Evaluation of Aging Resistance of Graphene Oxide Modified Asphalt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Asphalt
2.1.2. Graphene Oxide
2.2. Preparation of GO Modified Asphalt
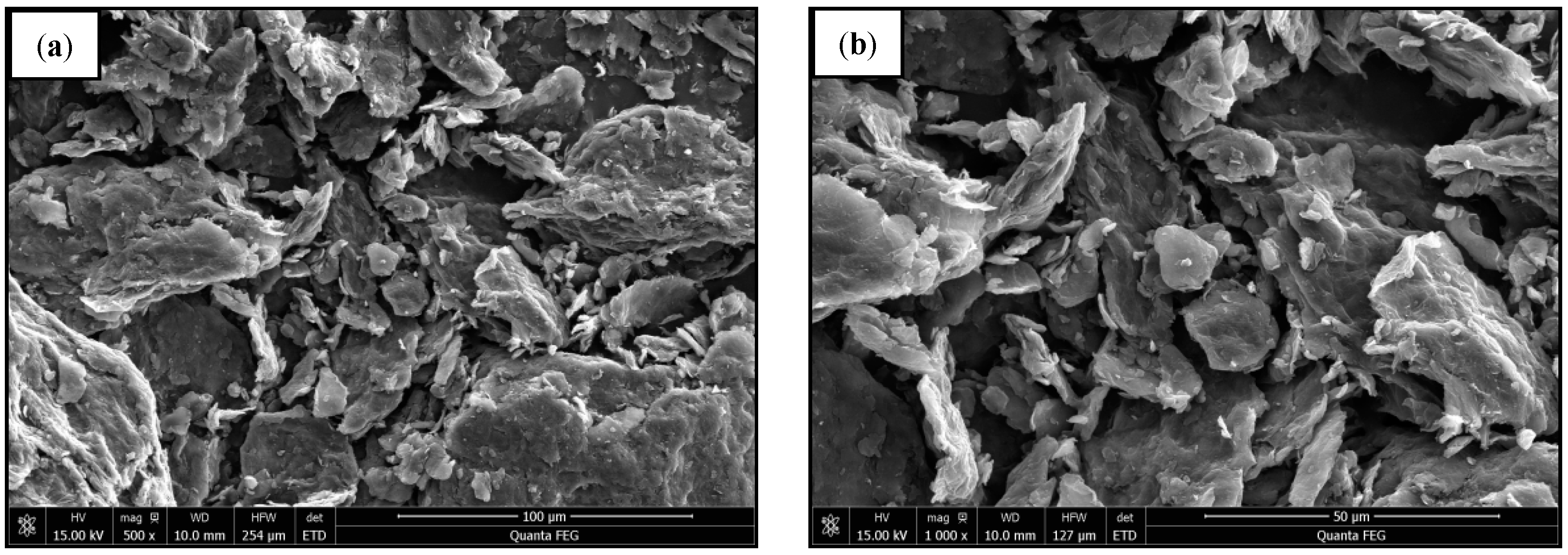
2.3. Microstructure of GO
2.4. Evaluation of Anti-Aging Performance
2.4.1. Aging Procedures
2.4.2. Physical Performance Tests
2.4.3. Characteristic Functional Group Test
2.4.4. Rheological Property Test
3. Results and Discussion
3.1. Characterization of the GO
3.2. UV Aging Resistance Performance
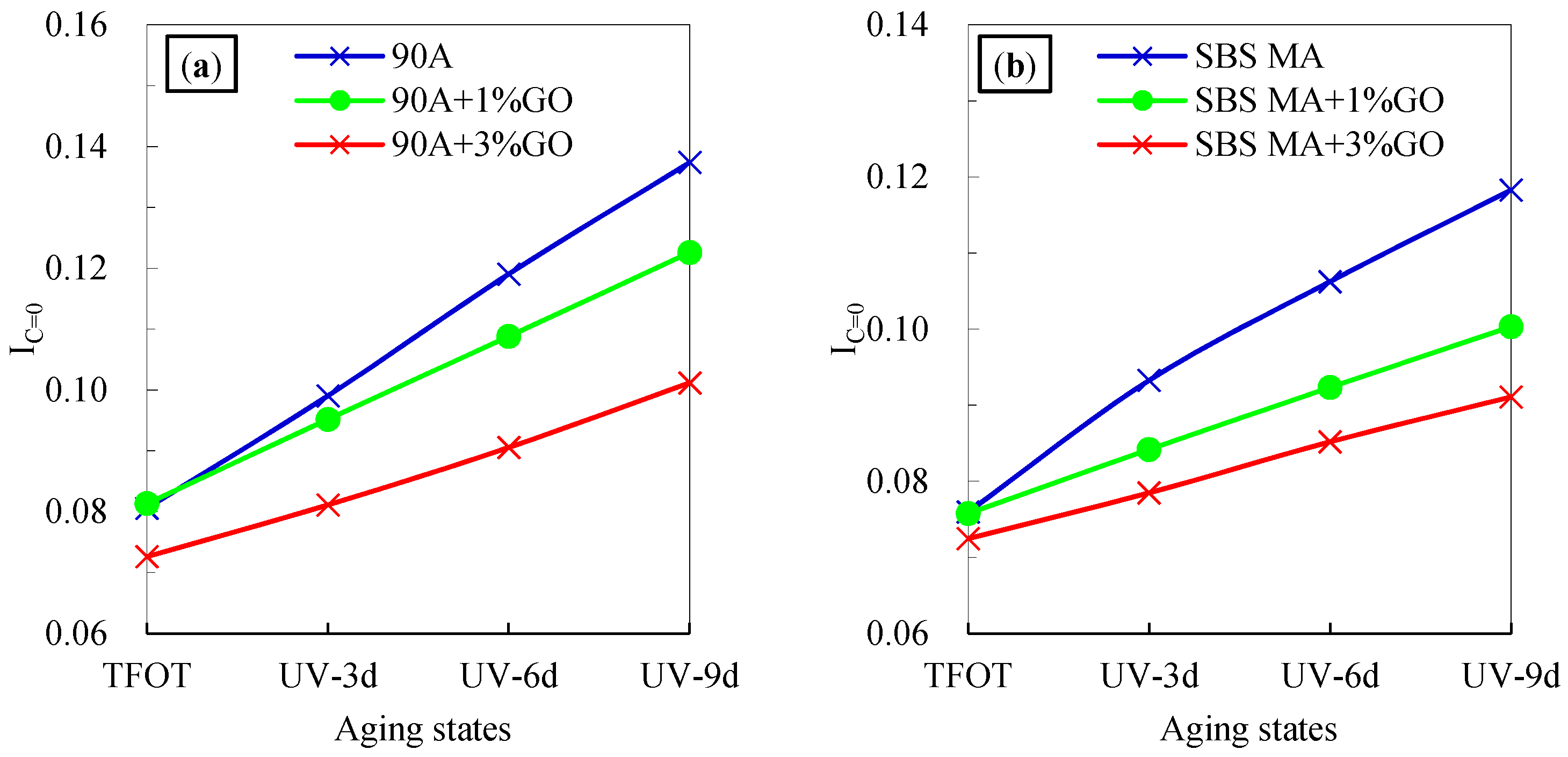
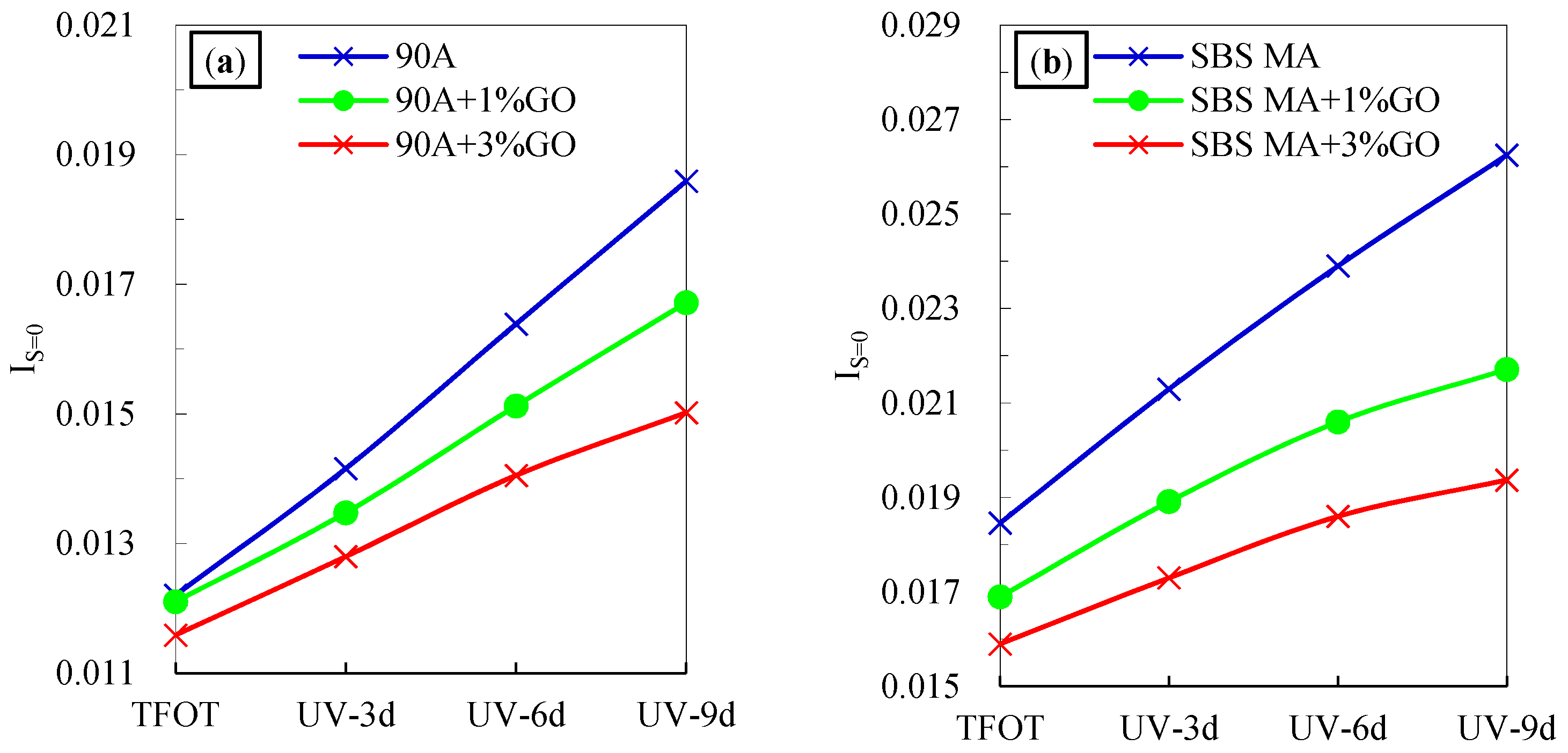
3.2.1. Chemical Structure
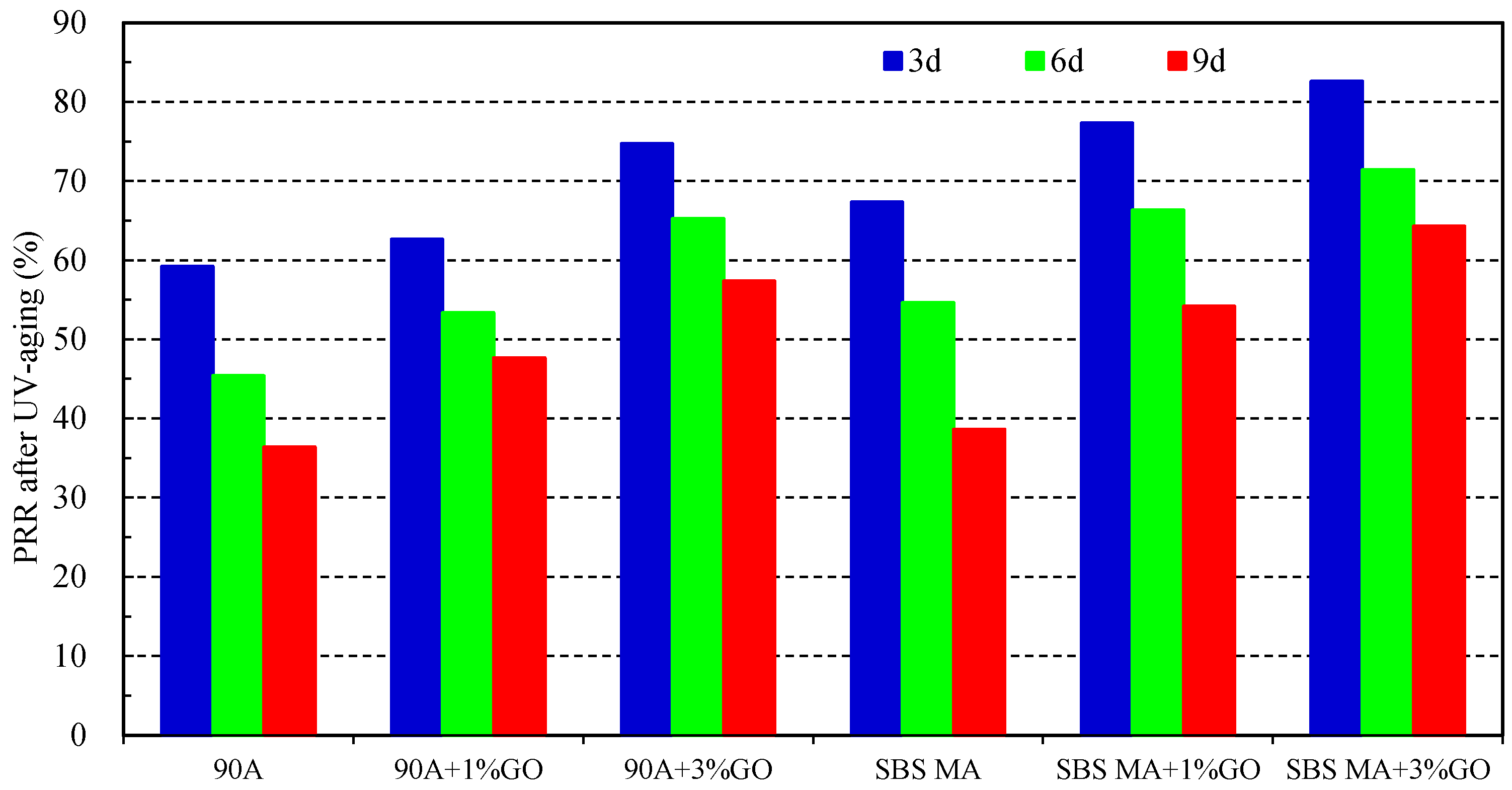
3.2.2. Physical Performance
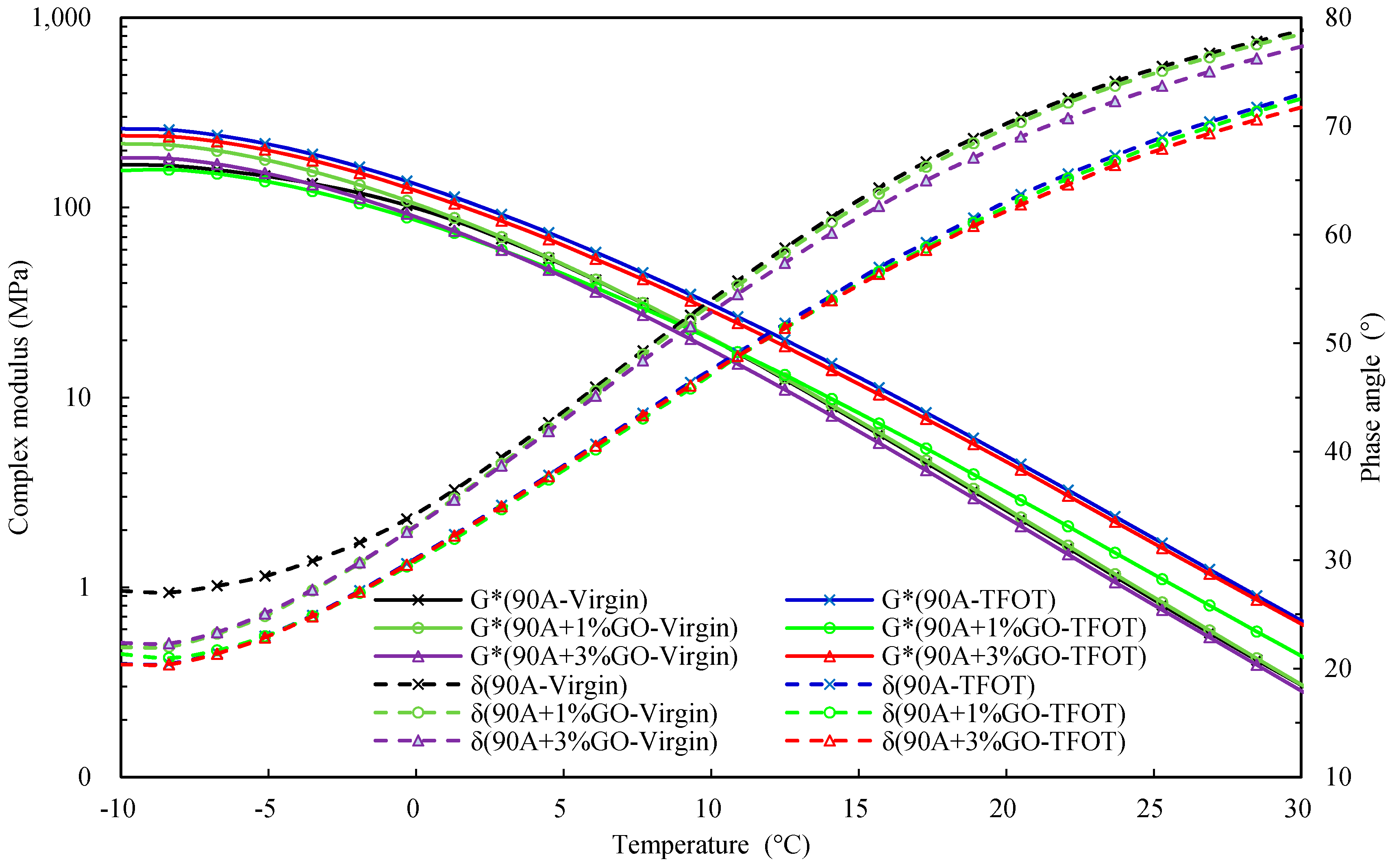
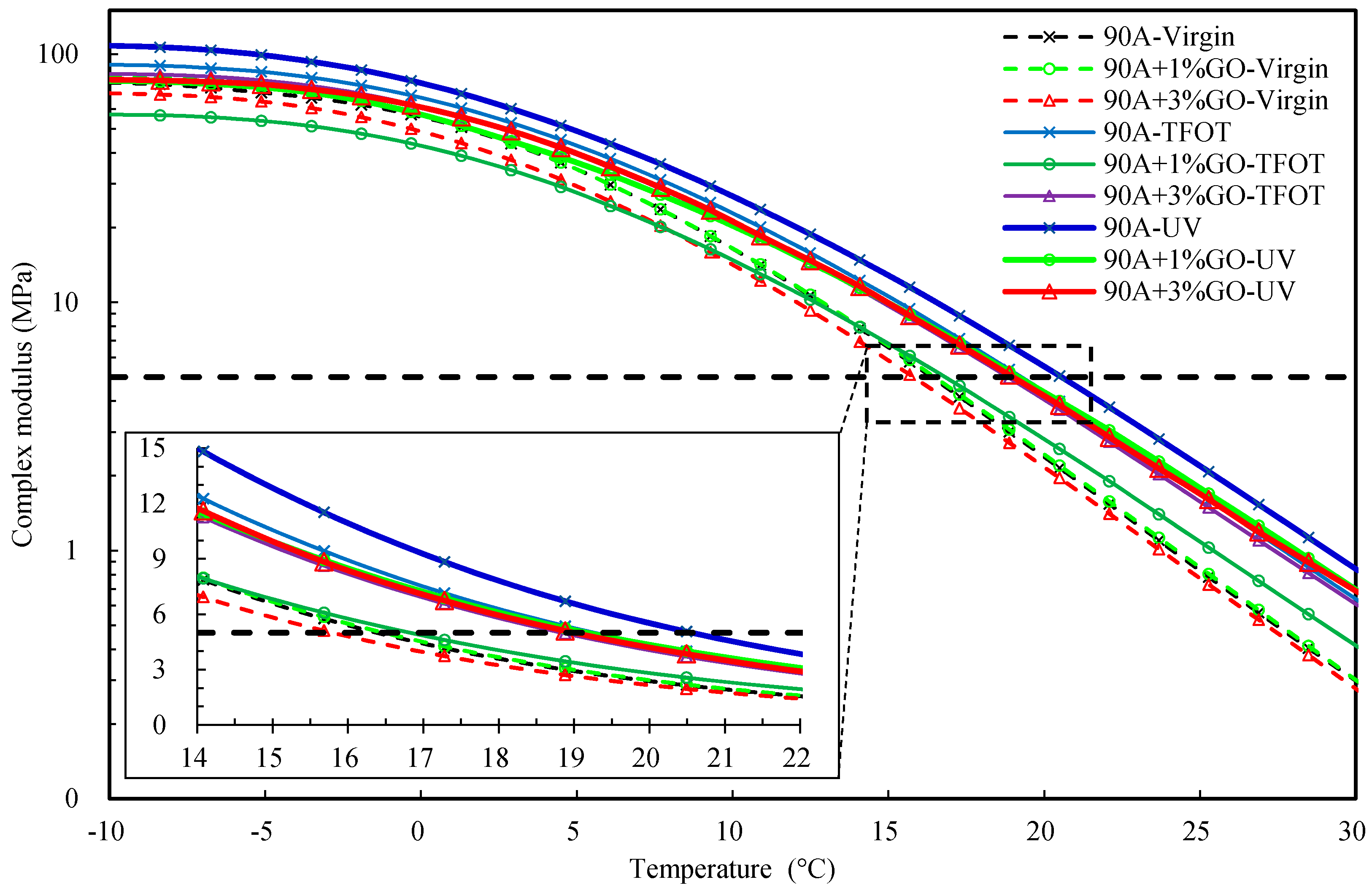
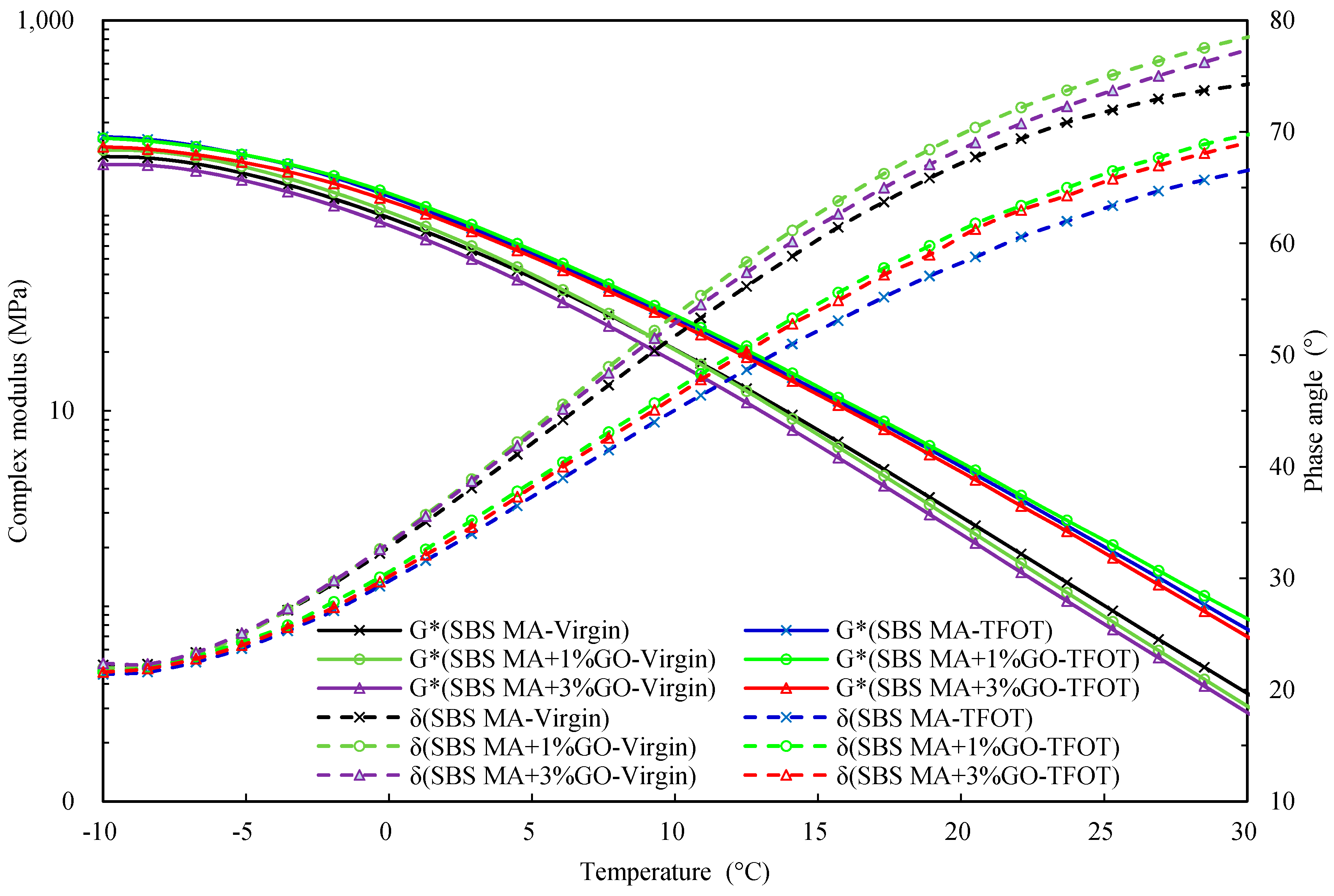
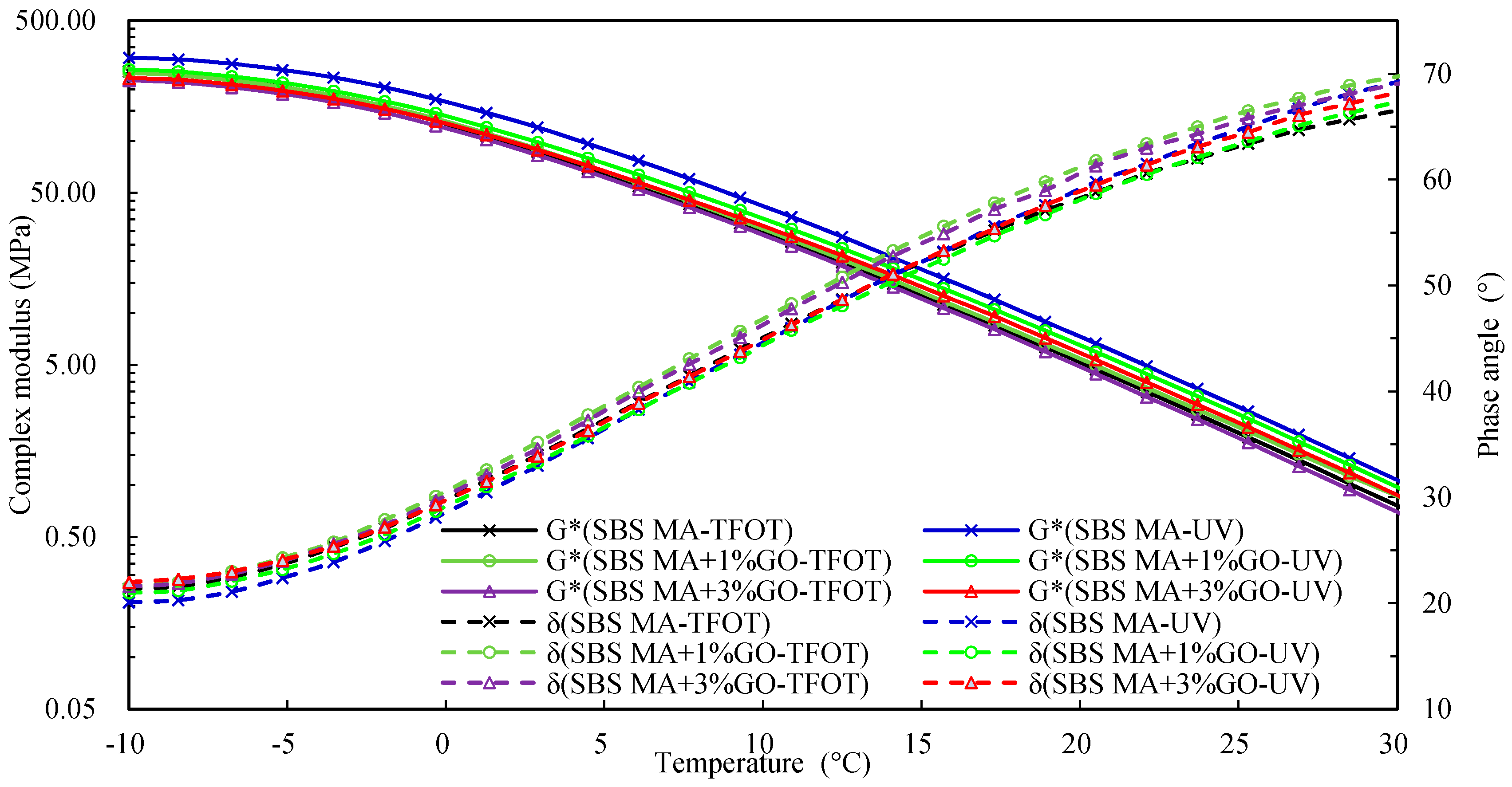
3.2.3. Evaluating the Rheological Properties
4. Conclusions
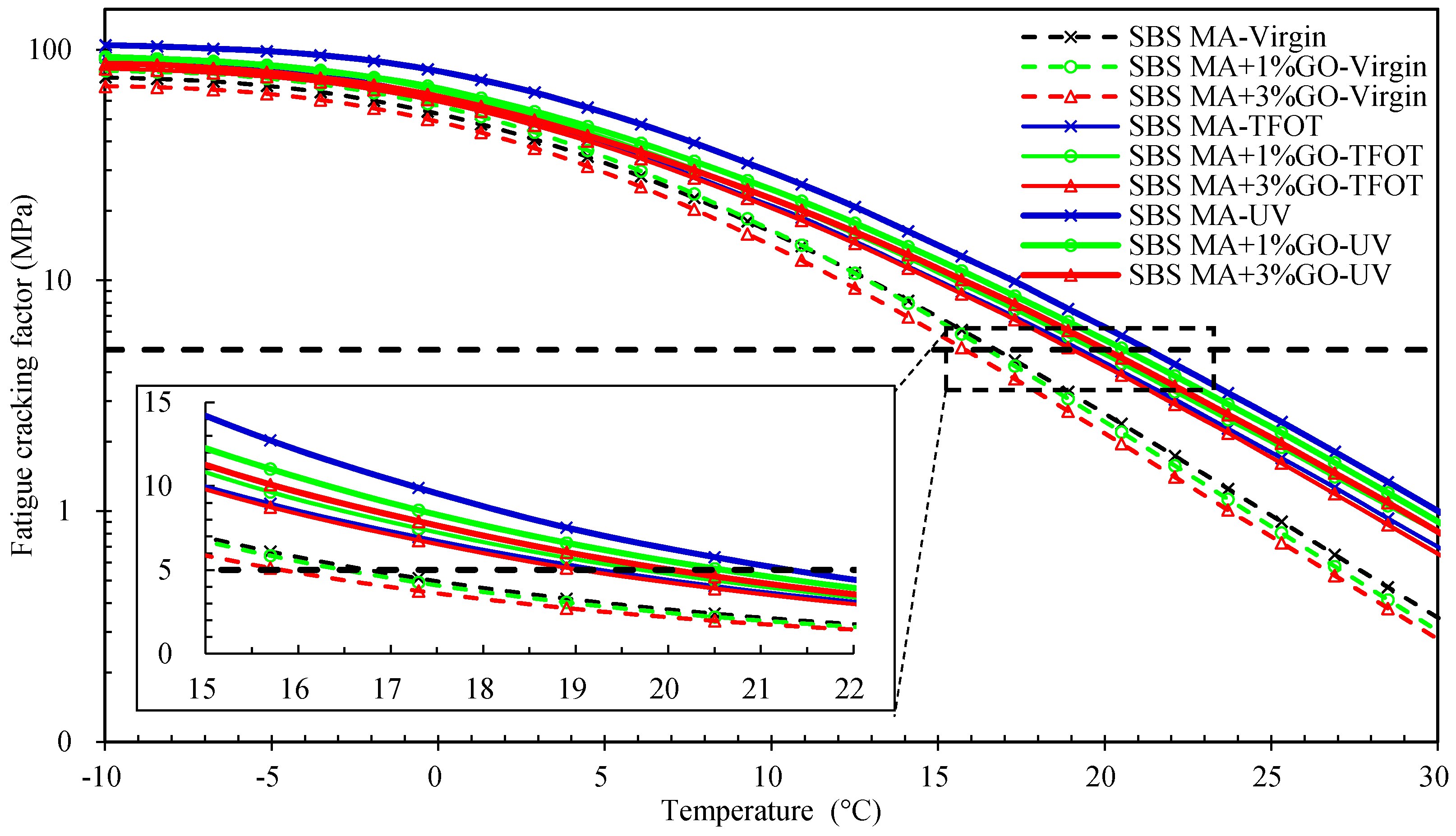
- Before aging, the GO could decrease the G* and slightly change the δ of 90 A and SBS MA; in total, a smaller G*sinδ value was obtained after being modified by the GO, which shows that the GO could improve the fatigue cracking resistance performance of the 90 A and SBS MA binders.
