Impact of the Fused Deposition (FDM) Printing Process on Polylactic Acid (PLA) Chemistry and Structure
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Fused Deposition (FDM) Printing
2.2. Optical Microscopy
2.3. Scanning Electron Microscopy (SEM)
2.4. Confocal Microscopy
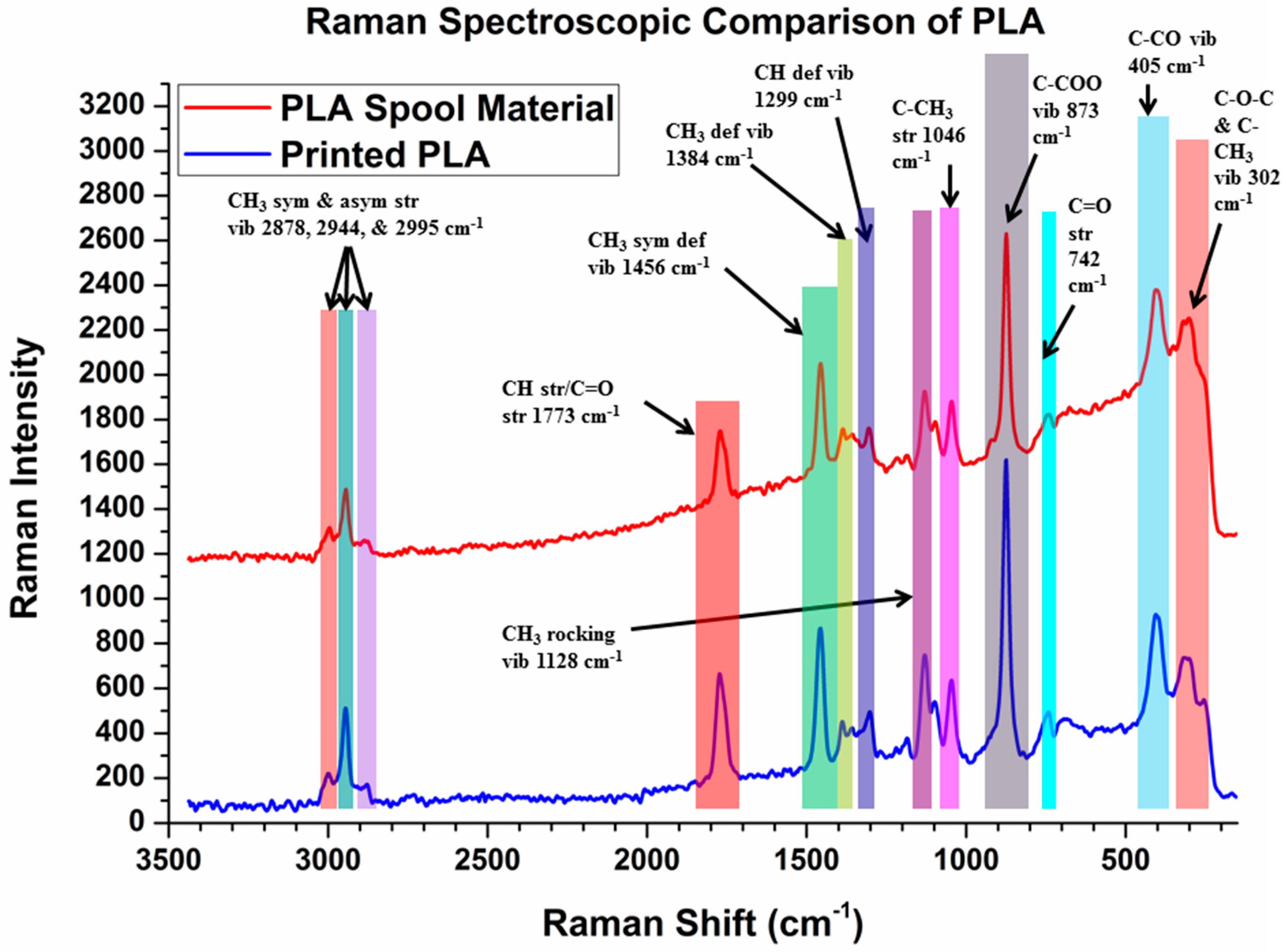
2.5. Raman Spectroscopy
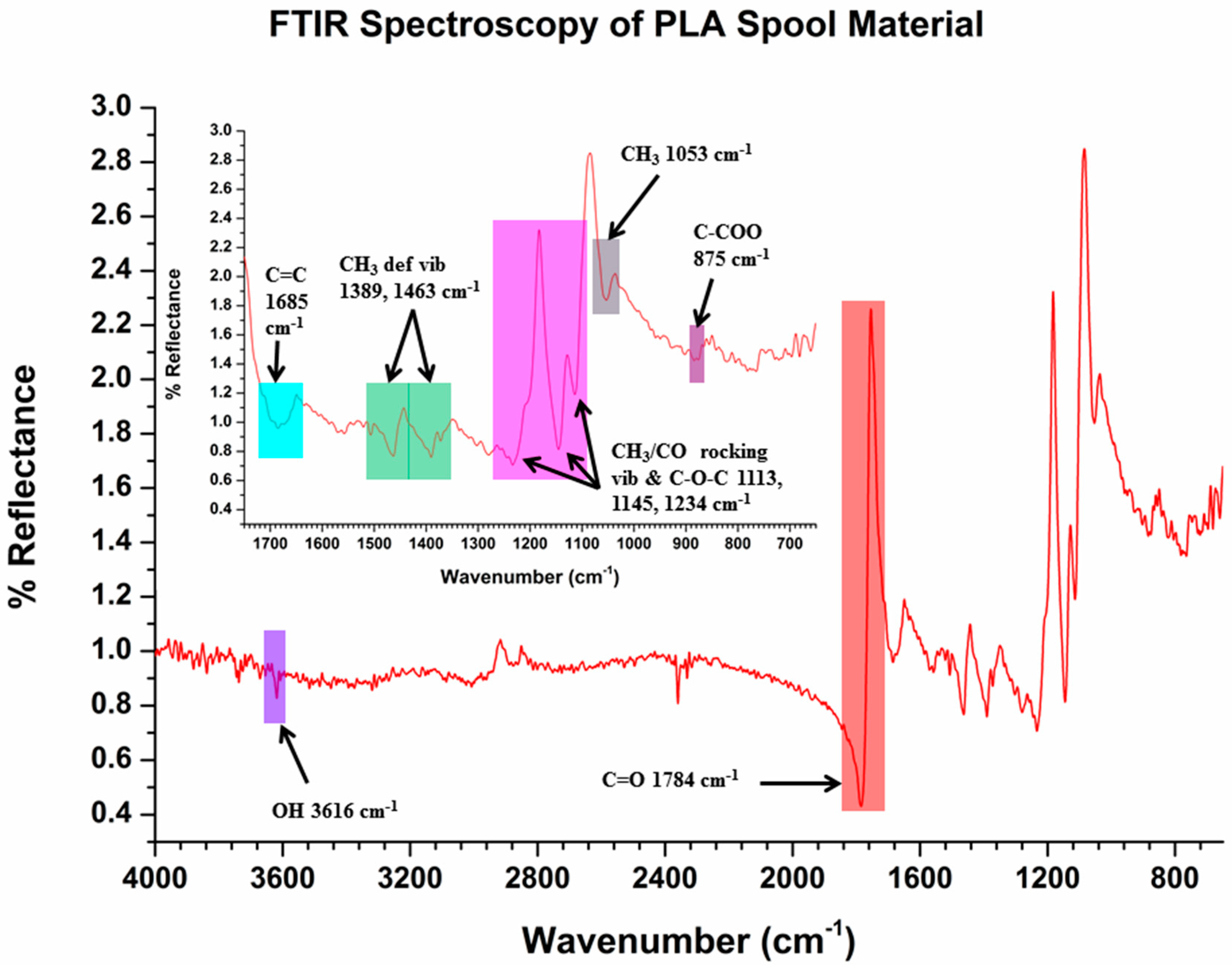
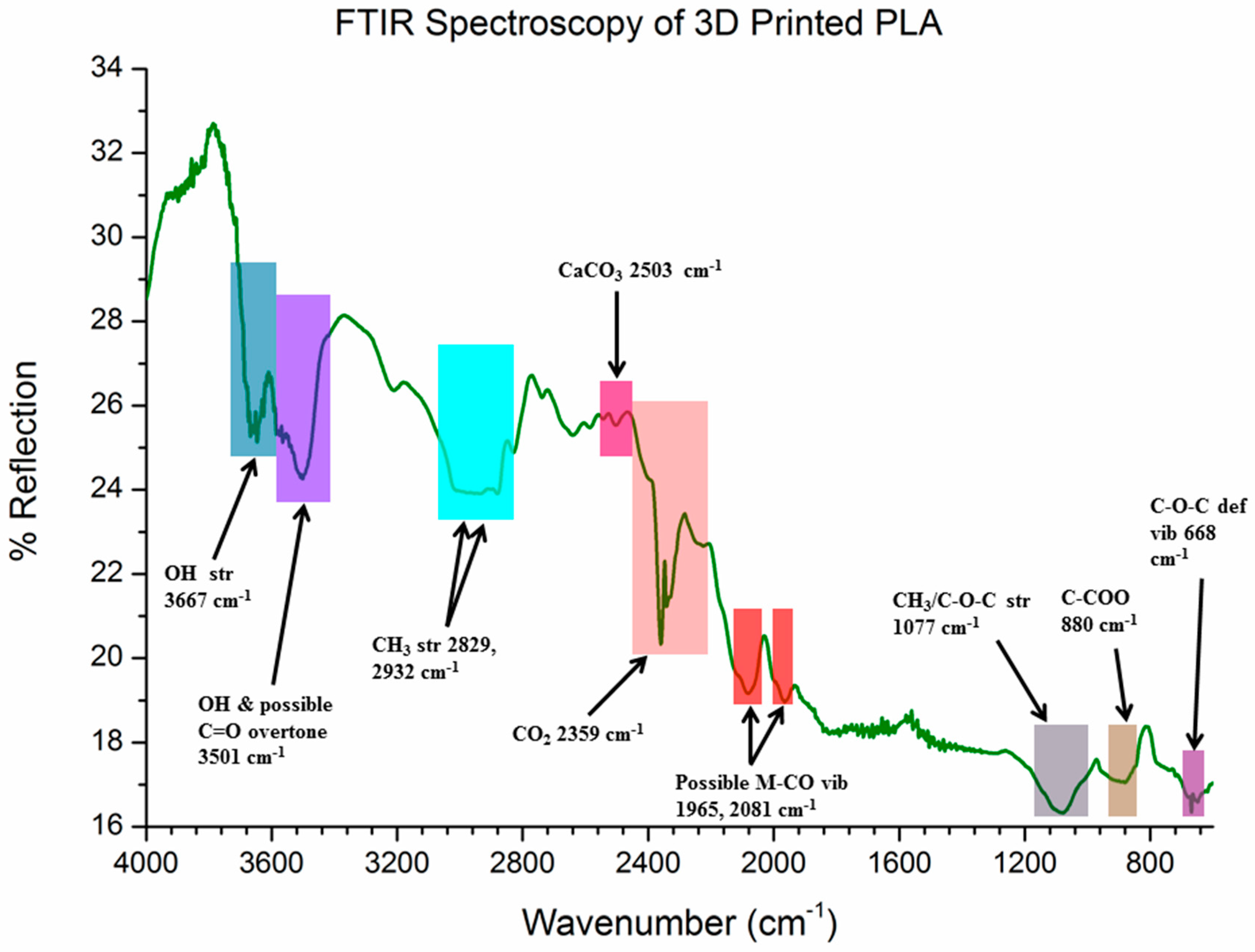
2.6. Fourier Transform Infrared (FTIR) Spectroscopy
