. Manag. 2003, 44, 2699–2712. [Google Scholar] [CrossRef]
- Ampomah, W.; Balch, R.; Cather, M.; Rose-Coss, D.; Dai, Z.; Heath, J.; Dewers, T.; Mozley, P. Evaluation of CO2 storage mechanisms in CO2 enhanced oil recovery sites: Application to Morrow sandstone reservoir. Energy Fuels 2016, 30, 8545–8555. [Google Scholar] [CrossRef]
- Dai, Z.; Viswanathan, H.; Middleton, R.; Pan, F.; Ampomah, W.; Yang, C.; Jia, W.; Xiao, T.; Lee, S.; McPherson, B.; et al. CO2 accounting and risk analysis for CO2 sequestration at enhanced oil recovery sites. Environ. Sci. Technol. 2016, 50, 7546–7554. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; McPherson, J.; Dai, J.; Jia, W.; Lee, S.; Ampomah, W.; Viswanathan, H.; Esser, R. Uncertainty analysis of carbon sequestration in an active CO2-EOR field. Int. J. of Greenh. Gas Control 2016, 51, 18–28. [Google Scholar] [CrossRef]
- Kirste, D.; Haese, R.; Boreham, C.; Schacht, U. Evolution of formation water chemistry and geochemical modeling of the CO2CRC Otway site residual gas saturation test. Energy Proc. 2014, 63, 2894–2902. [Google Scholar] [CrossRef]
- Mito, S.; Xue, Z.; Ohsumi, T. Case study of geochemical reactions at the Nagaoka CO2 injection site, Japan. Int. J. Greenh. Gas Control 2008, 2, 309–318. [Google Scholar] [CrossRef]
- Neele, F.; Quinquis, H.; Read, A.; Wright, M.; Lorsong, J.; Poulussen, D.F. CO2 storage development: Status of the large European CCS projects with EEPR funding. Energy Proc. 2014, 63, 6053–6066. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, J.; Li, C.; Hu, L.; Wu, X.; Wang, Y. A full chain CCS demonstration project in northeast Ordos basin, China: Operational experience and challenges. Int. J. Greenh. Gas Control 2016, 50, 218–230. [Google Scholar] [CrossRef]
- Miocic, J.M.; Gilfillan, S.M.V.; Roberts, J.J.; Edlmann, K.; McDermott, C.I.; Haszeldine, R.S. Controls on CO2 storage security in natural reservoirs and implications for CO2 storage site selection. Int. J. Greenh. Gas Control 2016, 51, 118–125. [Google Scholar] [CrossRef]
- Pereira, N.; Carneiro, J.F.; Araŭjo, A.; Bezzeghoud, M.; Borges, J. Seismic and structural geology constraint to the selection of CO2 storage sites-The case of the onshore Lusitanian basin, Portugal. J. Appl. Geophys. 2014, 102, 21–38. [Google Scholar] [CrossRef]
- Gunter, W.D.; Wiwehar, B.; Perkins, E.H. Aquifer disposal of CO2-rich greenhouse gases: Extension of the time scale of experiment for CO2-sequestering reactions by geochemical modeling. Mineral. Petrol. 1997, 59, 121–140. [Google Scholar] [CrossRef]