- According to the rheological property testing, before and after TFOT, the GO could improve the thermo-oxidative aging resistance performance of 90 A and SBS MA, and the improvement effect of the GO on 90 A was better than SBS MA.
- After UV aging, the IC=O and IS=O increment of the GO modified asphalt were smaller than that of the asphalt without GO. The GO could retard the formations of the carbonyl and sulfoxide groups during UV aging, and decrease the aging degree of 90 A and SBS MA.
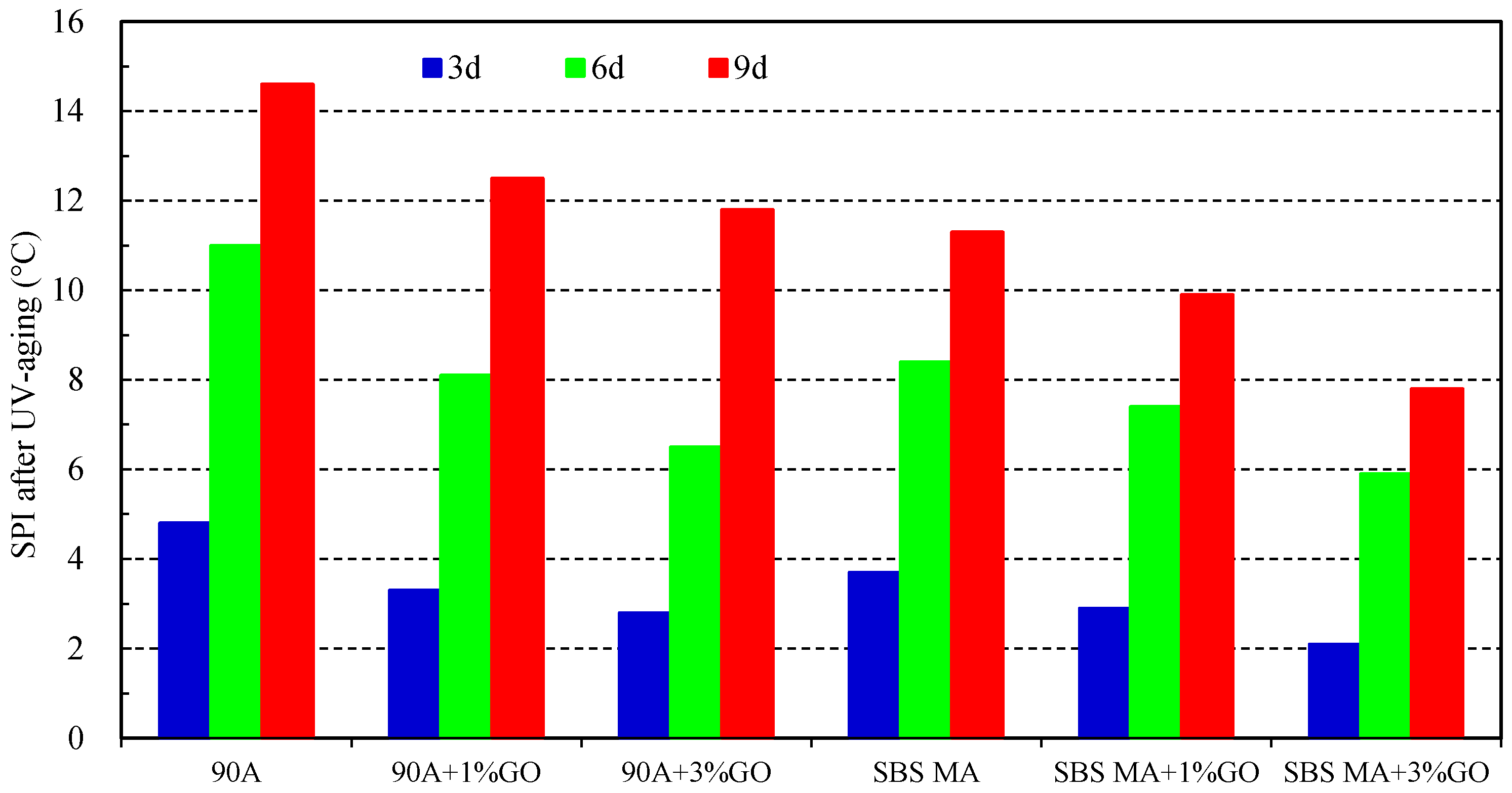
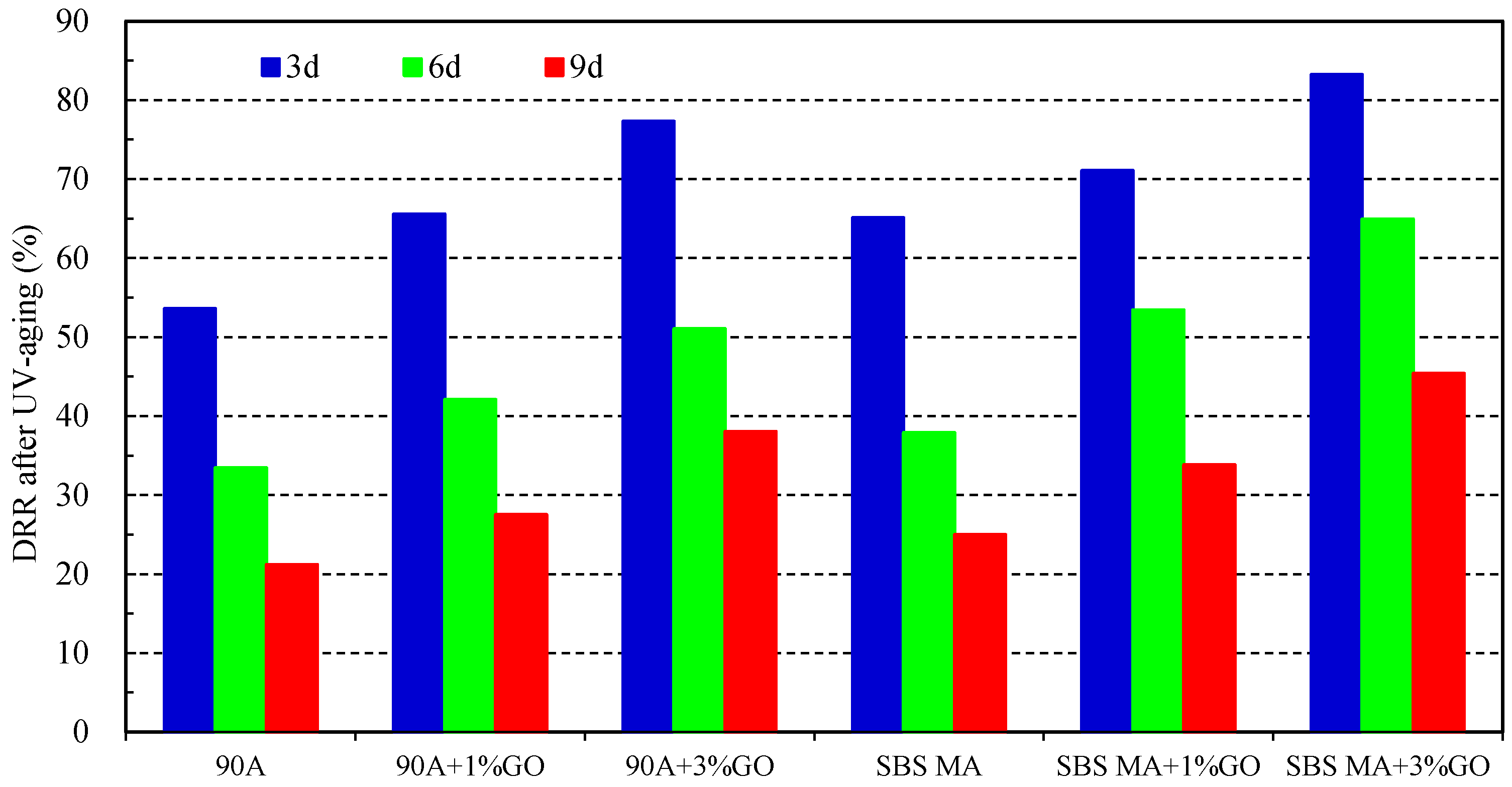
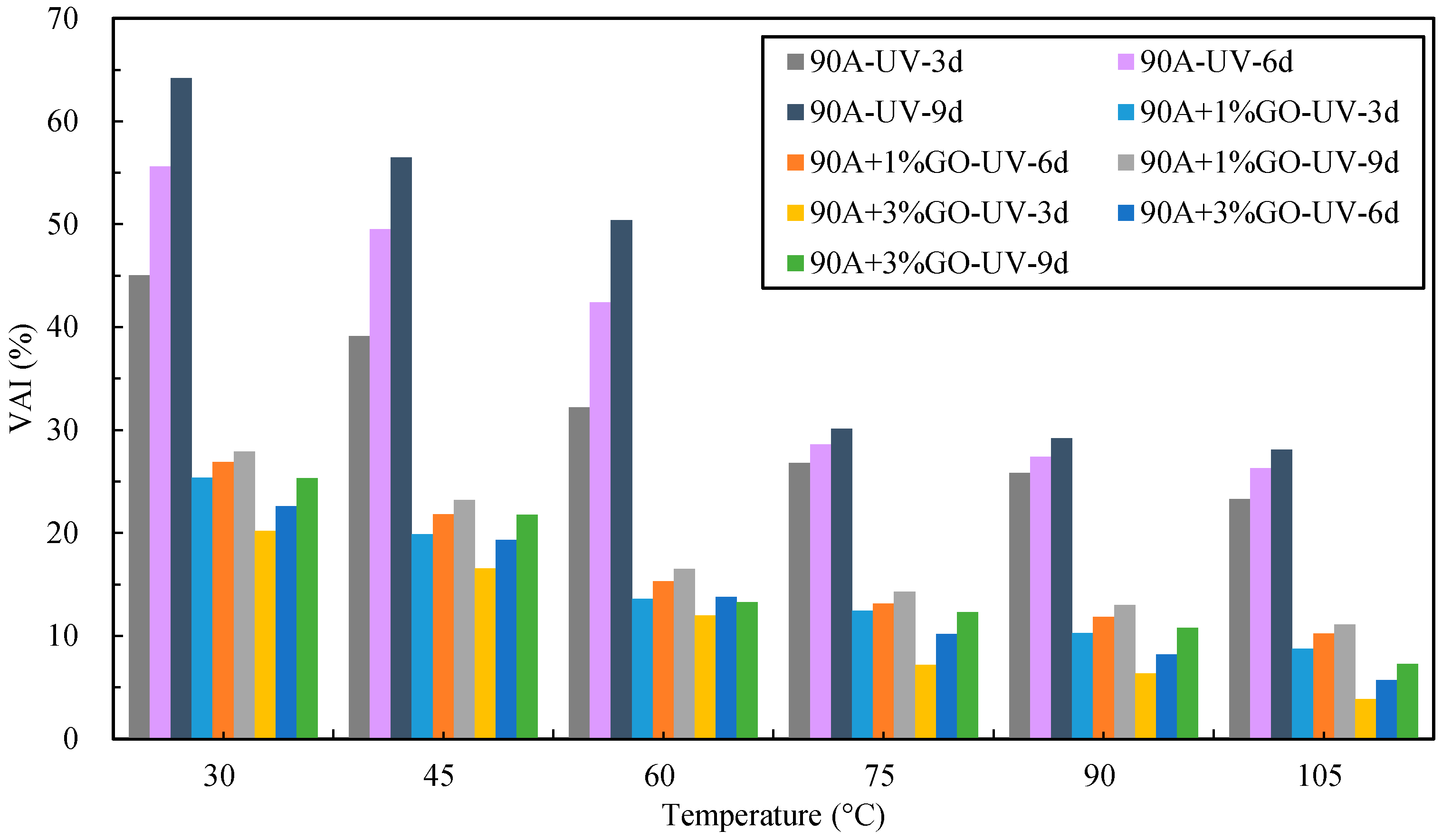
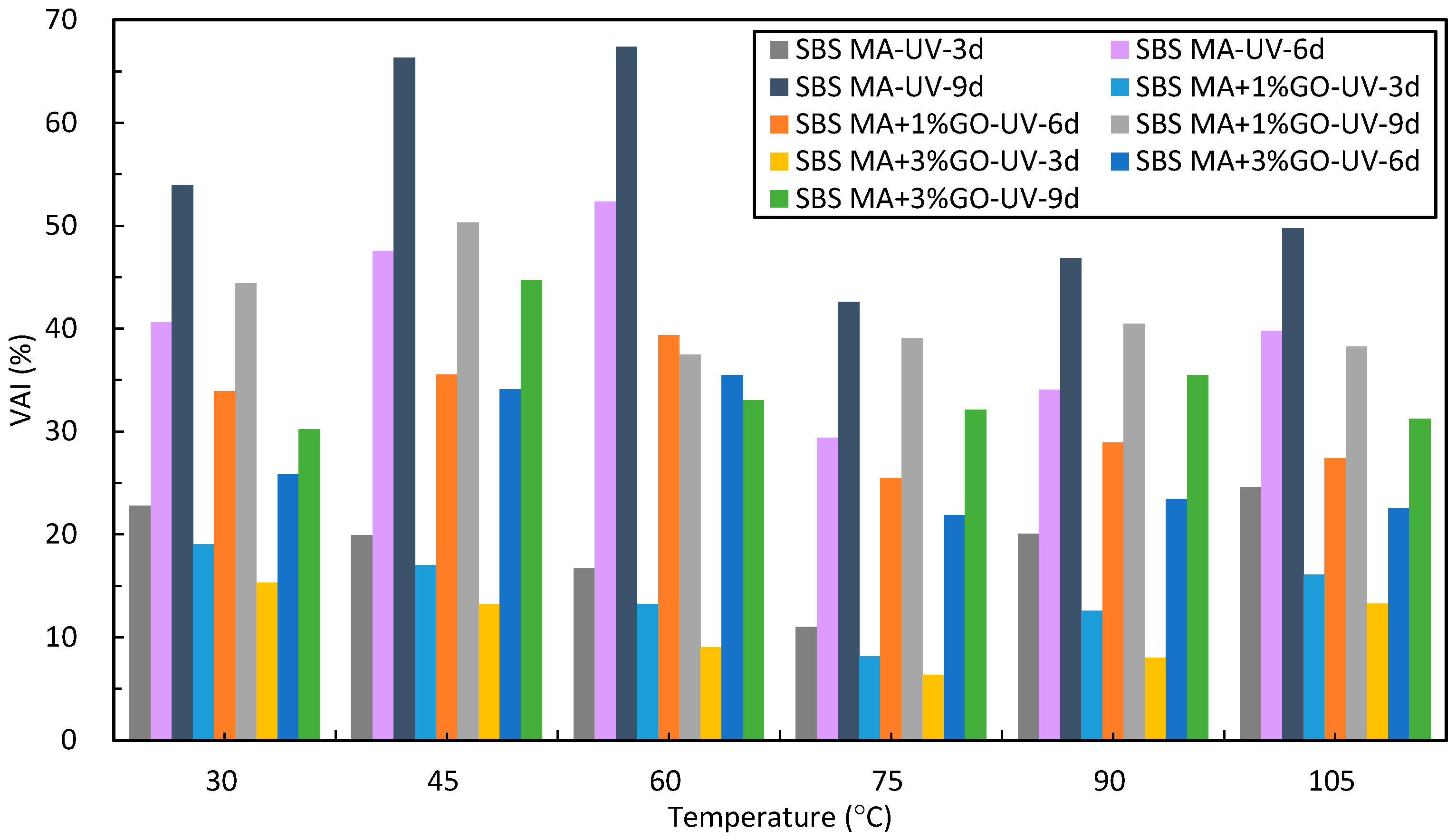
- After UV aging, the GO could increase the PRR and DRR of 90 A and SBS MA, meanwhile decreasing the SPI and VAI; the order of the G* was 3% GO-modified asphalt < 1% GO-modified asphalt < 90 A (or SBS MA). The increment of G* was obviously decreased after adding the GO, and the fatigue cracking resistance performance of GO-modified asphalt were better than that of the asphalt without the GO. The results showed that the GO could improve the stability of the asphalt physical performance. The GO could improve the UV aging resistance performance of 90 A and SBS MA obviously, and the improvement effect of the 3% GO was better than that of 1% GO.