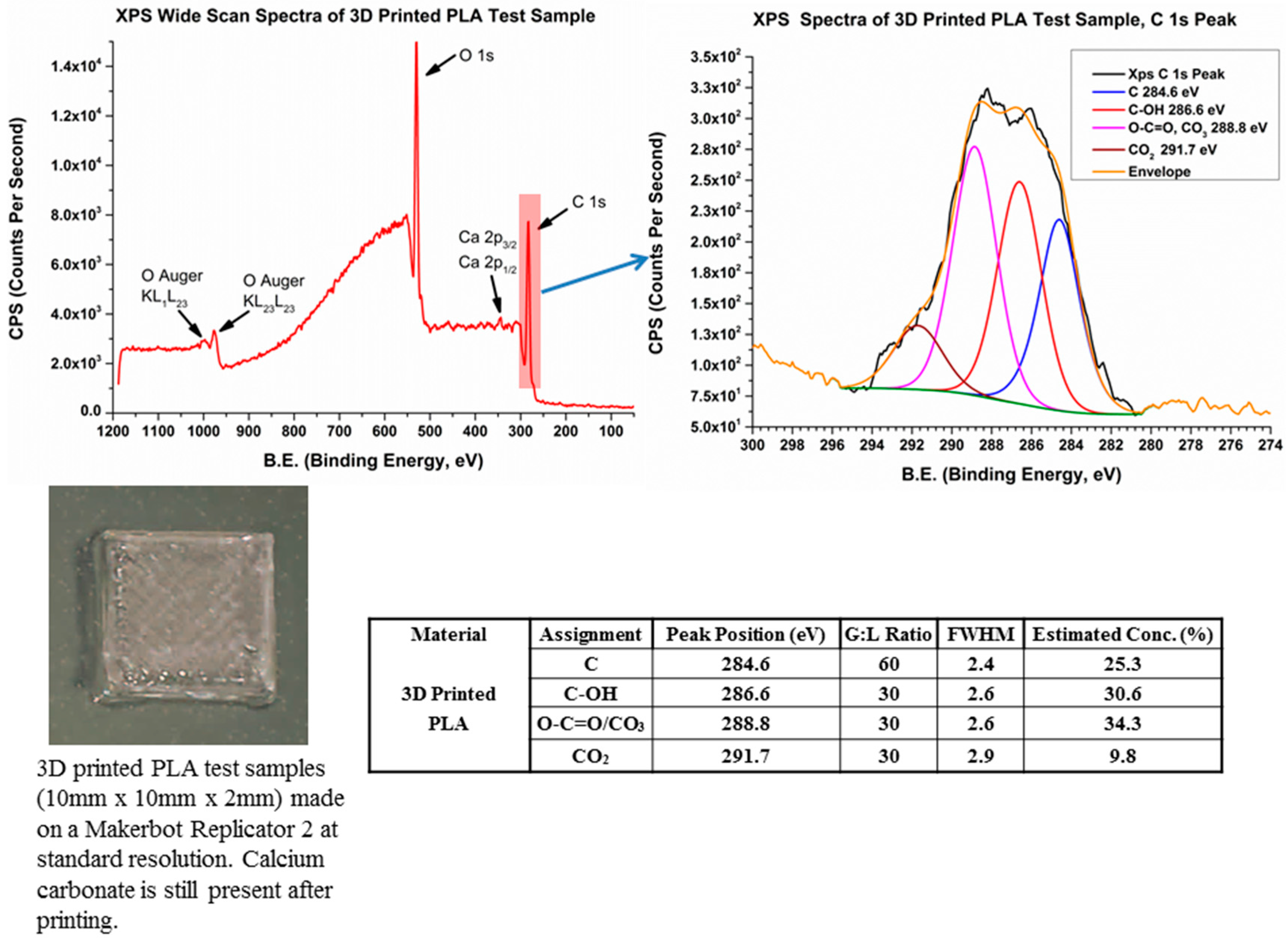
2.7. X-ray Photoelectron Spectroscopy (XPS)
2.8. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
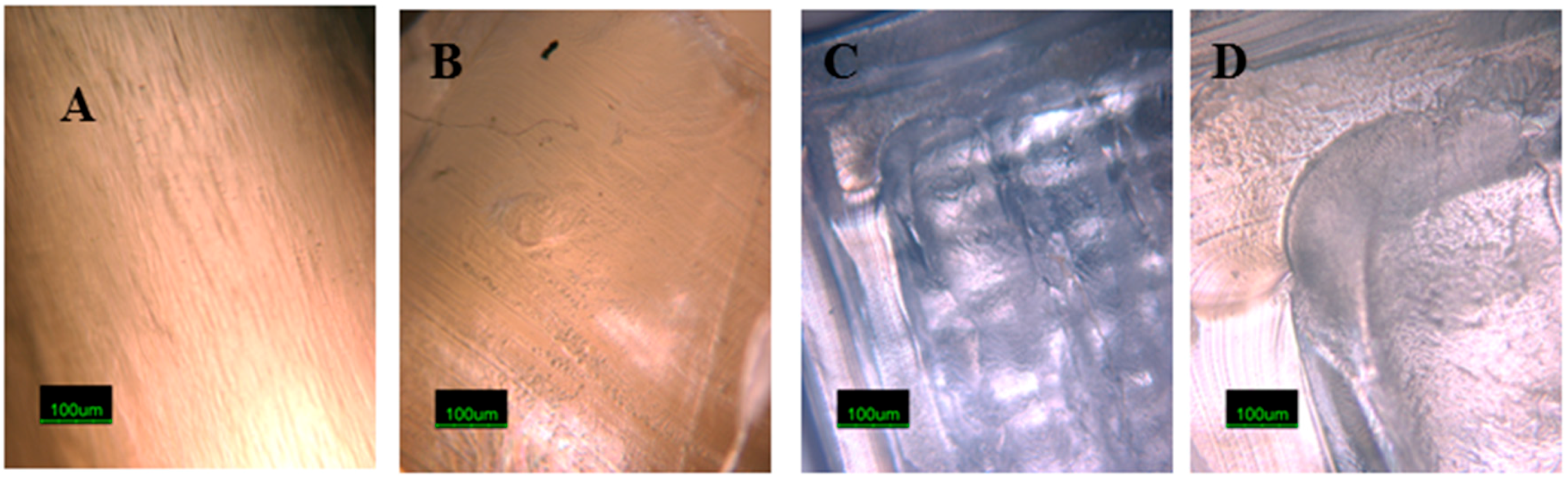
3.1. Optical Microscopy