- Koide, G.; Takahashi, M.; Tsukamoto, H. Self-trapping mechanisms of carbon dioxide. Energy Convers. Manag. 1995, 36, 505–508. [Google Scholar] [CrossRef]
- Perkins, E.; Lauriol, C.; Azaroual, M.; Durst, P. Long term predictions of CO2 storage by mineral and solubility trapping in the Weyburn Midale Reservoir. In Proceedings of the 7th International Conference on Greenhouse. Gas Control Technologies (GHGT-7), Vancouver, BC, Canada; 2005; pp. 2093–2096. [Google Scholar]
- Kampman, N.; Bickle, M.; Wigley, M.; Dubacq, B. Fluid flow and CO2-fluid-mineral interactions during CO2-storage in sedimentary basins. Chem. Geol. 2014, 369, 22–50. [Google Scholar] [CrossRef]
- Kang, H.; Baek, K.; Wang, S.; Park, J.; Lee, M. Study on the dissolution of sandstones in Gyeongsang basin and the calculation of their dissolution coefficients under CO2 injection condition. Econ. Environ. Geol. 2012, 45, 661–672. [Google Scholar] [CrossRef]
- Park, J.; Baek, K.; Lee, M.; Wang, S. Physical property changes of sandstones in Korea derived from the supercritical CO2-sandstone-groundwater geochemical reaction under CO2 sequestration condition. Geosci. J. 2015, 19, 313–324. [Google Scholar] [CrossRef]
- Pham, P.; Lu, P.; Agraard, P.; Zhu, C.; Hellevang, H. On the potential of CO2-water-rock interactions for CO2 storage using a modified kinetic model. Int. J. Greenh. Gas Control 2011, 5, 1002–1015. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.; Zhang, F.; Xu, T. Simulation of CO2–water–rock interactions on geologic CO2 sequestration under geological conditions of China. Mar.Pollut. Bull. 2013, 76, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, L.; Yang, S.; Li, S.; Yang, Y. An experimental study of CO2-brine-rock interaction at in situ pressure-temperature reservoir conditions. Chem. Geol. 2012, 326, 88–101. [Google Scholar] [CrossRef]
- Cao, P.; Karpyn, T.; Li, L. The role of host rock properties in determining potential CO2 migration pathways. Int. J. Greenh. Gas Control 2016, 45, 18–26. [Google Scholar] [CrossRef]
- Yasuhara, H.; Kinoshita, N.; Lee, D.S.; Choi, J.; Kishida, K. Evolution of mechanical and hydraulic properties in sandstone induced by simulated mineral trapping of CO2 geo-sequestration. Int. J. Greenh. Gas Control 2017, 56, 155–164. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, Y.; Li, W.; Wu, D.; Liu, Z. Influence of supercritical CO2 on pore structure and functional groups of coal: Implications for CO2 sequestration. J. Nat. Gas Sci. Eng. 2017, 40, 288–298. [Google Scholar] [CrossRef]
- Alonso, J.; Navarro, V.; Calvo, B.; Asensio, L. Hydro-mechanical analysis of CO2 storage in porous rocks using a critical state model. Int. J. Rock Mech. Min. 2012, 54, 19–26. [Google Scholar] [CrossRef]