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rasool, R.T.; Wang, S.; Zhang, Y.; Li, Y.; Zhang, G. Improving the aging resistance of SBS modified asphalt with the addition of highly reclaimed rubber. Constr. Build. Mater. 2017, 145, 126–134. [Google Scholar] [CrossRef]
- Behnood, A.; Olek, J. Rheological Properties of asphalt binders modified with styrene-butadiene-styrene (SBS), ground tire rubber (GTR), or polyphosphoric acid (PPA). Constr. Build. Mater. 2017, 151, 464–478. [Google Scholar] [CrossRef]
- Islam, M.R.; Tarefder, R.A. Study of asphalt aging through beam fatigue test. Transp. Res. Rec. J. Transp. Res. Board 2015, 2505, 115–120. [Google Scholar] [CrossRef]
- Pang, L.; Liu, K.; Wu, S.; Lei, M.; Chen, Z. Effect of LDHs on the aging resistance of crumb rubber modified asphalt. Constr. Build. Mater. 2014, 67, 239–243. [Google Scholar] [CrossRef]
- Apeagyei, A.K. Laboratory evaluation of antioxidants for asphalt binders. Constr. Build. Mater. 2011, 25, 47–53. [Google Scholar] [CrossRef]
- Petersen, J.C. A review of the fundamentals of asphalt oxidation: Chemical, physicochemical, physical property and durability relationships. Transportation research circular e-c140. Transp. Res. E-Circ. 2009. [Google Scholar] [CrossRef]