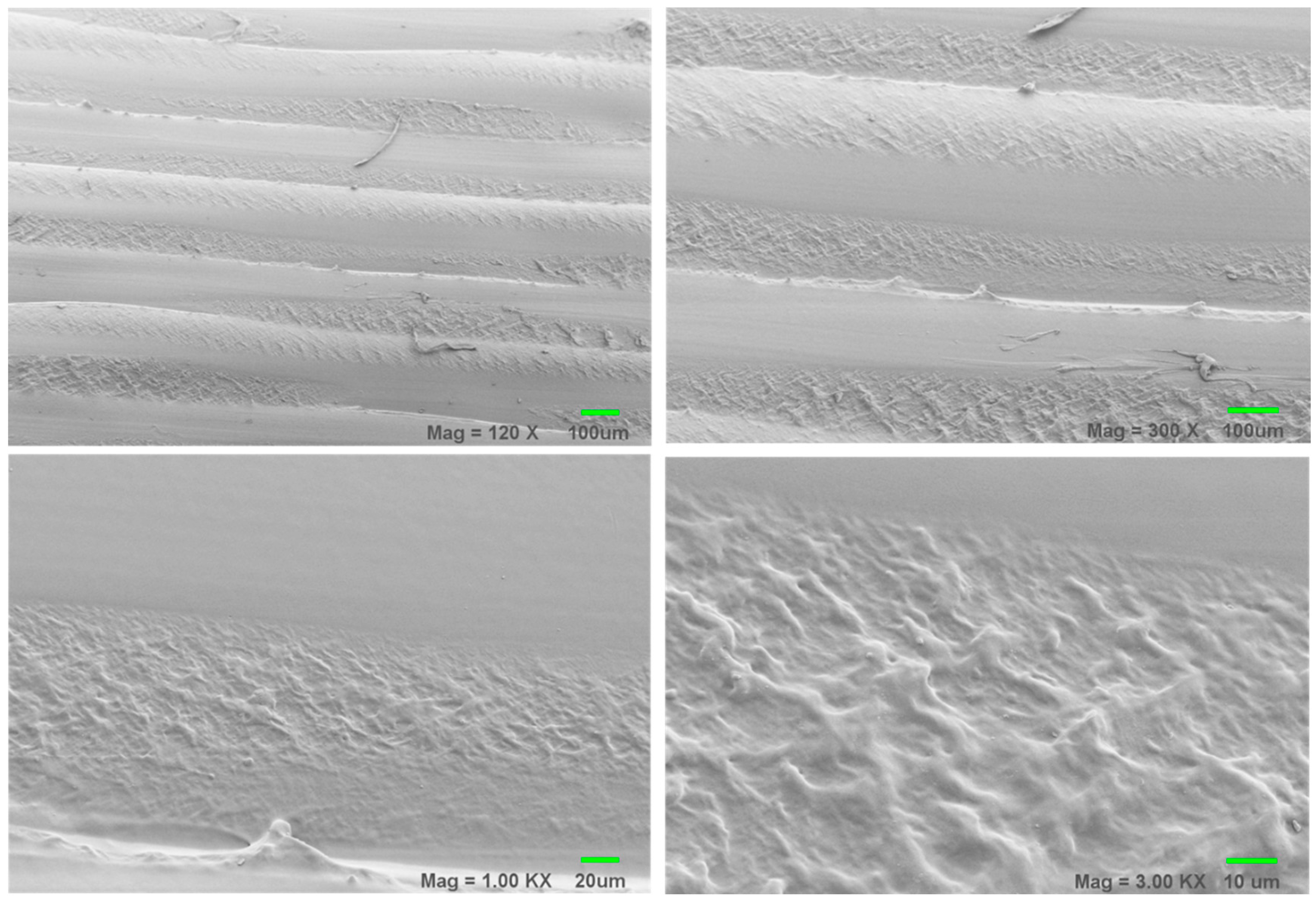
3.2. Scanning Electron Microscopy (SEM) Results
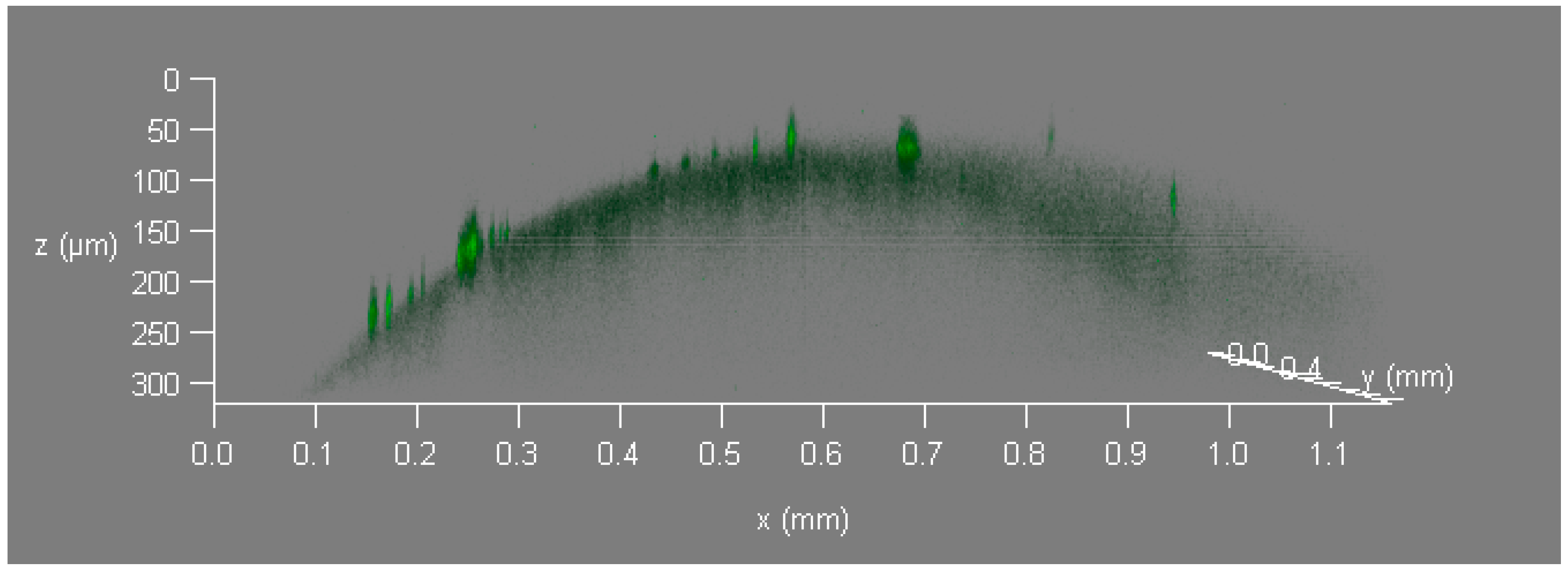
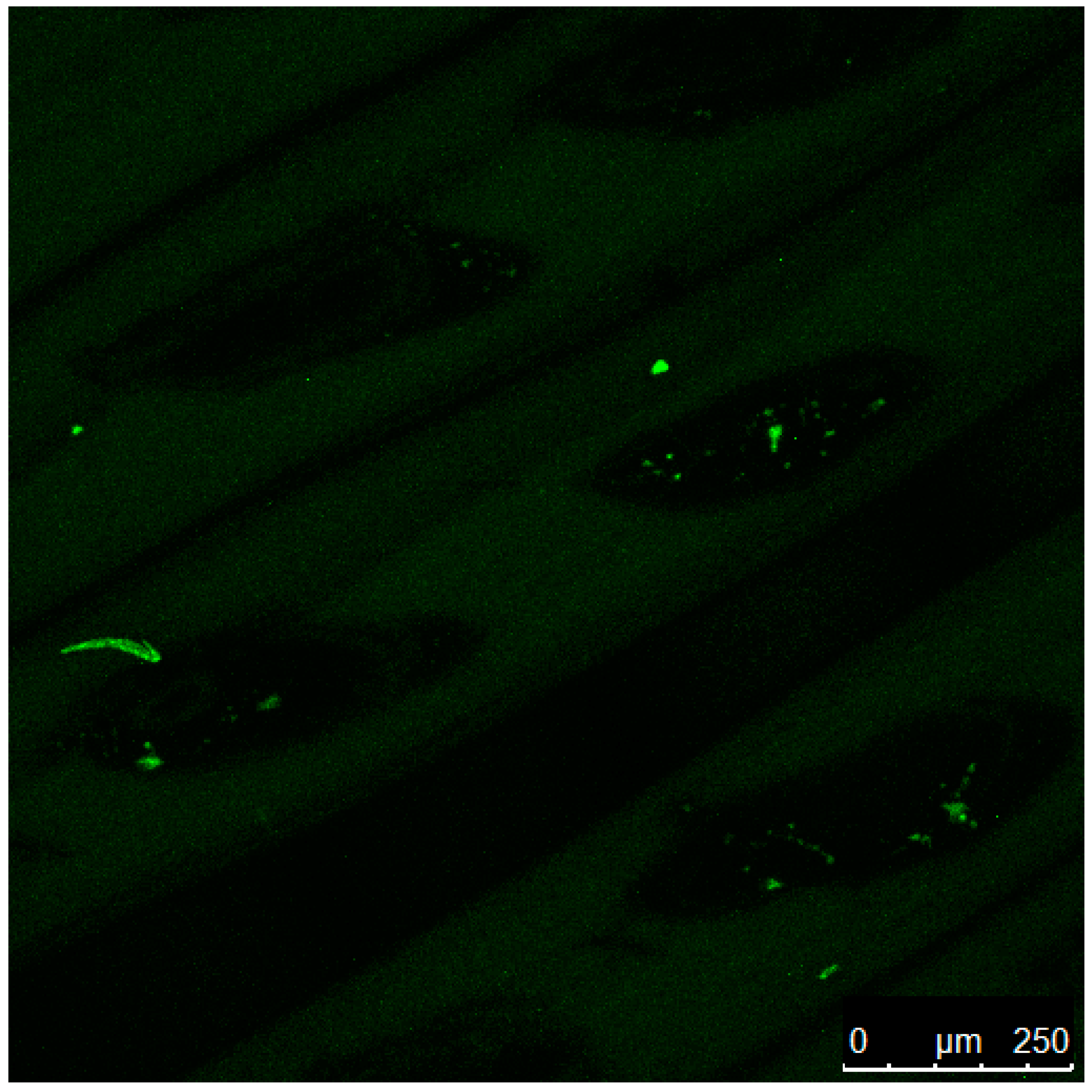
3.3. Laser Confocal Microscopy
3.4. Raman Spectroscopy
3.5. Diffuse Reflection FTIR (DRIFTS)
3.6. Differential Scanning Calorimetry
3.7. X-ray Photoelectron Spectroscopy (XPS)
4. Conclusions
- The chosen spool (source) material contains a calcium carbonate-based additive which is retained following printing and which becomes concentrated in pores or voids in the printed surface. Evidence indicates that these additives are, in some way, reacted with polymer components or with trace transition metals found to be present in the filament.