- Auquě, F.; Acero, P.; Gimeno, J.; Gŏmez, B.; Asta, P. Hydrogeochemical modeling of a thermal system and lessons learned for CO2 geologic storage. Chem. Geol. 2009, 268, 324–336. [Google Scholar] [CrossRef]
- Buscheck, A.; Sun, Y.; Chen, M.; Hao, Y.; Wolery, J.; Bourcier, L.; Court, B.; Celia, A.; Friedmann, S.J.; Aines, R.D. Active CO2, reservoir management for carbon storage: Analysis of operational strategies to relieve pressure buildup and improve injectivity. Int. J. Greenh. Gas Control 2012, 6, 230–245. [Google Scholar] [CrossRef]
- Choi, Y.; Yun, T.; Mayer, B.; Hong, Y.; Kim, H.; Jo, Y. Hydrogeochemical processes in clastic sedimentary rocks, South Korea: A natural analogue study of the role of dedolomitization in geologic carbon storage. Chem. Geol. 2012, 306, 103–113. [Google Scholar] [CrossRef]
- Irina, G. Role and impact of CO2–rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Koornneef, J.; Ramîres, A.; Urkenburg, W.; Faaij, A. The environmental impact and risk assessment of CO2 capture, transport and storage—An evaluation of the knowledge base. Prog. Energy Combust. Sci. 2012, 38, 62–86. [Google Scholar] [CrossRef]
- Xu, T.; Apps, A.; Pruess, K. Numerical simulation to study mineral trapping for CO2 disposal in deep aquifers. Appl. Geochem. 2004, 19, 917–936. [Google Scholar] [CrossRef]
- Bachu, S.; Bonijoly, D.; Bradshaw, J.; Burruss, R.; Holloway, S.; Christensen, P.; Mathiassen, M. CO2 storage capacity estimation: Methodology and gaps. Int. J. Greenh. Gas Control 2007, 1, 430–443. [Google Scholar] [CrossRef]
- Berrezueta, E.; González-Menéndez, L.; Breitner, D.; Luquot, L. Pore system changes during experimental CO2 injection into detritic rocks: Studies of potential storage rocks from some sedimentary basins of Spain. Int. J. Greenh. Gas Control 2013, 17, 411–422. [Google Scholar] [CrossRef]
- Zhao, F.; Liao, W.; Yin, D. An experimental study for the effect of CO2-brine-rock interaction on reservoir physical properties. J. Energy Inst. 2015, 88, 27–35. [Google Scholar] [CrossRef]
- Bader, G.; Thibeau, S.; Vincke, O.; Jannaud, D.; Saysset, S.; Joffre, H.; Giger, M.; David, M.; Gimenez, M.; Dieulin, A.; Copin, D. CO2 storage capacity evaluation in deep saline aquifers for an industrial pilot selection, methodology and results of the France Nord project. Energy Proc. 2014, 63, 2779–2788. [Google Scholar] [CrossRef]
- Li, P.; Zhou, D.; Zhang, C.; Chen, G. Assessment of the effective CO2 storage capacity in the Beibuwan Basin, offshore of southwestern P. R. China. Int. J. Greenh. Gas Control 2015, 37, 325–339. [Google Scholar] [CrossRef]