- Feng, Z.G.; Wang, S.J.; Bian, H.J.; Guo, Q.L.; Li, X.J. FTIR and rheology analysis of aging on different ultraviolet absorber modified bitumens. Constr. Build. Mater. 2016, 115, 48–53. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, J.; Xue, L.; Sun, Y. Investigation of γ-(2,3-Epoxypropoxy) propyltrimethoxy Silane Surface Modified Layered Double Hydroxides Improving UV Ageing Resistance of Asphalt. Materials 2017, 10, 78. [Google Scholar] [CrossRef]
- Yu, J.Y.; Feng, P.C.; Zhang, H.L.; Wu, S.P. Effect of organo-montmorillonite on aging properties of asphalt. Constr. Build. Mater. 2009, 23, 2636–2640. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, J.; Wu, S. Effect of montmorillonite organic modification on ultraviolet aging properties of SBS modified bitumen. Constr. Build. Mater. 2012, 27, 553–559. [Google Scholar] [CrossRef]
- Cong, P.; Xu, P.; Chen, S. Effects of carbon black on the anti aging, rheological and conductive properties of SBS/asphalt/carbon black composites. Constr. Build. Mater. 2014, 52, 306–313. [Google Scholar] [CrossRef]
- Feng, Z.; Yu, J.; Wu, S. Rheological evaluation of bitumen containing different ultraviolet absorbers. Constr. Build. Mater. 2012, 29, 591–596. [Google Scholar] [CrossRef]