- The FDM printing process results in chemical changes in PLA (indicated by changes in the vibrational frequencies associated with CH3, C-O-C, and C=O ligands), as well as increased hydroxyl groups (which may be due to absorbed water).
- The FDM printing process likely results in changes to the structure of the PLA, which is reflected in shifts in the temperature range and potential mechanism of cold crystallization, as well as the resulting melting characteristics.
- Surface analytical studies indicate a surface which is likely more chemically reactive, in particular for processes which involve interaction of sorbed species with oxidized carbon ligands.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lipson, H.; Kurman, M. Fabricated: The New World of 3D Printing; John Wiley and Sons, Inc.: Indianapolis, IN, USA, 2013. [Google Scholar]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2002, 9, 36–84. [Google Scholar]
- Henton, D.E.; Patrick, G.; Lunt, J.; Randall, J. Polylactic Acid Technology, Natural Fibers, Biopolymers, and Biocomposites; Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 527–578. [Google Scholar]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, Toxicity, Biocompatibility and Clinical Applications of Polylactic Acid/Polyglycolic Acid Copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Proikakis, C.S.; Tarantili, P.A.; Andreopoulos, A.G. The role of Polymer/Drug interactions on the Sustained Release from Poly(d, l-Lactic acid) Tablets. Eur. Polym. J. 2006, 4, 3269–3276. [Google Scholar] [CrossRef]
- Kulkarni, R.K.; Pani, K.C.; Neuman, C.; Leonard, F. Polylactic Acid for Surgical Implants; Technical Report 6608; U.S. Army Medical Biomechanical Research Laboratory/Walter Reed Army Medical Center: Washington, DC, USA, April 1966. [Google Scholar]
- Timrak, B.M.; Kreiger, M.; Pearce, J.M. Mechanical properties of components fabricated with open-source 3-D printers under realistic environmental conditions. Mater. Des. 2014, 58, 242–246. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venancio Silva, S.; Oliveira, M.T. Raman Microspectroscopy of Some Iron Oxides and Oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Hanesch, M. Raman Spectroscopy of Iron Oxides and (oxy)hydroxides at Low laser Power and Possible Applications in Environmental Magnetic Studies. Geophys. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Luo, L.; Wilhelm, C.; Young, C.N.; Grey, C.P.; Halada, G.P.; Xiao, K.; Ivanov, I.N.; Howe, J.Y.; Geohegan, D.B.; Goroff, N.S. Characterization and Carbonization of Highly Oriented Poly(diiododiacetylene) Nanofibers. Macromolecules 2011, 44, 2626–2631. [Google Scholar] [CrossRef]
- Savutsky, A.; Golay, M.J. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Sherwood, P.M.A. Practical Surface Analysis; Briggs, D., Seah, M.P., Eds.; Wiley, John and Sons: New York, NY, USA, 1993. [Google Scholar]
- Halada, G.P.; Clayton, C.R. Comparison of Mo-N and W-N Synergism During Passivation of Stainless Steel through X-ray Photoelectron Spectroscopy and Electrochemical Analysis. J. Vac. Sci. Technol. A 1993, 11, 2342–2347. [Google Scholar] [CrossRef]
- Halada, G.P.; Kim, D.; Clayton, C.R. The Influence of Nitrogen on the Electrochemical Passivation of High Ni Stainless Steels and Thin Mo-Ni Films. Corrosion 1996, 52, 36–46. [Google Scholar] [CrossRef]
- Chidambaram, D.; Clayton, C.R.; Halada, G.P. A Duplex Mechanism-Based Model for the Interaction Between Chromate Ions and the Hydrated Oxide Film on Aluminum Alloys. J. Electrochem. Soc. 2003, 150, B224–B237. [Google Scholar] [CrossRef]
- Solarski, S.; Ferreira, M.; Devaux, E. Charicterization of the Thermal Properties of PLA Fibers by Modulated Differential Scanning Calorimetry. Polymer 2005, 46, 11187–11192. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Mozetic, P.; Trombetta, M.; Rainer, A. Combined additive manufacturing approaches in tissue engineering. Acta Biomater. 2015, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Favi, P.; Cheng, X.; Golshan, N.H.; Ziemer, K.S.; Keidar, M.; Webster, T.J. Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration. Acta Biomater. 2016, 46, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.J.; Kearns, J.R.; Halada, G.P.; Clayton, C.R. Spatially resolved microchemical analysis of chromate-conversion-coated aluminum alloy AA2024-T3. Surf. Interface Anal. 2002, 33, 796–806. [Google Scholar] [CrossRef]
- Berg, B.L.; Ronholm, J.; Applin, D.M.; Mann, P.; Izawa1, M.; Cloutis, E.A.; Whyte, L.G. Spectral features of biogenic calcium carbonates and implications for astrobiology. Int. J. Astrobiol. 2014, 13, 353–365. [Google Scholar] [CrossRef]
- Roberson, D.A.; Carmen, R.R.; Piñon, M. Evaluation of 3D Printable Sustainable Composites. Available online: https://sffsymposium.engr.utexas.edu/sites/default/files/2015/2015-75-Roberson.pdf (accessed on 15 March 2017).