- IWR (International Economic Platform for Renewable Energies). Climate: Global CO2 emissions rise to new record level in 2011. Pressed on Renewable Energy Industry, Germany. Available online: http://www.cerina.org/en/co2-global/co2-2011 (accessed on 1 June, 2017).
- Park, Y.; Huh, D.; Yoo, D.; Hwang, S.; Lee, H.; Roh, E. A review of business model for CO2 geological storage project in Korea. J. Geol. Soc. Korea 2009, 45, 579–587. [Google Scholar]
- Egawa, K.; Hong, S.; Lee, H.; Choi, T.; Lee, M.; Kang, J.; Yoo, K.; Kim, J.; Lee, Y.; Kihm, J.; Kim, J. Preliminary evaluation of geological storage capacity of CO2 in sandstones of the Sindong Group, Gyeongsang Basin (Cretaceous). J. Geol. Soc. Korea 2009, 45, 463–472. [Google Scholar]
- Alemu, B.L.; Aagaard, P.; Munz, I.A.; Skurtveit, E. Caprock interaction with CO2: A laboratory study of reactivity of shale with supercritical CO2 and brine. Appl. Geochem. 2011, 26, 1975–1989. [Google Scholar] [CrossRef]
- Dawson, W.; Pearce, K.; Biddle, D.; Golding, D. Experimental mineral dissolution in Berea Sandstone reacted with CO2 or SO2-CO2 in NaCl brine under CO2 sequestration conditions. Chem. Geol. 2015, 399, 87–97. [Google Scholar] [CrossRef]
- Farquhar, M; Pearce, K; Dawson, W; Golab, A; Sommacal, S; Kirste, D; Biddle, D; Golding, D. A fresh approach to investigating CO2 storage: Experimental CO2-water-rock interactions in a low-salinity reservoir system. Chem. Geol. 2015, 399, 98–122. [Google Scholar] [CrossRef]
- Liu, F.; Lu, P.; Griffith, C.; Hedges, S.W.; Soong, Y.; Hellevang, H.; Zhu, C. CO2-brine-caprock interaction: Reactivity experiments on Eau Claire shale and a review of relevant literature. Int. J. Greenh. Gas Control 2012, 7, 153–167. [Google Scholar] [CrossRef]
- Ko, M.; Kang, H.; Wang, S.; Lee, M. The weathering process of olivine and chlorite reacted with the supercritical CO2 on the sequestration condition. J. Geol. Soc. Korea 2011, 45, 463–472. [Google Scholar]
- Senetakis, K.; Coop, R.; Todisco, C. The inter-particle coefficient of friction at the contacts of Leighton Buzzard sand quartz minerals. Soils Found. 2013, 53, 746–755. [Google Scholar] [CrossRef]
- Yang, H.; Baudet, A.; Yao, T. Characterization of the surface roughness of sand particles using an advanced fractal approach. Proc. R. Soc. A 2016, 472, 1–20. [Google Scholar] [CrossRef]
- Degarmo, P.; Black, T.; Kohser, A. Materials and Processes in Manufacturing, ninth ed.Wiley: New Jersy, NJ, USA, 2003. [Google Scholar]