- Nazari, M.H.; Shi, X. Polymer-Based Nanocomposite Coatings for Anticorrosion Applications. In Industrial Applications for Intelligent Polymers and Coatings; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Park, S.; Lee, K.S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene oxide papers modified by divalent ions—Enhancing mechanical properties via chemical cross-linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, D.; Dong, X.Y.; Zhang, Z.J.; Pei, X.F.; Chen, X.J.; Chen, B.; Jin, J. Layered assembly of graphene oxide and Co–Al layered double hydroxide nanosheets as electrode materials for supercapacitors. Chem. Commun. 2011, 47, 3556–3558. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wu, X.; Guo, B.; Zhang, L.; Jia, D. Preparation of butadiene–styrene–vinyl pyridine rubber–graphene oxide hybrids through co-coagulation process and in situ interface tailoring. J. Mater. Chem. 2012, 22, 7492–7501. [Google Scholar] [CrossRef]
- ASTM D5/D5M. Standard Test Method for Penetration of Bituminous Materials; American Society for Testing and Materials: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM D36/D36M. Standard Test Method for Softening Point of Bitumen (Ring and Ball Apparatus); American Society for Testing and Materials: West Conshohocken, PA, USA, 2012. [Google Scholar]
- ASTM D113. Standard Test Method for Ductility of Bituminous Materials; American Society for Testing and Materials: West Conshohocken, PA, USA, 2007. [Google Scholar]
- ASTM D4402. Standard Test Method for Viscosity Determination of Asphalt at Elevated Temperatures Using a Rotational Viscometer; American Society for Testing and Materials: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Yoo, B.M.; Shin, H.J.; Yoon, H.W.; Park, H.B. Graphene and graphene oxide and their uses in barrier polymers. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically derived graphene oxide: Towards large-area thin-film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cote, L.J.; Kim, F.; Yuan, W.; Shull, K.R.; Huang, J. Graphene oxide sheets at interfaces. J. Am. Chem. Soc. 2010, 132, 8180–8186. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, G.; Bai, X.; Sun, X.; Wang, X.; Wang, E.; Dai, H. Highly conducting graphene sheets and Langmuir–Blodgett films. Nat. Nanotechnol. 2008, 3, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’Homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhu, M.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Xu, Z.; Zhu, H. Selective ion penetration of graphene oxide membranes. ACS Nano 2012, 7, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Lim, Y.S.; Han, J.H.; Kim, K.; Kim, J.Y.; Choi, S.Y.; Shin, K. A graphene oxide oxygen barrier film deposited via a self-assembly coating method. Synth. Met. 2012, 162, 710–714. [Google Scholar] [CrossRef]
- Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Synthesis of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1179–1184. [Google Scholar] [CrossRef]
- Priolo, M.A.; Gamboa, D.; Holder, K.M.; Grunlan, J.C. Super gas barrier of transparent polymer−clay multilayer ultrathin films. Nano Lett. 2010, 10, 4970–4974. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, S.; Pang, L.; Sun, Y.; Chen, Z. The utilization of graphene oxide in traditional construction materials: Asphalt. Materials 2017, 10, 48. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef] [PubMed]
- AASHTO T315. Determining the Rheological Properties of Asphalt Binder Using a Dynamic Shear Rheometer; American Association of State Highway and Transportation Officials: Washington, DC, USA, 2010. [Google Scholar]

| Asphalt | Technical Parameters | Unit | Test Results | Method |
|---|---|---|---|---|
| 90 A | 25 °C Penetration | 0.1 mm | 84.6 | ASTM D5 [17] |
| Softening point | °C | 47.8 | ASTM D36 [18] | |
| 10 °C ductility | cm | >100 | ASTM D113 [19] | |
| 60 °C viscosity | Pa⋅s | 206 | ASTM D4402 [20] | |
| SBS MA | 25 °C Penetration | 0.1 mm | 62.3 | ASTM D5 [17] |
| Softening point | °C | 57.5 | ASTM D36 [18] | |
| 5 °C ductility | cm | 55.8 | ASTM D113 [19] | |
| 135 °C viscosity | Pa⋅s | 1.337 | ASTM D4402 [20] |
| Aging Degree | Virgin | TFOT Aging | UV Aging | ||||||
|---|---|---|---|---|---|---|---|---|---|
| GO content (%) | 0 | 1 | 3 | 0 | 1 | 3 | 0 | 1 | 3 |
| FFT (°C) | 16.5 | 16.5 | 15.8 | 19.3 | 16.7 | 18.8 | 20.6 | 19.2 | 19.0 |
| Aging Degree | Virgin | TFOT Aging | UV Aging | ||||||
|---|---|---|---|---|---|---|---|---|---|
| GO content (%) | 0 | 1 | 3 | 0 | 1 | 3 | 0 | 1 | 3 |
| FFT (°C) | 16.8 | 16.6 | 15.8 | 19.3 | 19.8 | 19.1 | 21.4 | 20.6 | 20.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Zhao, Z.; Li, Y.; Pang, L.; Amirkhanian, S.; Riara, M. Evaluation of Aging Resistance of Graphene Oxide Modified Asphalt. Appl. Sci. 2017, 7, 702. https://doi.org/10.3390/app7070702
Wu S, Zhao Z, Li Y, Pang L, Amirkhanian S, Riara M. Evaluation of Aging Resistance of Graphene Oxide Modified Asphalt. Applied Sciences. 2017; 7(7):702. https://doi.org/10.3390/app7070702
Chicago/Turabian StyleWu, Shaopeng, Zhijie Zhao, Yuanyuan Li, Ling Pang, Serji Amirkhanian, and Martin Riara. 2017. "Evaluation of Aging Resistance of Graphene Oxide Modified Asphalt" Applied Sciences 7, no. 7: 702. https://doi.org/10.3390/app7070702