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, Tables and Charts, 3rd ed.; John Wiley and Sons: West Sussex, UK, 2001. [Google Scholar]
- Suzuki, T.; Takahashi, K.; Uehara, H.; Yamanobe, T. Applications and Analysis of a DSC-Raman Spectroscopy for Indium and Poly(lactic acid). J. Therm. Anal. Calorim. 2013, 113, 1543–1549. [Google Scholar] [CrossRef]
- Kister, G.; Cassanas, G.; Vert, M. Effects of Morphology, Conformation and Configuration on the IR and Raman Spectra of Various Poly(lactic acid)s. Polymers 1998, 39, 267–273. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolding in Tissue Engineering Bone and Cartilage. Biomaterial 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Cao, X.; Mohamed, A.; Gordon, S.H.; Willett, J.L.; Sessa, D.J. DSC Study of Biodegradable Poly(lactic acid) and Poly(hydroxyl ester ether) Blends. Thermochim. Acta 2003, 406, 115–127. [Google Scholar] [CrossRef]
- Jalali, A.; Huneault, M.A.; Elkoun, S.J. Effect of Thermal History on Nucleation and Crystallization of Poly(lactic acid). Mater. Sci. 2016, 51, 7768–7779. [Google Scholar] [CrossRef]
- Halada, G.P.; Jha, P.; Cuiffo, M.; Acquah, K.; Carl, S. Aqueous Electrochemical Synthesis of Stable Silver Metal Nanoparticles onto a Chitosan Matrix on Stainless Steel. ECS Trans. 2014, 58, 19–32. [Google Scholar] [CrossRef]
- Li, S.; McCarthy, S. Further investigations on Hydrolytic Degradation of Poly (dl-Lactide). Biomaterial 1999, 20, 34–44. [Google Scholar] [CrossRef]
- De Jong, S.J.; Arias, E.R.; Rijkers, D.T.S.; Van Nostrum, C.F.; Kettenes-Van den Bosch, J.J.; Hennink, W.E. New Insights into Hydrolytic Degradation of Poly (lactic acid) Participation of the Alcohol Terminus. Polymers 2001, 42, 2795–2802. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of Thermal Stability, Rheology and Mechanical Properties of PLA, PBAT and Their Blends by Reactive Extrusion with Functionalized Epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Carrasco, F.; Pages, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of Poly(lactic acid): Characterization of chemical Structure, Thermal Stability and Mechanical Properties. Polym. Degrad. Stab. 2010, 90, 116–125. [Google Scholar] [CrossRef]
- Yuzay, I.E.; Auras, R.; Soto-Valdez, H.; Selke, S. Effects of Synthetic and Natural Zeolites on Morphology and Thermal Degradation of Poly(lactic acid) Composites. Polym. Degrad. Stab. 2010, 95, 1769–1777. [Google Scholar] [CrossRef]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 700–711. [Google Scholar]


| Element | Source Filament (Averaged over Three Samples) (ppm) | Printed PLA (Averaged over Three Areas) (ppm) |
|---|---|---|
| P | 2100 ± 50 | 1183 ± 70 |
| Ca | 528 ± 40 | 585 ± 25 |
| Si | 310 ± 35 | 180 ± 15 |
| Cr | 110 ± 10 | 61 ± 5 |
| Ti | 80 ± 10 | 40 ± 10 |
| Cu | 35 ± 10 | 13 ± 4 |
| Sn | (below detection) | 18 ± 5 |
| Trace amounts of W, Cd, Zr, Nb, Mo | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuiffo, M.A.; Snyder, J.; Elliott, A.M.; Romero, N.; Kannan, S.; Halada, G.P. Impact of the Fused Deposition (FDM) Printing Process on Polylactic Acid (PLA) Chemistry and Structure. Appl. Sci. 2017, 7, 579. https://doi.org/10.3390/app7060579
Cuiffo MA, Snyder J, Elliott AM, Romero N, Kannan S, Halada GP. Impact of the Fused Deposition (FDM) Printing Process on Polylactic Acid (PLA) Chemistry and Structure. Applied Sciences. 2017; 7(6):579. https://doi.org/10.3390/app7060579
Chicago/Turabian StyleCuiffo, Michael Arthur, Jeffrey Snyder, Alicia M. Elliott, Nicholas Romero, Sandhiya Kannan, and Gary P. Halada. 2017. "Impact of the Fused Deposition (FDM) Printing Process on Polylactic Acid (PLA) Chemistry and Structure" Applied Sciences 7, no. 6: 579. https://doi.org/10.3390/app7060579