- Den Outer, A.; Kaashoek, F.; Hack, K. Difficulties of using continuous fractal theory for discontinuity surfaces. Int. J. Rock Mech. Min. 1995, 32, 3–9. [Google Scholar] [CrossRef]
- Lee, M.; Wang, S.; Kim, S.; Park, J. Investigation of the relationship between CO2 reservoir rock property change and the surface roughness change originating from the supercritical CO2-sandstone-groundwater geochemical reaction at CO2 sequestration condition. Energy Proc. 2015, 76, 495–502. [Google Scholar] [CrossRef]
- Javadpour, F. CO2 injection in geological formations: Determining macroscale coefficients from pore scale processes. Transp. Porous Med. 2009, 79, 87–105. [Google Scholar] [CrossRef]
- Kjøller, C.; Weibel, R.; Bateman, K.; Laier, T.; Nielsen, H.; Frykman, P.; Springer, N. Geochemical impacts of CO2 storage in saline aquifers with various mineralogy-results from laboratory experiments and reactive geochemical modeling. Energy Proc. 2011, 4, 4724–4731. [Google Scholar] [CrossRef]
- Silva, D.; Ranjith, G.; Perera, A. Geochemical aspects of CO2 sequestration in deep saline aquifers: A review. Fuel 2015, 155, 128–143. [Google Scholar] [CrossRef]
- Mitiku, B.; Li, D.; Bauer, S.; Beyer, C. Geochemical modeling of CO2-water-rock interactions in a potential storage formation of the North German sedimentary basin. Appl. Geochem. 2013, 36, 168–186. [Google Scholar] [CrossRef]
 ) and photographs of sandstone cores and a slab for the experiment (modified from [22]).
) and photographs of sandstone cores and a slab for the experiment (modified from [22]).
 ) and photographs of sandstone cores and a slab for the experiment (modified from [22]).
) and photographs of sandstone cores and a slab for the experiment (modified from [22]).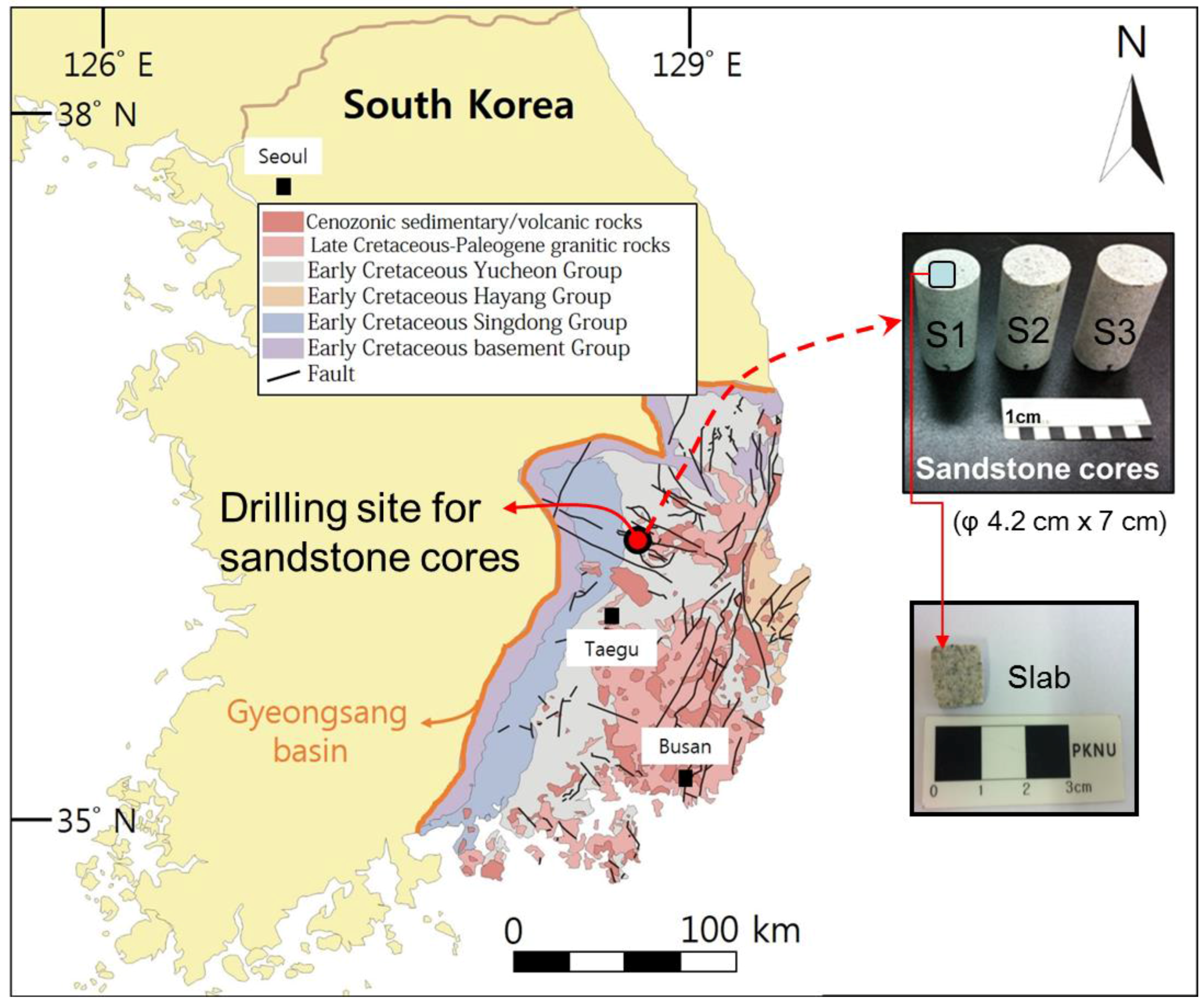



| Thin Section No. | Mineral Portion (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Quartz | Plagioclase | K-Feldspar | Muscovite | Biotite | Calcite | Chlorite | Others | |
| S1-1 | 50.7 | 7.8 | 25.5 | 0.3 | 2.3 | 3.1 | 3.1 | 7.2 |
| S1-2 | 43.4 | 9.2 | 27.6 | 0.3 | 1.5 | 5.3 | 5.1 | 7.6 |
| S1-3 | 50.5 | 11.3 | 23.5 | 1.1 | 0.2 | 4.3 | 3.2 | 5.9 |
| S1-4 | 46.1 | 8.1 | 23.2 | 1.4 | 2.1 | 6.2 | 4.7 | 8.2 |
| Average ± standard deviation | 47.7 ± 3.6 | 9.1 ± 1.6 | 25.0 ± 2.0 | 0.8 ± 0.6 | 1.5 ± 0.9 | 4.7 ± 1.3 | 4.0 ± 1.0 | 7.2 ± 1.0 |
| S2-1 | 38.7 | 13.4 | 17.9 | 0.7 | 2.2 | 9.5 | 0.1 | 17.5 |
| S2-2 | 35.6 | 15.5 | 13.4 | 1.5 | 2.7 | 12.4 | 0.0 | 18.9 |
| S2-3 | 40.5 | 11.7 | 16.9 | 0.1 | 1.4 | 6.4 | 0.0 | 23.0 |
| S2-4 | 41.9 | 16.8 | 19.8 | 1.4 | 1.9 | 4.1 | 0.1 | 14.0 |
| Average ± standard deviation | 39.2 ± 2.7 | 14.4 ± 2.3 | 17.0 ± 2.7 | 0.9 ± 0.7 | 2.1 ± 0.5 | 8.1 ± 3.6 | 0.1 ± 0.1 | 18.4 ± 3.7 |
| S3-1 | 61.6 | 7.6 | 13.2 | 3.3 | 0.4 | 0.3 | 0.1 | 13.5 |
| S3-2 | 57.5 | 5.1 | 7.7 | 5.9 | 0.6 | 3.5 | 0.3 | 19.4 |
| S3-3 | 55.1 | 8.7 | 14.3 | 2.7 | 0.4 | 3.8 | 0.2 | 14.8 |
| Average ± standard deviation | 58.1 ± 3.3 | 7.1 ± 1.8 | 11.7 ± 3.5 | 4.0 ± 1.7 | 0.5 ± 0.1 | 2.5 ± 1.9 | 0.2 ± 0.1 | 15.9 ± 3.1 |
| Temp. (°C) | pH | Average Concentration (mg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | Ca2+ | K+ | Mg2+ | Si4+ | Al3+ | Fe2+ | Cl− | SO42− | NO3− | HCO3− | ||
| 40.6 | 8.6 | 633.2 | 547.1 | 23.3 | 20.9 | 12.4 | 4.3 | 0.0 | 2109.1 | 259.3 | 212.0 | 51.7 |
| Slab Type | Mineral Type | Reaction Time (day) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 60 | 90 | ||
| S1 | Quartz-1 | 0.878 | 1.057 | 1.176 | 1.136 | 1.223 | 1.261 |
| Quartz-2 | 0.322 | 1.164 | 1.438 | 1.541 | 1.441 | 1.446 | |
| Quartz-3 | 0.518 | 0.945 | 0.976 | 1.201 | 1.242 | 1.041 | |
| Plagioclase-1 | 0.164 | 1.007 | 1.012 | 1.711 | 1.143 | 2.017 | |
| Plagioclase-2 | 0.442 | 1.598 | 1.133 | 1.748 | 2.760 | 3.068 | |
| Plagioclase-3 | 0.427 | 1.130 | 1.294 | 1.905 | 2.160 | 3.391 | |
| Biotite-1 | 0.908 | 1.173 | 1.752 | 2.052 | 1.815 | 2.091 | |
| Biotite-2 | 0.749 | 0.994 | 1.259 | 1.078 | 1.612 | 2.071 | |
| Biotite-3 | 0.658 | 1.250 | 1.435 | 1.326 | 1.273 | 1.297 | |
| S1 average ± std | 0.563 ± 0.25 | 1.146 ± 0.20 | 1.275 ± 0.24 | 1.522 ± 0.35 | 1.630 ± 0.54 | 1.965 ± 0.82 | |
| S2 | Quartz-1 | 0.275 | 0.169 | 1.612 | 1.512 | 1.997 | 2.011 |
| Quartz-2 | 0.276 | 0.663 | 1.035 | 1.362 | 1.540 | 1.581 | |
| Quartz-3 | 0.271 | 0.590 | 1.274 | 1.420 | 2.055 | 1.998 | |
| Plagioclase-1 | 0.157 | 0.436 | 1.287 | 1.909 | 1.201 | 2.318 | |
| Plagioclase-2 | 0.192 | 1.151 | 1.205 | 1.955 | 1.854 | 2.101 | |
| Plagioclase-3 | 0.184 | 0.840 | 1.203 | 1.679 | 1.788 | 1.991 | |
| Biotite-1 | 0.698 | 1.166 | 1.988 | 1.747 | 1.689 | 2.485 | |
| Biotite-2 | 0.666 | 1.122 | 1.131 | 1.244 | 1.215 | 1.303 | |
| Biotite-3 | 0.612 | 0.746 | 1.499 | 1.585 | 1.810 | 2.13 | |
| S2 average ± std | 0.370 ± 0.22 | 0.765 ± 0.34 | 1.359 ± 0.30 | 1.601 ± 0.24 | 1.683 ± 0.31 | 1.991 ± 0.36 | |
| S3 | Quartz-1 | 0.259 | 0.681 | 0.702 | 0.739 | 0.865 | 0.998 |
| Quartz-2 | 0.142 | 0.661 | 0.898 | 0.980 | 0.939 | 1.103 | |
| Quartz-3 | 0.226 | 0.571 | 0.860 | 0.801 | 0.888 | 0.998 | |
| Plagioclase-1 | 0.084 | 0.623 | 0.635 | 0.684 | 0.754 | 1.774 | |
| Plagioclase-2 | 0.224 | 0.922 | 0.600 | 0.635 | 1.189 | 1.696 | |
| Plagioclase-3 | 0.196 | 0.702 | 0.852 | 0.843 | 0.828 | 1.033 | |
| Biotite-1 | 0.554 | 0.782 | 1.110 | 1.017 | 1.358 | 1.551 | |
| Biotite-2 | 0.394 | 0.656 | 0.797 | 0.836 | 1.179 | 0.966 | |
| Biotite-3 | 0.362 | 0.804 | 0.977 | 0.955 | 0.899 | 0.954 | |
| S3 average ± std | 0.271 ± 0.14 | 0.711 ± 0.11 | 0.826 ± 0.16 | 0.832 ± 0.13 | 0.989 ± 0.20 | 1.230 ± 0.25 | |
| Sandstone | Reaction Time (day) | Porosity Change Rate (%) during 150 Days (1) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 30 | 60 | 90 | 150 | ||
| S1 | 8.183 | 8.321 | 8.464 | 8.569 | 8.553 | 8.649 | 5.7 |
| 8.121 | 8.149 | 8.293 | 8.299 | 8.350 | 8.39 | 3.3 | |
| 7.986 | 8.298 | 8.705 | 8.767 | 8.791 | 8.888 | 11.3 | |
| S1 average ± std | 8.097 ± 0.10 | 8.256 ± 0.09 | 8.487 ± 0.21 | 8.545 ± 0.23 | 8.565 ± 0.22 | 8.642 ± 0.25 | 6.7 |
| S2 | 8.760 | 8.991 | 9.360 | 9.460 | 9.462 | 9.459 | 8.0 |
| 8.889 | 9.334 | 9.673 | 9.857 | 9.811 | 9.859 | 10.9 | |
| 8.680 | 8.713 | 8.919 | 9.148 | 9.163 | 9.160 | 5.5 | |
| S2 average ± std | 8.776 ± 0.11 | 9.013 ± 0.31 | 9.318 ± 0.38 | 9.488 ± 0.36 | 9.479 ± 0.32 | 9.493 ± 0.35 | 8.2 |
| S3 | 3.121 | 3.278 | 3.221 | 3.305 | 3.353 | 3.412 | 9.3 |
| 3.056 | 3.282 | 3.33 | 3.38 | 3.336 | 3.358 | 9.9 | |
| 3.093 | 3.349 | 3.337 | 3.361 | 3.402 | 3.419 | 10.5 | |
| S3 average ± std | 3.090 ± 0.03 | 3.303 ± 0.04 | 3.296 ± 0.07 | 3.349 ± 0.04 | 3.364 ± 0.03 | 3.396 ± 0.03 | 9.9 |
| Property | Reaction Time (day) | Property Change Rate (%) during 150 Days (1) | ||||
|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 150 | ||
| Dry density (g/cm3) | 2.390 ± 0.0003 | 2.387 ± 0.0007 | 2.386 ± 0.0003 | 2.384 ± 0.0004 | 2.384 ± 0.0008 | 0.25 |
| P-wave velocity (m/s) | 3.578 ± 31 | 3.467 ± 25 | 3.412 ± 62 | 3.410 ± 12 | 3.375 ± 45 | 5.67 |
| S-wave velocity (m/s) | 1.288 ± 65 | 1.252 ± 45 | 1.226 ± 23 | 1.231 ± 57 | 1.214 ± 55 | 5.75 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Baek, K.; Lee, M.; Chung, C.-W.; Wang, S. The Use of the Surface Roughness Value to Quantify the Extent of Supercritical CO2 Involved Geochemical Reaction at a CO2 Sequestration Site. Appl. Sci. 2017, 7, 572. https://doi.org/10.3390/app7060572
Park J, Baek K, Lee M, Chung C-W, Wang S. The Use of the Surface Roughness Value to Quantify the Extent of Supercritical CO2 Involved Geochemical Reaction at a CO2 Sequestration Site. Applied Sciences. 2017; 7(6):572. https://doi.org/10.3390/app7060572
Chicago/Turabian StylePark, Jinyoung, Kyoungbae Baek, Minhee Lee, Chul-Woo Chung, and Sookyun Wang. 2017. "The Use of the Surface Roughness Value to Quantify the Extent of Supercritical CO2 Involved Geochemical Reaction at a CO2 Sequestration Site" Applied Sciences 7, no. 6: 572. https://doi.org/10.3390/app7060572